Abstract
Background: Osteoporosis is an important cause of morbidity in beta-thalassemia patients. Bisphosphonates are potent inhibitors of osteoclast activity and have been recently used for the treatment of osteoporosis in beta-thalassemia. The aim of this study is to assess the efficacy and safety of zoledronic acid in Lebanese thalassemics with osteoporosis.
Methods: Eighteen thalassemic patients (13 thalassemia major and 5 intermedia) with osteoporosis defined as Z-score <−2.5 were given zoledronic acid 4 mg i.v. every 3 months over a period of 12 months (Total of 4 doses administered). The efficacy of treatment was assessed by measuring Bone Mineral Density (BMD) at the lumbar spine, femoral neck and hip at baseline, 6 and 12 months. Other efficacy measurements included markers of bone formation and resorption (bone alkaline phosphatase (BAP), osteocalcin (OC), and urinary deoxypyridinoline (Dpd)), assessment of pain score, analgesic score, and performance score measured at baseline and at 3-month intervals. Safety assessment included regular physical exams, standard hematology and clinical chemistry tests, and adverse events recording. All patients were on Ca/Vitamin D supplementation prior to and during the study. Ten thalassemic osteoporotic patients were followed up only with serial BMDs as controls.
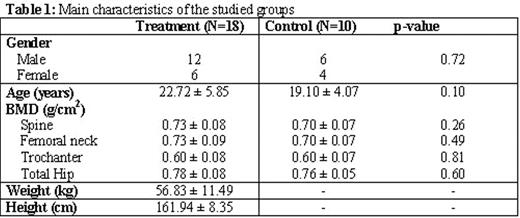
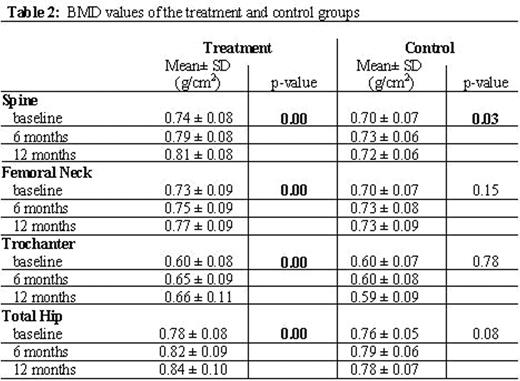
Results: The characteristics of all patients are shown in Table 1. Both groups had no significant difference with respect to age, gender and baseline BMD. Patients taking zoledronic acid had a significant increase in their spine, femoral neck, trochanter and total hip BMD measurements over the 12-month period (all p-values<0.05). Patients in the control group, on the other hand, did not have any significant change except in the spine BMD. The BMD values are presented in Table 2. There was a significant change in the levels of the OC and BAP over the 12-month follow-up in the treatment group (p=0.00 for both). Dpd levels did not significantly change overall (p=0.06) although they decreased throughout the study. Reported adverse events included joint pain in 9 patients (50%) after the 1st dose and in 2 (11.1%) after the 2nd dose and responding very well to oral analgesics. Two patients (11.1%) had perioral numbness and 3 (16.7%) had low grade fever after the 1st dose. No treatment-related adverse events were reported after the 3rd and 4th doses. No patients withdrew from the study.
Conclusions: Treatment of Lebanese thalassemic osteoporotic patients with zoledronic acid 4 mg every 3 months is effective in increasing BMD at the lumbar spine and hip and is well-tolerated. Well-controlled studies with longer follow-up are needed to determine the fracture-reduction benefits and the most optimal zoledronic acid treatment dose and frequency in this patient population.
Author notes
Corresponding author