Abstract
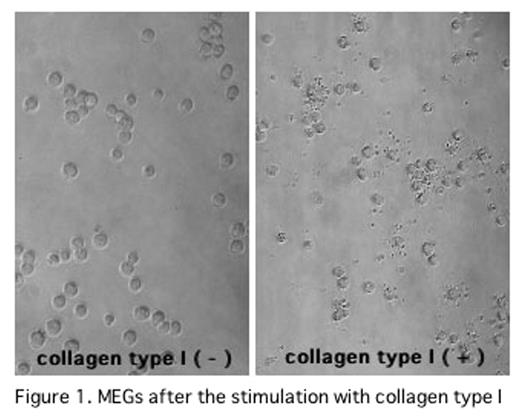
In the previous ASH Meetings, we have reported that thrombin/antithrombin III complex in association with high density lipoprotein directly stimulated PPF of human or murine megakaryocytes through Rho pathway. Recently, we found that CTI also induced PPF of murine megakaryocytes and PP of MEGs in vitro. In this study, we investigated the signal transduction pathways of cytoskeltal reorganization responsible for PP in MEGs after the stimulation with CTI. Murine megakaryocytes were incubated for 48 hrs in serum free condition on CTI dishes. PPF of murine megakayocytes were significantly increased (collagen (−): 25.5±5.0%, collagen (+): 36.0±1.0%, mean±SD, P<0.05). In the culture of murine megakaryocytes on CTI dishes with phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002) or Src family kinase inhibitor (PP1, SU6656), PPFs of murine megakayocytes were inhibited (LY294002:0μM:31.7±1.0%,1μM:25.8±2.2%, 10μM:28.1±8.2%,100nM:26.1±5.1% (p<0.05), PP1:0nM:32.4±1.0%,0.2μM:24.5 ±5.2%,2μM:21.3±2.5%,20 μM:15.9±5.7% (p<0.01), SU6656: 0nM: 32.2±1.0%, 2.5μM:30.0±1.3%,25μM:15.5±4.5% (p<0.01) ). These results suggest that PPF of megakaryocytes was induced by CTI through Rac activation pathway, based on that Rac is activated by two pathways including PI3K and Src family kinese. As we could not obtain enough murine megakaryocytes, MEGs (cell line) were used to determine whether or not the Rac is activated after the stimulation by CTI. PP of MEGs was significantly increased after the incubation of MEGs on the CTI dishes (collagen (−): 16.0±5.0%, collagen (+): 58.3±7.2%, (p<0.01)) (shown in Figure1). In the culture of MEGs on CTI dishes with LY294002, PP1 or SU6656, PPs of MEGs were significantly inhibited(0nM:54.9± 1.0%, LY294002:0.5μM:48.3±7.2%, PP1:20μM:47.9±1.1%, SU6656:5μM:44.9±6.7%,(p<0.05) ). These results were similar to those of murine megakaryocytes. Therefore, activation of Rac was investigated using MEGs. MEGs, which were incubated in the serum free conditon on the CTI dishes at 0, 60, 90, 120, 150 and 180 min. Activated Rac in the whole lysate were co-precipitated with p21-activated kinase-p21Binding domain (PAK-PBD) beads. The amount of activated Rac was detected by a Western blot using anti Rac specific antibody. Rac was activated at 60min and tapered at 120min after incubation on CTI dishes, shown in Figure 2. Phospholylation of cofilin (Ser3) was also evaluated by a Western blot using anti phospholylated cofilin (Ser3) antibody. Phospholylated cofilin (Ser3) expression began to be detected at 60 min, peaked at 120min, and tapered at 180 min. These data suggests that integrin alpha2beta1 on megakaryocytes was stimulated by CTI, followed by the activation of Rac through PI3K and Src family kinase, and by phospholylation of cofilin (Ser3). These pathways may play an important role in platelet production in vitro.
Author notes
Corresponding author