Abstract
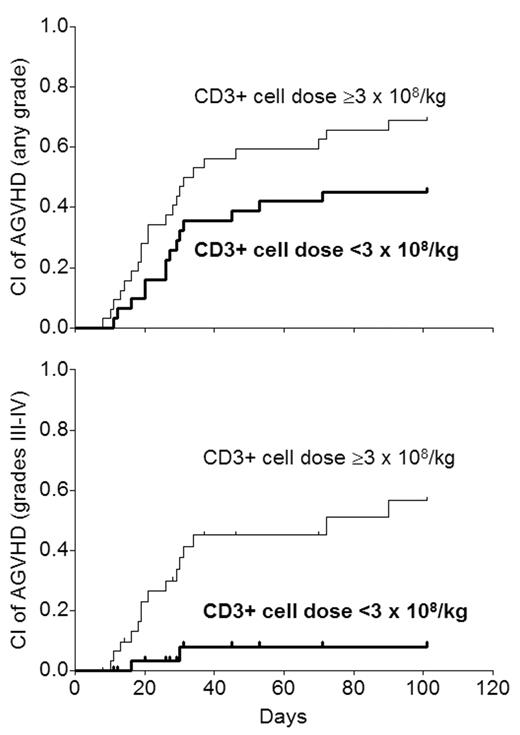
Graft CD3+ cell dose has been shown to affect the incidence of GVHD after myeloablative allogeneic HSCT. It is not known if this is so after submyeloablative HSCT. 63 patients (27–66 y, median 52) underwent submyeloablative HSCT from HLA-matched siblings (n=37), 10/10 allele-matched unrelated donors (n=19), or 1-locus/allele mismatched donors (n=7) for hematologic malignancies from 3/01 to 5/04 (>1 y minimum follow-up). The conditioning regimen was 100 mg/m2 melphalan with (n=42; no prior autograft) or without (n=21; prior autograft) 50 mg/kg cyclophosphamide. GVHD prophylaxis comprised cyclosporine-mycophenolate with HLA-matched siblings and tacrolimus-mycophenolate with the rest. G/GM-CSF were not used. Supportive care was uniform. While the target CD34+ cell dose was 2–8 x 106/kg ideal body weight (IBW), the actual dose was 1.4–11.8 (median 5). The CD3+ cell dose was not controlled, and was 0.9–14.9 x 108/kg IBW (median 3). There was no significant correlation between CD3+ and CD34+ cell doses (r=0.17; P=0.18). Exploratory univariate analysis of various CD3+ cell dose thresholds showed that a dose of <3 x 108/kg (n=31) was associated with better survival than ≥3 (P=0.032). This level was chosen for further analysis for its effect on GVHD. The 100-day cumulative incidence (CI) of acute GVHD (AGVHD) was 57% for any grade (I–IV) and 34% for grades III–IV. As the curves show, a CD3+ cell dose <3 was associated with significantly lower incidence of any grade of AGVHD (RR 0.48; P=0.032) and grades III–IV acute GVHD (RR 0.10; P=0.003).
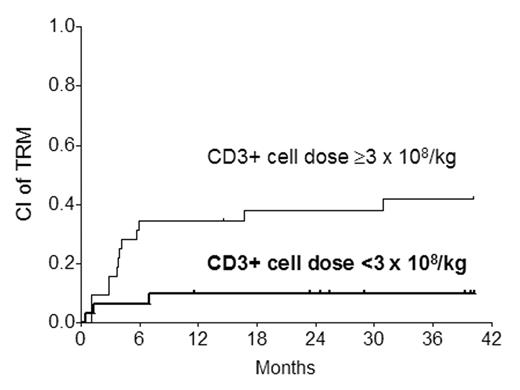
In Cox analysis, the CD3+ cell dose was the only variable affecting the incidence of AGVHD (any grade or grades III–IV) significantly. When the CD3+ cell dose was analyzed as a numeric (continuous) variable rather than categoric, the significant relationship with any grade of AGVHD (RR 1.16; P=0.04) and grade III–IV AGVHD (RR 1.36; P=0.0005) was maintained. 9 of 16 patients with grades III–IV AGVHD experienced transplant-related mortality (TRM) compared with 7 of 47 not getting severe AGVHD (P<0.0001). As a result, there was a significant relationship between a higher CD3+ cell dose and TRM (RR 5.58; P=0.008).
The significant relationship between the CD3+ cell dose and grades III–IV AGVHD as well as TRM was maintained when the 7 HLA-mismatched cases were excluded, but the relationship with any grade AGVHD became non-significant (RR 0.56; P=0.11). The 2-y CI of chronic GVHD (CGVHD) was 25%. No variable including the CD3+ cell dose was found to influence the incidence of CGVHD independently. Our findings suggest that CD3+ cell doses ≥3 x 108/kg IBW are associated with a higher incidence of severe AGVHD and TRM after submyeloablative HSCT. These findings, if confirmed in a larger - ideally prospective - study, may provide the basis for relatively simple graft manipulation to achieve a more desirable T cell dose and reduce the likelihood of AGVHD and TRM.
Author notes
Corresponding author