Abstract
Background: Darbepoetin alfa (Aranesp®; DA) has been shown to be safe and effective for treating chemotherapy-induced anemia (CIA). The ability to administer darbepoetin alfa every 3 weeks (Q3W) (coincident with chemotherapy) would simplify the treatment of CIA. We report results from the first multicenter, randomized, double-blind, placebo-controlled, phase 3 clinical trial evaluating efficacy and safety of fixed Q3W administration of an erythropoietic agent.
Methods: This study enrolled subjects ≥18 years, diagnosed with anemia (hemoglobin [Hb]<11g/dL) and a nonmyeloid malignancy with ≥12 weeks of planned chemotherapy. Patients (N=391) were randomized 1:1 to receive DA 300 μg or placebo Q3W for 15 weeks. Dose adjustment rules included: increase (to 500 μg Q3W) if Hb concentration was <9 g/dL at week 4 or <10 g/dL (and had <1-g/dL increase) at week 7, or decrease (dependent on previous dose) if Hb concentration was ≥13 g/dL or had ≥1-g/dL increase in any 2-week period. Efficacy was assessed by incidence of red blood cell (RBC) transfusions and achievement of target Hb of ≥11 g/dL (not exceeding 13 g/dL), consistent with ASH/ASCO, NCCN, EORTC evidence-based practice guidelines.
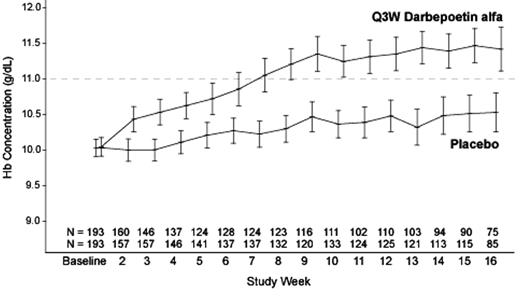
Results: A total of 386 randomized patients were included in the analysis. Demographic characteristics were similar between the 2 groups. Mean (SD) Hb levels at baseline were 10.03 (0.86) and 10.05 (0.92) g/dL in the placebo and DA groups, respectively. The most common tumor types were breast (23%), colon (11%), nonsmall-cell-lung cancer (10%), and hematologic malignancies (11%; 8% Non-Hodgkin’s Lymphoma). The incidence of RBC transfusions from week 5 to the end of treatment phase (EOTP) (the primary endpoint) was significantly lower for the DA group than for the placebo group (P<0.001) (see Table). Hb levels rose steadily in the DA group through approximately week 9, increasing by a mean (SD) of 1.08 (1.28) g/dL from baseline, and then remained relatively stable (see Figure). The proportion of patients achieving Hb target range from week 5 to EOTP was significantly higher for the DA group than for the placebo group (P<0.001). Dose adjustment rules helped to maintain Hb levels within target range. The safety profile of DA was consistent with that observed in previous studies. Rapid increases in Hb concentration or increases to ≥13 g/dL were not associated with adverse events.
Conclusions: Fixed Q3W administration of DA is well tolerated and effective for the treatment of CIA.
Summary of Results
| . | Placebo . | Darbepoetin alfa . |
|---|---|---|
| KM = Kaplan-Meier estimate | ||
| Week 5 to EOTP | N=185 | N=181 |
| Transfusions, KM (95% CL) (primary endpoint) | 41% (34, 49) | 24% (18, 30) |
| Achievement of target Hb, KM (95% CL) | 48% (41, 56) | 82% (76, 88) |
| Week 1 to EOTP | N=193 | N=193 |
| Transfusions, KM (95% CL) | 47% (40, 54) | 30% (23, 36) |
| Median time to target Hb, weeks (95% CL) | 12 (9, 16) | 6 (3, 7) |
| . | Placebo . | Darbepoetin alfa . |
|---|---|---|
| KM = Kaplan-Meier estimate | ||
| Week 5 to EOTP | N=185 | N=181 |
| Transfusions, KM (95% CL) (primary endpoint) | 41% (34, 49) | 24% (18, 30) |
| Achievement of target Hb, KM (95% CL) | 48% (41, 56) | 82% (76, 88) |
| Week 1 to EOTP | N=193 | N=193 |
| Transfusions, KM (95% CL) | 47% (40, 54) | 30% (23, 36) |
| Median time to target Hb, weeks (95% CL) | 12 (9, 16) | 6 (3, 7) |
Figure
Author notes
Corresponding author