Abstract
Studies have revealed that many patients developing deep vein thrombosis (DVT) during a hospitalization have not received any prior DVT prophylaxis despite a lack of medical contraindications. Many measures have been proposed to increase the use of DVT prophylaxis, including computer assisted reminders, standing admission orders, educational activities, etc. Another measure to increase the use of DVT prophylaxis has been to study special patient populations and extended duration of DVT prophylaxis. We present the prevalence of contraindications to the use of a low-molecular-weight-heparin (LMWH) prophylaxis regimen that we encountered in screening patients for admission to a clinical trial of DVT prophylaxis using LMWH with extended treatment at home after discharge.
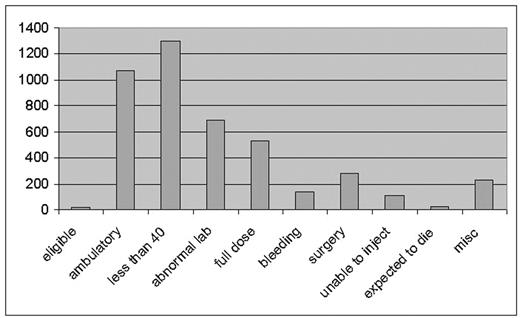
From November 2002 through July 2005 patients admitted to the general medical service of a major tertiary care academic medical center were screened for trial eligibility. Of 4388 patients screened only 19 (0.4%) were eligible for the trial. Of the 4369 patients excluded, 1072 (25%) were not confined to bed and were not felt to need DVT prophylaxis. Individuals under the age of 40 [1305 (30%)] were considered to be at low risk and thus not in need of DVT prophylaxis. An abnormal baseline laboratory value (either creatinine, platelet count or prothrombin time) precluded LMWH treatment in 687 patients (16%). There were 534 patients (12%) who already had an indication for full dose anticoagulation and were excluded. Active bleeding or a pre-existing bleeding disorder was present in 136 patients (3%). There were 276 patients (6%) with a history of recent surgery who were either considered high risk for bleeding complications or were already receiving DVT prophylaxis or treatment, and 109 patients (2.5%) who were judged by themselves or the medical staff to be unable to self-inject the LMWH at home. Individuals expected to die before completion of the trial [21 (0.5%)] were excluded. Miscellaneous exclusions such as demented or comatose patients unable to give consent, surgical or trauma patients admitted to a medical unit or need for an invasive procedure accounted for an additional 229 individuals (5%) of the total.
These results do not reflect any lack of adherence to guidelines in the use of DVT prophylaxis at our institution, rather they indicate that the study design included criteria strict enough to restict eligibility to very few individuals. Strict eligibility criteria serve to limit a trial to those individuals most likely to show safety and efficacy of the study drug and avoid diluting the results with groups in which safety and efficacy might not be better than the control treatment. The limitation of that strategy is that the results may not be generalizable to a larger population. Labelling, marketing and treatment decisions based on this and other trials require a knowledge of the generalizability of the data. This in turn requires a knowledge of the inclusion and exclusion criteria and screening data. Based upon this experience we would recommend that screening data be made available when studies are eiither registered or published.
Author notes
Corresponding author