Tumor necrosis factor (TNF)-α, a potent stimulus of nuclear factor-κB (NF-κB), is up-regulated in myelodysplastic syndrome (MDS). Here, we show that bone marrow mononuclear cells (BMMCs) and purified CD34+ cells from patients with low-grade/early-stage MDS (refractory anemia/refractory anemia with ring sideroblasts [RA/RARS]) have low levels of NF-κB activity in nuclear extracts comparable with normal marrow, while patients with RA with excess blasts (RAEB) show significantly increased levels of activity (P = .008). Exogenous TNF-α enhanced NF-κB nuclear translocation in MDS BMMCs above baseline levels. Treatment with arsenic trioxide (ATO; 2-200 μM) inhibited NF-κB activity in normal marrow, primary MDS, and ML1 cells, even in the presence of exogenous TNF-α (20 ng/mL), and down-regulated NF-κB-dependent antiapoptotic proteins, B-cell leukemia XL (Bcl-XL), Bcl-2, X-linked inhibitor of apoptosis (XIAP), and Fas-associated death domain (FADD)-like interleukin-1β-converting enzyme (FLICE) inhibitory protein (FLIP), leading to apoptosis. However, overexpression of FLIP resulted in increased NF-κB activity and rendered ML1 cells resistant to ATO-induced apoptosis. These data are consistent with the observed up-regulation of FLIP and resistance to apoptosis with advanced MDS, where ATO as a single agent may show only limited efficacy. However, the data also suggest that combinations of ATO with agents that interfere with other pathways, such as FLIP autoamplification via NF-κB, may have considerable therapeutic activity.
Introduction
No “standard” therapy for the treatment of myelodysplastic syndrome (MDS) has been established. Recent efforts have focused on pathophysiology-based approaches.1-4 We and others have shown high rates of apoptosis in MDS marrow cells, especially with less advanced disease.5-7 Apoptosis occurs in both clonal and nonclonal precursors, as determined by fluorescent in situ hybridization (FISH).8 We have also shown up-regulation of tumor necrosis factor (TNF)-α in marrow plasma from patients with MDS.5 As the disease advances, the rate of apoptosis in clonal cells declines, and proliferation prevails. Concurrently, there appears to be a shift in the expression of TNF-α receptors R1 (p55) and R2 (p75) in favor of R2,9 which transmits cytoprotective signals via nuclear factor-κB (NF-κB), whereas R1 transmits both cytoprotective (via NF-κB) and proapoptotic signals (via TNF receptor-associated death domain/Fas-associated death domain [TRADD/FADD]/caspase-8). In addition, we observed dysregulation of FADD-like interleukin-1β-converting enzyme (FLICE) inhibitory protein (FLIP) in MDS marrow.10 FLIP modulates death signals triggered by various pathways, including TNF-α and its receptors.11,12 The transcription factor NF-κB, which consists of homo- or heterodimers of the NF-κB/Rel family members, is activated in response to TNF-α, and regulates transcription of a great diversity of genes involved in differentiation, inflammatory responses, and regulation of apoptosis and cell growth,13-15 including the antiapoptotic gene products FLIP, cIAPs, Bcl-2, and Bcl-XL.12,15-17 In most normal cells, NF-κB complexes are present in the cytoplasm but remain inactive due to their interaction with inhibitor of κB (IκB) proteins. Upon cell activation, IκB kinases phosphorylate IκBα, which is degraded and frees NF-κB to translocate to the nucleus. In contrast, many neoplastic cells (Hodgkin disease, lymphomas, and acute leukemias) show constitutive NF-κB activation, which contributes to abnormal proliferation, resistance to apoptosis, and disease progression.16,18-21 As TNF-α, which is up-regulated in MDS, is a potent stimulus of NF-κB,22,23 and FLIP has been shown to be under NF-κB regulation, we hypothesized that this transcription factor was involved in the pathophysiology of MDS.
Arsenic trioxide (ATO), which has been shown to have therapeutic efficacy in patients with MDS,24-26 induces apoptosis through several mechanisms: release of cytochrome c, modulation of cellular redox potential, down-regulation of Bcl-2, and inhibition of NF-κB by interfering with IκB kinases, thereby preventing spontaneous and TNF-induced NF-κB translocation to the nucleus.27-30 The mechanism by which ATO induces therapeutic responses in MDS has not been defined. In this study, we assessed NF-κB activation in MDS marrow and determined the effect of ATO on NF-κB activity. NF-κB activity correlated with MDS disease stage, and ATO inhibited NF-κB translocation to the nucleus.
