Abstract
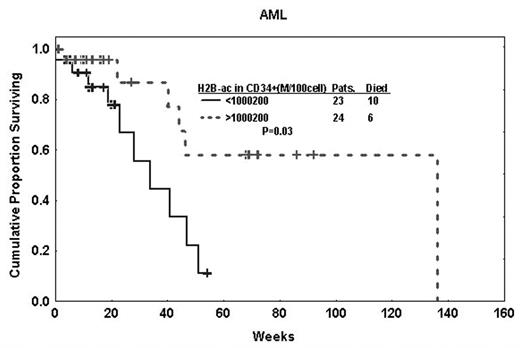
Histone acetylation, along with DNA methylation, is a major epigenetic silencing mechanism believed to play a role in oncogenesis. Agents counteracting histone acetylation, such as the histone deacetylase (HDAC) inhibitors sodium phenylbutyrate and valproic acid, have shown promise in some types of leukemia and MDS. Despite the interest in HDAC inhibitors as therapeutic agents, little is known about the levels of histone acetylation and its clinical relevance in leukemia. In this study we used flow cytometry to quantify the levels of histone 2B acetylation (H2B-ac) and H3-ac in CD34+ and CD3+ cells in AML and MDS patients. Quantification using QuantiBRITE and PE (phycoerythrin)-labeled antibodies with a 1:1 ratio allowed us to specifically measure the antibody binding capacity in 100 CD34+ or CD3+ cells (molecules/100 CD34+ or CD3+ cells). While there was no significant difference in H2B-ac in CD3+ cells between previously untreated AML (50 patients) and previously untreated MDS (11 patients), a significantly higher percentage of CD34+ cells in MDS patients were positive for H2B-ac (P=0.03). In contrast, H3-ac levels were significantly higher in CD34+ and CD3+ cells in MDS than in AML (P=0.02 and P=0.03, respectively). This supports the concept that MDS is not simply a preleukemic disease and the blast population in MDS has characteristics different from those of blasts in AML. In patients with AML, higher levels of H2B-ac in CD34+ cells were associated with longer survival (P=0.03; Figure); multivariate analysis showed that this association was independent of cytogenetic abnormalities. This data confirms that epigenetic changes play a role in clinical behavior in patients with AML and that these patients may benefit from therapy that modifies histone deacetylation.
Author notes
Corresponding author