Abstract
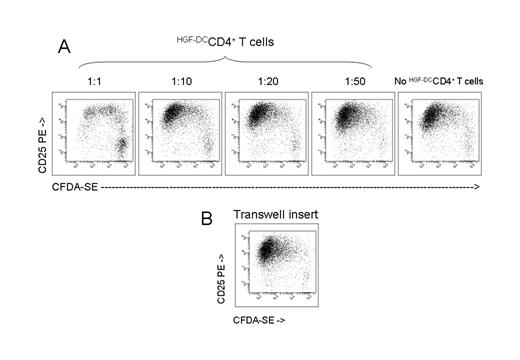
Dendritic cells (DCs) are highly specialized antigen presenting cells (APCs) with unique capacity to fully activate and induce clonal expansion of naive and memory T cells. Recently, DCs have been implicated in the maintenance of antigen (Ag)-specific unresponsiveness or tolerance through the induction of regulatory T (Treg) cells. Several hematopoietic growth factors, including interleukin-10 (IL-10), transforming growth factor-β1 (TGF-β1) and granulocyte colony-stimulating factor (G-CSF), might promote the differentiation of regulatory dendritic cells (DCs) in vivo and/or in vitro. Hepatocyte growth factor (HGF) is a pleiotropic cytokine and its effects on human DC differentiation and function have not been investigated. Monocytes cultured with HGF (HGF-DCs) differentiated into accessory cells with DC features, released low amounts of IL-12p70 and up-regulated IL-10 both at the mRNA and at the protein level. HGF-DCs displayed a CD14+CD1a−costimulationlow phenotype, and expressed the inhibitory receptor ILT3. Upon activation with HGF-DCs, allogeneic CD4+CD25− T cells up-regulated CD25 and the Treg-associated transcription factor FoxP3, proliferated poorly and released high levels of IL-10 but trace amounts of IL-2 and IFN-≤ . Interestingly, blockade of surface ILT3 on HGF-DCs or neutralization of secreted IL-10 translated into partial restoration of T-cell proliferation. Addition of exogenous IL-2 to CD4+ T cells initially primed with HGF-DCs was not associated with the enhancement of T cell proliferation. Secondary stimulation of HGF-DC-primed CD4+ T cells with immunogenic DCs differentiated with GM-CSF and IL-4 from monocytes of the same donor resulted in optimal T-cell proliferation. Interestingly, HGF-DC-primed CD4+ T cells significantly inhibited the proliferation of naive CD4+CD25− T cells in a cell contact-dependent manner, as shown by co-culture experiments with transwell inserts.
Finally, DNA microarray analysis revealed a unique gene expression profile of HGF-activated monocytes. Specifically, HGF-DCs over-expressed a set of 217 genes implicated in cellular metabolism, signal transduction, cell adhesion, cell-to-cell signaling, inflammation and immune response.
Genes up-regulated by HGF in freshly isolated monocytes are categorized according to their biological function
| Defense response | 19 |
| Cell adhesion and signaling | 12 |
| Signal transduction | 32 |
| Transcription regulation | 17 |
| Metabolism | 35 |
| Protein synthesis and degradation | 24 |
| Cytoskeletal organization | 7 |
| Transport | 16 |
| Chemotaxis | 9 |
| Cell proliferation and differentiation | 6 |
| Diverse physiological functions | 9 |
| Response to stress | 9 |
| Cell growth | 4 |
| Cell cycle | 4 |
| Morphogenesis | 13 |
| Unclassified | 31 |
| Defense response | 19 |
| Cell adhesion and signaling | 12 |
| Signal transduction | 32 |
| Transcription regulation | 17 |
| Metabolism | 35 |
| Protein synthesis and degradation | 24 |
| Cytoskeletal organization | 7 |
| Transport | 16 |
| Chemotaxis | 9 |
| Cell proliferation and differentiation | 6 |
| Diverse physiological functions | 9 |
| Response to stress | 9 |
| Cell growth | 4 |
| Cell cycle | 4 |
| Morphogenesis | 13 |
| Unclassified | 31 |
Of interest, HGF-DCs also upregulated mRNA signals for genes involved in tryptophan catabolisms, e.g., indoleamine 2,3-dioxygenase (IDO).
Collectively, our findings point to a novel role for HGF in the regulation of monocyte/DC functions. From a therapeutic standpoint, HGF might represent an attractive target for immunotherapy.
Author notes
Corresponding author