Abstract
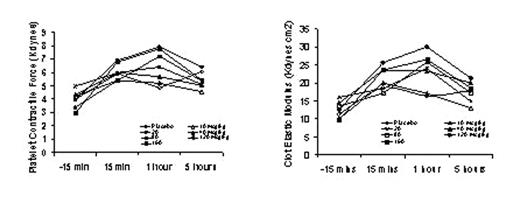
The generation of thrombin is a critical step in clot formation and securing hemostasis. Inadequate thrombin generation may result in excessive bleeding, while uncontrolled thrombin production has the potential to induce thromboses. Recombinant FVIIa has been shown to control bleeding in hemophilia patients with inhibitors to coagulation factors VIII and IX, and several other clinical conditions in which life- and limb-threatening bleeding may occur. However, for non-hemophilia patients the dose and dosing regimen still require refinement. The use of ex-vivo assessment systems (Thromboelastograph assay, Hemodyne Hemostasis Analyzer) to generate an in vitro model of hemostasis may be useful to determine the effect of rFVIIa on the dynamics of clot formation, particularly in a patient population where clinical trials are difficult. A series of pilot studies were performed to establish an optimal punch biopsy location and biopsy size to create an effective bleeding site for further study. The current study (48 subjects consented for an IRB approved protocol) examined, in an ascending dose-escalation manner, the effect of an individual dose of rFVIIa on bleeding time, blood loss volume, coagulation parameters and coagulation status in healthy volunteers. All patients underwent three punch biopsies: the first (baseline biopsy) with no treatment, the second and third biopsy with either a low or high rFVIIa dose administered prior to biopsy. The treatment pair sequences were placebo/10, 10/20, 20/40, 40/80, 80/120, and 120/160 μg/kg. Blood samples were drawn 15 minutes pre-, 15 minutes post-, 1 hour and 5 hours post-biopsy to conduct ex-vivo assessments of hemostasis. The results indicate that overall there was a trend towards an increase in platelet contractile force and clot elastic modulus with an apparent maximal effect at 1 hour post-biopsy. At the higher doses this effect, although reduced, does not return to pre-treatment values by 5 hours post-biopsy. For the lower doses these values decreased to levels comparable to those at pre-treatment. These observations are consistent with the reports of a dose-related half-life of rFVIIa. These data imply that the administration of rFVIIa may have an effect in non-coagulopathic individuals. Although the heterogenity observed in this sample of patients may limit interpretations, it is proposed that this ex-vivo model with further refinements may be useful for evaluating the effects of future pro-hemostatic agents.
Author notes
Corresponding author