Abstract
Purpose: Extracellular antagonists of the cellular WNT signalling pathway can be divided into two classes depending on the mechanism by which ligand-receptor interactions are inhibited. Members of the first class including SFRP (secreted frizzled-related proteins), WIF1 (WNT inhibitory factor 1) and CER1 (cerberus homolog 1) bind to WNT proteins in the extracellular milieu. Members of the second class including Dickkopf (DKK) proteins bind to the WNT receptor subunits LRP5/6. Reduced WNT signalling may play a role in the natural history of the bone disease that is a hallmark of multiple myeloma (MM). Gene expression profiling of MM plasma cells has shown that over-expression of the Dickkopf family member DKK1 is linked with abundance and size of osteolytic lesions (N Engl J Med 2003, 349:2483–94). The biological mechanism of DKK1-mediated bone destruction is not known.
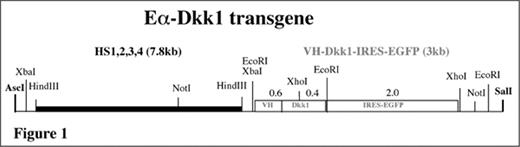
Experimental procedure: To elucidate the mechanism by which DKK1 facilitates osteolysis, we generated transgenic mice in which mouse Dkk1 is expressed under control of the mouse immunoglobulin Eα enhancer. This enhancer is located 3′ of the immunoglobulin heavy-chain locus and comprised of four individual elements that span ~30 kb of genomic DNA. These elements were subcloned into a contiguous stretch of DNA, which we refer to as “reconstituted” Eα enhancer or HS1234 (Int Immunol 2001, 13:1003–12). Here we appended HS1234 to a Dkk1 cDNA whose expression is controlled by a mouse immunoglobulin VH promoter inserted 5′ of the transcriptional start site of Dkk1. Because of an internal ribosome entry site (IRES) 3′ of Dkk1, the gene encoding enhanced green fluorescence protein (EGFP) is co-expressed with Dkk1; i.e., it is a reporter of Dkk1 expression. A scheme of the structure of the Eα-Dkk1 transgene is depicted in Figure 1.
Results: We generated 14 founder lines of Eα-Dkk1 transgenic mice that expressed Dkk1 and the EGFP-encoding gene by RT-PCR (Figure 2).
The mice contained a variable number of Eα-Dkk1 copies, ranging from 2–3 to more than 100. There was but a loose association of transgene expression (qPCR of Dkk1 mRNA levels) and copy number (qPCR of genomic Ea-Dkk1 fragments); i.e., the high-copy number mice exhibited higher average mRNA levels than the low-copy number mice, although there were outliers in each group. Consistent with Eα-dependent restriction of Dkk1 expression to B/plasma cells, Dkk1 mRNA was readily detectable in lymphoid tissues, such as spleen, mesenteric lymph node and bone marrow, but not in liver.
Conclusion: The newly developed Eα-Dkk1 transgenics may provide a valuable model system to elucidate DKK1-dependent bone disease in human MM.
Author notes
Corresponding author