Abstract
Background &Aims Scleroderma is an immune mediated vasculopathy of unknown etiology. There is no known therapy that could alter the natural course of the disease. Patients with diffuse cutaneous disease or visceral involvement has an overall 10-year survival of 35 to 68%. Autologous hematopoietic stem cell transplantation (HSCT) with myeloablative regimen has been performed, but with substantial mortality and morbidity especially pulmonary and renal toxicity. We have performed autologous HSCT utilizing non-myeloablative, but lymphoablative conditioning.
Methods
We conducted a phase 1 HSCT study in 9 patients with diffuse scleroderma with poor clinical features. Candidates were less than 65 years old with Rodman score of more than 14 or diffusion capacity less than 80%, interstitial lung disease, elevated ESR, renal involvement, or abnormal electrocardiogram. Patients with pulmonary hypertension (systolic pressure > 45 mm Hg) were excluded. Peripheral blood stem cells were mobilized with cyclophosphamide and granulocyte colony-stimulating factor. The graft was not manipulated. The conditioning regimen consisted of 200mg/kg cyclophosphamide (CY), and 7.5mg/kg rabbit antithymocyte globulin (ATG).
Results
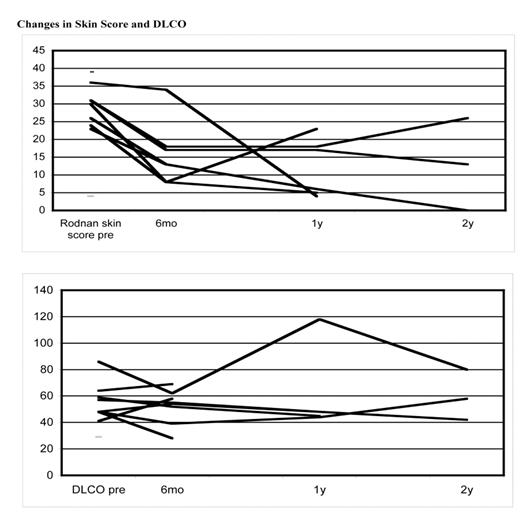
The procedure was well tolerated with anticipated cytopenias, neutropenic fever, and fluid overload that were easy to manage and without mortality. The median days for neutrophil and platelet engraftment were 8 (range 7–9) and 8 (range 0–10), respectively. The median infused CD34+ and CD3+ cell counts were 8.31 x 106/kg (range 2.35–14.7) and 2.03 x 108/kg (range 0.41–6.83), respectively. There was a marked improvement of skin score in all subjects, whereas cardiac (ejection fraction, pulmonary arterial pressure), pulmonary function (DLCO) and renal function (creatinine) remained stable. One patient with advanced disease with poor performance status died 2 years after the transplant with progressive disease. After median follow-up of 20 months (range 5–32), the overall survival is 89% (eight out of nine) and progression-free survival with continuing improvement is 67% (six out of nine. 2 patients developed recurrence of skin tightness without compromising organ function. Both of them were placed on mycophenolate mofetil with gradual improvement of skin tightness.
Conclusion
Autologous HSCT with CY/ATG is safe and is effective. A randomized study (ASSIST: American Scleroderma Stem Cell vs. Immune Suppression Trial), comparing HSCT to intravenous pulse cyclophosphamide is now enrolling patients.
Author notes
Corresponding author