Abstract
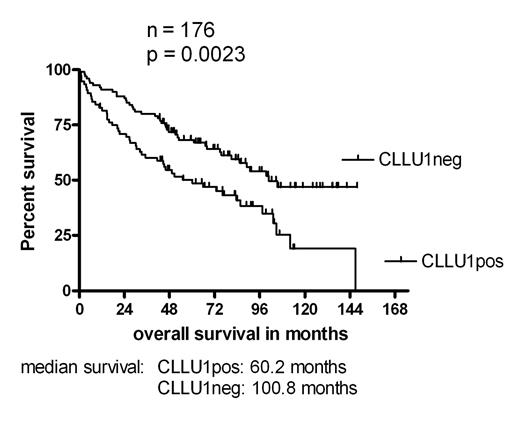
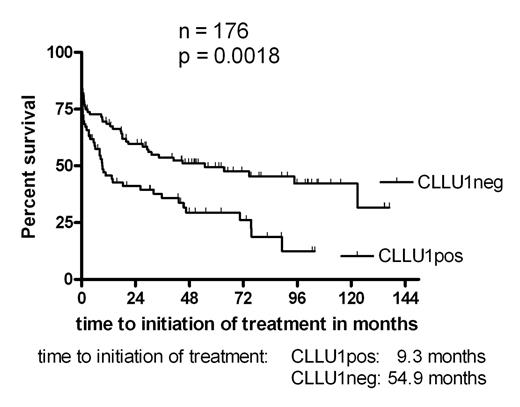
We have recently identified and cloned a novel disease specific gene CLLU1, with a strong prognostic significance in a small test cohort of CLL patients (ASH 2004, #770). To validate this finding in a larger series, 176 newly diagnosed, previously untreated CLL patients referred to Rigshospitalet, Copenhagen in 1991–1999, were investigated. Clinical characteristics of the population were: 116 stage A patients (66 %), median age 69.9 years, 98 males (56%), median follow up 59 months. The end points for the statistical analysis were overall survival and time to initiation of treatment. At time of analysis 91 (52%) patients had died and 104 (59%) had commenced treatment. Frozen patient samples, taken at time of diagnosis, were investigated for CLLU1 expression, as well as for mutational status, CD38 expression, ZAP-70 expression and cytogenetic aberrations. CLLU1 expression was assayed by QRT-PCR using the cDNA1 splice variant, and expressed as fold upregulation above the CLLU1 level in normal B-cells. CD38 and ZAP-70 analysis was performed by flow cytometry, using a 20 % cut-off level. Cytogenetic analysis was performed by FISH. The previously reported (ASH 2004, #770) median upregulation of CLLU1 in CLL cells was accurately reproduced and confirmed (25.5-fold (N = 176) vs. 27.7-fold (N = 59) in test population). Also in accordance with the pilot study, segregation of the patients based on the median CLLU1 expression level divided the population in two groups with significantly different times to initiation of treatment and borderline significant difference in overall survival. However, further analysis found an optimal cut-off level at 40-fold upregulation of CLLU1 expression; use of this cut-off demonstrated a highly significant difference in overall survival for patients with upregulated expression of CLLU1 (p = 0.0023). The median overall survival was 60.2 months for patients with CLLU1 expression above 40-fold compared to 100.8 months for the group with lower expression level. Likewise the time to initiation of first treatment was significantly shorter in the high-level expression group (p = 0.0018, median time to first treatment 9.3 months vs. 54.9 months). Furthermore, CLLU1 expression was upregulated in all poor prognostic subgroups investigated: Binet stage B or C, IgVH unmutated, unfavorable cytogenetics, high CD38 expression and high ZAP-70 expression. Our new study confirms that CLLU1 is a strong prognostic factor in CLL, comparable to other established prognostic markers. The exclusive upregulation of CLLU1 expression in CLL suggests a putative role for CLLU1 in the pathogenesis of CLL, and makes this gene a strong candidate for future gene-targeted treatment strategies.
Author notes
Corresponding author