Materials and methods
Materials
ATO was obtained from CTI (Seattle, WA), and etanercept (Enbrel) was obtained from Immunex (Seattle, WA) and Amgen (Thousand Oaks, CA). The competitive IκB kinase inhibitor, BMS345541, was kindly provided by Dr S. Nadler (Bristol-Myers Squibb, Princeton, NJ). TNF-α was purchased from PeproTech (Rocky Hill, NJ), TNF-related apoptosis-inducing ligand (TRAIL) from Alexis (San Diego, CA) and l-buthionine sulphoximine (BSO) from Sigma (Saint Louis, MO).
Cell cultures and sample preparation
The human myeloid leukemia cell line ML1 (a gift from Dr D. Banker, Fred Hutchinson Cancer Research Center [FHCRC]) was maintained in RPMI 1640 medium, containing 10% heat-inactivated fetal bovine serum in a humidified 5% CO2 environment at 37°C. ML1 cells stably expressing FLIPLong and control-vector green fluorescent protein (GFP) were cultured under the same conditions as the wild-type cells.
Marrow aspirates were obtained from healthy volunteers and patients with MDS who had given informed consent according to procedures approved by the Institutional Review Board of the FHCRC. Bone marrow mononuclear cells (BMMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. CD34+ selection was performed by magnetic-activated cell sorting (MACS) according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Cell numbers varied considerably from patient to patient, and not all tests could be carried out on every patient. Samples for experiments were selected such that the spectrum of MDS categories was represented.
Lentivirus construct
We used a plasmid encoding the lentiviral vector pRRLMSCV.ires2eGFP.sin, which was kindly provided by Dr H. P. Kiem (FHCRC). To insert the FLIPLong coding sequence, the vector was digested with AgeI and BamHI. cDNA was prepared from total RNA extracted from KG-1a cells, which show constitutively high FLIP expression. AgeI and BglII sites were added to the 5′ and 3′ ends, respectively, of the polymerase chain reaction (PCR) product prepared with the use of Hifi Taq polymerase (Invitrogen, Carlsbad, CA). PCR products were gel purified and digested with the above enzymes, ligated into the vector, and checked for sequence integrity. As a control, we used the lentiviral vector pRRLsin-cPPT-MSCV-GFP (GFP) (provided by Dr H. P. Kiem, FHCRC). To produce lentivirus, 293T cells were cotransfected with the pRRLMSCV.FLIPL.IRES2eGFP.sin/pRRLsin-cPPT-MSCV-GFP along with the constructs containing the gag/pol and the vesicular stomatitis virus G protein (VSV-G) envelope using calcium phosphate precipitation. Lentiviral supernatants were collected at 18, 30, and 42 hours after cotransfection in Dulbecco modified Eagle medium (DMEM; containing 10% heat inactivated fetal bovine serum [FBS] 1% penicillin/streptomycin (P/S) + 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]), filtered through a 0.22-μm filter, concentrated by centrifugation for 24 hours at 6800g and resuspended in 1/100 of the volume in Iscove medium. ML1 cells were transduced either with FLIPLong or control vector (GFP).
Treatment and determination of apoptosis
Reagents including ATO, etanercept, and TNF-α were tested in ancillary experiments for optimum concentrations and time courses. Based on those data, cells were exposed to ATO (at concentrations of 2-200 μM) alone or in the presence of etanercept (at 5-10 μg/mL) for 1 hour, followed by TNF-α (20 ng/mL, a predetermined dose which induces NF-κB activation31 ) for 16 to 20 hours. To determine apoptotic changes, cells were stained with Annexin V-fluoroscein isothiocyanate (FITC) or phycoerythrin (PE) (Becton Dickinson, San Jose, CA) and propidium iodide or, alternatively, with 7-amino-actinomycin (7AAD) and examined on a FACScan (Becton Dickinson, Mountainview, CA). Results were analyzed using CellQuest software (Becton Dickinson, San Jose, CA). To assess apoptosis induced by ATO, the proportion of apoptotic cells was calculated as follows: (drug-induced apoptosis - apoptosis in medium) × 100/(100 - apoptosis in medium) as described by Frelin et al.32 Student t test was applied for statistical analysis using GraphPadPrism (version 3) software (GraphPad Software, San Diego, CA).
Cell lysates and nuclear extracts
Cell lysates for Western blots were obtained using Chaps Cell Extract Buffer (Cell Signaling Technology, Beverly, MA) according to manufacturer's protocol. Nuclear extracts were prepared using fresh nonsorted BMMCs (5-10 × 106 cells) or CD34+ cells selected by MACS (1-10 × 106 cells): after washing with cold phosphate-buffered saline (PBS), cells were lysed with lysis buffer (20 mM HEPES, pH 7.6; 20% glycerol; 1.5 mM MGCl2, 0.2 mM EDTA (ethylenediaminetetraacetic acid); 0.1% Triton X-100; 10 mM NaCl; 100 μg/mL aprotinin; 10 μg/mL leupeptin; 10 μg/mL pepstatin; 1 mM phenylmethylsulfonyl fluoride [PMSF]; and 1 mM dithiothreitol [DTT]). After centrifugation at 700g for 5 minutes, the pellet was resuspended in nuclear extract buffer (same as lysis buffer but with 0.5 M NaCl), then centrifuged at 10 000g for 10 minutes. Supernatants were stored at -80°C. A Bio-Rad assay (Hercules, CA) was used to measure protein concentration.
