Comment on Collet et al, page 3824
In this issue, Collet and colleagues identified αC as the major determinant of viscoelastic clot properties and unambiguously highlighted its polymerization and fibrinolysis roles.
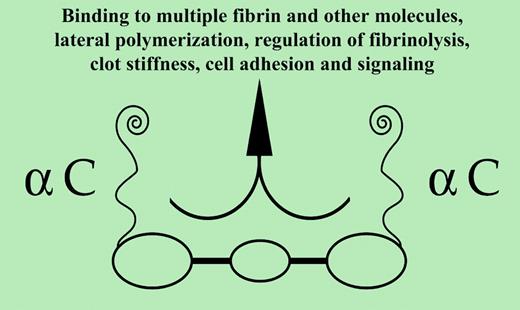
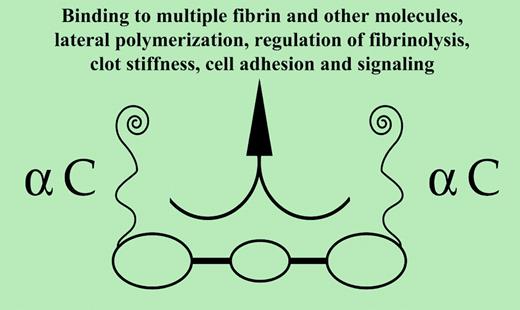
Fibrinogen1 consists of 3 pairs of disulfide-linked polypeptide chains, Aα,Bβ, and γ, and possesses globular regions at each end and in its N terminal center. The carboxyl terminal Aα-chain region, commencing with residue 220, is termed αC and is required for certain fibrin functions (see figure). Following release by thrombin of amino terminal fibrinopeptides, fibrin monomers formed polymerize in a half-staggered manner, the central domain of one binding the outer domain of another. When 600 to 800 nm long, the 2-stranded, twisting protofibrils assemble laterally forming fibrils. Lateral contact pairs include B knobs–b holes; αC–αC; and possibly2 γ(350-360)–(370-380).
Viscoelastic properties of thrombus are regarded essential to its physiologic functions.3 The markedly decreased storage modulus (stiffness) by α251 clots implies that interactions among juxtaposed molecules via their αC domains largely determine the normal level of clot stiffness. Additionally, αC domains play a significant role in minimizing the irreversible deformation (plasticity) of clots. Also, γ-γ bonds confer appreciable stiffness even before clots are cross-linked. Thus, αC-αC compared with γ-γ bonds constitute the major determinant of stiffness.FIG1
Other likely minor determinants are γ-α and A knob–a hole bonds.4 Not surprisingly, stiffness from the first 2 sets of determinants is maximized by factor XIIIa–catalyzed cross-links.
Decreased porosity in α251 clots, relative to that of normal clots, underscores the role of αC. Under certain in vivo conditions (eg, variable thrombin concentrations), a thrombus is likely to contain both increased and decreased porosity domains. The latter may be enhanced by the high-fibrinogen excess at blood-thrombus interface(s). Decreased porosity domains, by limiting intrathrombus circulatory flow, may shield intrathrombus microenvironments and by extension enhance hemostatic/tissue repair effectiveness and limit fibrinolysis. Along with decreased porosity, the distinctly fine α251 networks are consistent with long-held views on the network of fibrin lack αC, while emphasizing the αC role in lateral polymerization. The latter process is poorly understood, in contrast to protofibril formation and branching. The αC pair, self-associated at their ends and tethered to the central region, become untethered following fibrinopeptide B release. Whether they participate in lateral polymerization in tethered, untethered, or both forms remains unclear. Reptilase clots display ample lateral polymerization, suggesting that either tethered αC participates by an unknown mechanism or other parts of the molecule are involved. Be that as it may, participation in lateral polymerization, while significant, may not be a major function of αC domains.
Decreased plasmin resistance by fibrin 251 clots appears inconsistent with reports of increased plasmin resistance by normal fine network clots. However, fibrin 251 results imply that interactions among αC domains are more important for plasmin resistance than other clot network structure(s). Additionally, αC regulates fibrinolysis, evident from its binding of an impressive array of plasminogen activation and inhibitory proteins.5 These new results, moreover, support the view that the αC domains contribute directly to fibrinolysis resistance. The impressive αC repertoire tempts speculation that more scenes remain undiscovered. ▪