Abstract
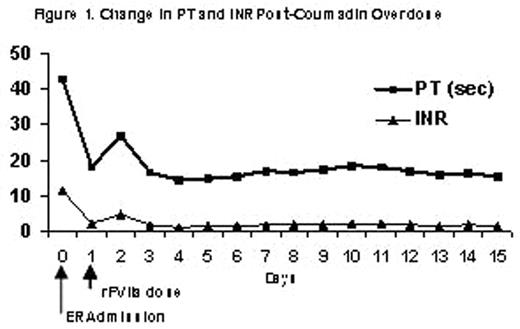
Coumadin is typically prescribed as prophylaxis to prevent extension of emboli or for patients with mechanical heart valves and atrial fibrillation in order to reduce the risk of strokes. Coumadin toxicity is common and usually results from changes in therapy or drug interactions. However, there are very few overdose cases reported in the literature. Typically, immediate reversal of coumadin toxicity can be achieved by the infusion of prothrombin complex concentrate (PCC), large volumes of fresh frozen plasma (FFP) or Vitamin K. However concerns still exist regarding the potential for transmission of blood-borne pathogens, large infusion volumes, and the thrombogenic potential of PCC use. Patients who are Jehovah’s Witness cannot receive blood products. Vitamin K is unsuitable to correct acute bleeding episodes, as there is a 4 to 6 hour delay in onset of action. Recombinant FVIIa (rFVIIa), a Vitamin-K dependent glycoprotein, is currently licensed for the treatment of bleeding episodes in hemophilia A or B patients with inhibitors to FVIII or FIX. Structurally identical to plasma-derived FVII, rFVIIa complexes with tissue factor (TF) initiating the activation of several other coagulation factors, eventually leading to the conversion of prothrombin to thrombin, a key component of clot formation and stabilization. This process can also occur on the surface of platelets. In coumadin-treated rats, rFVIIa has been shown to normalize prothrombin time (PT) as well as to stop acute bleeding. In healthy volunteers treated with the oral anticoagulant acenocoumarol in whom the international normalized ratio (INR, defined as a ratio of the patient PT to the international reference PT) was greater than 2.0, correction of the elevated INR was achieved using doses of rFVIIa between 5 and 320 mcg/kg. Therefore rFVIIa could potentially be used for the correction of PT and INR in patients overdosed with oral anticoagulants. In this report, an 83-year-old male Jehovah’s Witness diagnosed with metastatic prostate cancer, diabetes mellitus, hypertension, and deep vein thrombosis (DVT), received 4.5 mg coumadin daily. This patient presented to the emergency room with epistaxis, elevated PT of 42.7 and INR of 11.48, as a result of an unintentional overdose of coumadin. Hemoglobin was 7.7, hematocrit 22.8, and platelet count 284,000. Coumadin therapy was discontinued and general supportive care was started including Vitamin B12, Vitamin K, iron and erythropoietin. As bleeding did not stop, a dose of rFVIIa (90 mcg/kg) was administered. Thirty minutes after the dose of rFVIIa the PT/INR was 18/2.05, and hemostasis was achieved. The next day, there was no evidence of active bleeding, but since the hemoglobin and hematocrit dropped, (6.8/20.3) the patient was given a second dose of rFVIIa. The patient’s PT and INR remained at normal levels during the 2 week follow-up period. The figure below describes the time course of the change in PT and INR as a result of rFVIIa treatment.