Abstract
Ribavirin has been used in combination with interferon-alpha (IFN-α) to treat chronic hepatitis C. This combination therapy has been reported to be more effective than IFN-α monotherapy for eradicating hepatitis C virus (HCV), including patients with concomitant hemophilia. Eight consecutive hemophilia patients were treated for HCV infection with IFN-α and ribavirin between June 2002 and May 2005 at Nagoya University Hospital as outpatients.
Characteristics of patients and responses to anti-HCV treatment
| Patient . | Age (yrs) . | Hemophilia type . | Severity of hemophilia . | HIV infection . | Ribavirin load (mg/day) . | HCV genotype . | Eradication of HCV . |
|---|---|---|---|---|---|---|---|
| The eradication of HCV was considered positive when the absence of serum HCV RNA was maintained for 24 weeks after treatment was completed. | |||||||
| 1 | 28 | A | moderate | N | 800 | 3a | Yes |
| 2 | 61 | A | severe | N | 800 | 3a | Yes |
| 3 | 50 | A | severe | N | 600 --> 400 | 1b | No |
| 4 | 42 | B | mild | N | 800 | 2a +1b | Yes |
| 5 | 44 | A | severe | N | 800 | 3a | Yes |
| 6 | 52 | A | mild | N | 600 | 2b | Yes |
| 7 | 37 | A | mild | P | 800 --> 600 | 1a | No |
| 8 | 44 | B | moderate | N | 800 | 1a | Yes |
| Patient . | Age (yrs) . | Hemophilia type . | Severity of hemophilia . | HIV infection . | Ribavirin load (mg/day) . | HCV genotype . | Eradication of HCV . |
|---|---|---|---|---|---|---|---|
| The eradication of HCV was considered positive when the absence of serum HCV RNA was maintained for 24 weeks after treatment was completed. | |||||||
| 1 | 28 | A | moderate | N | 800 | 3a | Yes |
| 2 | 61 | A | severe | N | 800 | 3a | Yes |
| 3 | 50 | A | severe | N | 600 --> 400 | 1b | No |
| 4 | 42 | B | mild | N | 800 | 2a +1b | Yes |
| 5 | 44 | A | severe | N | 800 | 3a | Yes |
| 6 | 52 | A | mild | N | 600 | 2b | Yes |
| 7 | 37 | A | mild | P | 800 --> 600 | 1a | No |
| 8 | 44 | B | moderate | N | 800 | 1a | Yes |
All patients were men with a mean age (SD) of 44.8 (10.0) years. Seven patients had hemophilia A, and 2 had hemophilia B. Hemophilia was severe in 4 patients, moderate in 2 and mild in 3. Four patients had been previously treated with IFN-α-2b alone (Intron A®, Schering Plough, K.K., Osaka, Japan) but HCV had not been eradicated. During this study, all patients were treated with the same 24-week regimen of IFN-α. Oral ribavirin (Rebetol, Schering-Plough, Kenilworth, N.J.) was administered at a dose of 600 mg/day for patients who weighed 60 kg or less and 800 mg/day for those who weighed more than 60 kg during 24 weeks. We observed the reduction of the frequency and dose of infusion with clotting factors as a hemostatic therapy in HCV-positive hemophilia patients who were administered with ribavirin.
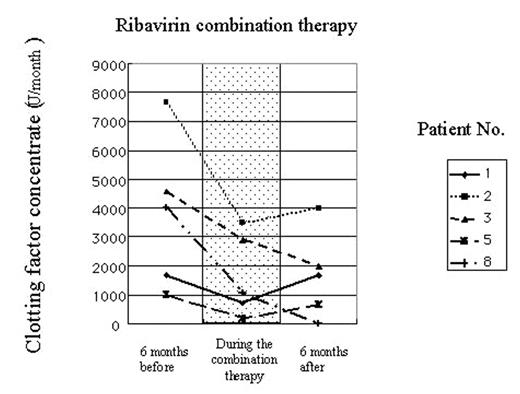
(Use of clotting factor concentrates 6 months before, during and 6 months after combination therapy with ribavirin and IFN-α. Use of clotting factors is presented as the average use per month.) In order to investigate the mechanism of this prophylactic effect of ribavirin to bleeding in hemophiliacs, we analyzed ribavirin-induced changes in the activity of factor VII in patients’ plasma. The clotting activity of factor VII in plasma has been elevated at 15% on an average in 9 HCV-positive hemophilia patients during treatment with ribavirin (without ribavirin: 86.3±7.6%; with ribavirin: 102.0±10.3%). Furthermore, a significant induction of factor VII mRNA was demonstrated in cultured normal human hepatocytes or HepG2 cells when treated with ribavirin at the therapeutic concentration. These observations indicate that ribavirin can elevate factor VII procoagulant activity in plasma, possibly due to the induction of factor VII in hepatocytes, thus, contributing to decreased events of bleeding in HCV-positive hemophiliacs.
Author notes
Corresponding author