Electrophoretic mobility shift assays
To determine NF-κB activation, electrophoretic mobility shift assay (EMSA) was carried out as previously described.33 Briefly, NF-κB consensus oligonucleotide sequences from Santa Cruz Biotechnologies (Santa Cruz, CA) were labeled with T4 polynucleotide kinase and γ-[32P]-ATP. Protein nuclear extracts were incubated with 2 μg poly d(I-C) and 32P-labeled probe (5000 counts per minute [cpm]) in 20 mM HEPES, pH7.9; 40 mM KCl; 10% glycerol; 0.05 mM EDTA; and 1.6 mM MgCl2 for 30 minutes at room temperature. For competitive inhibition, 50-fold molar excess of unlabeled (cold) probe was used. For supershift assays, antibody for p65 (sc-372; Santa Cruz Biotechnologies) was added to the reaction mixture 30 minutes before adding the probe. Protein/DNA complexes were resolved on 5% Tris (tris(hydroxymethyl)aminomethane)-borate-EDTA (TBE) gels in 1 × TBE. Gels were dried and exposed at -80°C to x-ray films. Protein/DNA complexes were analyzed densitometrically using ImageQuant software (Molecular Dynamics, Sunnyvale, CA) for NF-κB activation. Results represent intensity ratios of patient samples against healthy controls.
Western blot analysis
Cell lysates (using 25 μg of protein) were resolved on 4% to 20% sodium dodecyl sulfate (SDS)-polyacrylamide gels and processed according to standard protocols. The antibodies used were p-IκBα (Ser32), p-Akt (Ser473), Bcl-XL, Bcl-2, XIAP (1:1000; Cell Signaling Technology), anti-FLIP (NF6; 1:500) and antiactin (Sigma, St Louis). The secondary antibodies (antirabbit and antimouse) were conjugated to horseradish peroxidase (dilution, 1:20 000). Signals were detected using the electro-chemiluminescence (ECL) system (PIERCE, Rockford, IL).
Quantitative real-time PCR
cDNA was prepared from a pool of total RNA obtained from 2 independent experiments with ML1 or primary MDS BMMCs using oligo-dT20 and Thermoscript RT-PCR System (Invitrogen, Carlsbad, CA). PCR primers for each gene were designed as previously reported for FLIPLong, FLIPShort, and β2-microglobulin,10 with melting temperatures at 52 to 56°C and resulting products of 124, 93, and 338 base pair (bp), respectively. Each PCR was carried out in triplicate in a 25 μL volume using SYBR Green Master Mix (Invitrogen) for 2 minutes at 50°C, 2 minutes at 95°C, and 50 cycles of 95°C for 30 seconds, and 60°C for 45 seconds in the ABI Prism 7700 sequence Detection System (Applied Biosystems, Foster City, CA). Cloning of FLIPLong and FLIPShort cDNA into a pcDNA3.1 plasmid was performed as previously described10 to construct standard curves for each transcript analyzed. β2-microglobulin was cloned into pcr2.1 TOPOVector (Invitrogen) using a standard protocol. Values for each gene were normalized to expression levels of β2-microglobulin.
Glutathione-SH determination
Intracellular glutathione-SH (GSH) was measured using a glutathione detection kit according to the manufacturer's protocol (Oncogene, San Diego, CA). In brief, cell lysates from 3 × 106 cells were incubated with monochlorobimane, a high-affinity dye for glutathione in the presence of glutathione S-transferase. GSH levels were then determined fluorometrically by using a Biosystems Cytofluor4000 plate reader (PerSeptive Biosystems, Framingham, MA) and expressed as nmol GSH per mg protein. Protein concentration was assessed using the Bio-Rad protein assay.
Flow cytometry
Flow cytometric analysis of membrane TNF-α receptors R1 (p55) and R2 (p75) was performed using PE-conjugated monoclonal antibodies TNF R1 and R2 according to the manufacturer's protocol (R&D Systems). Mouse immunoglobulin G (IgG)-PE isotype control was obtained form Becton Dickinson. Analysis was carried out with a FACScan flow cytometer (Becton Dickinson). Results were expressed as the ratio of the mean fluorescence intensity of specific and isotype control antibody.
Proliferation assay
As a measure for the inhibitory effect of ATO on hemopoietic cells, we determined 3H-thymidine incorporation. Briefly, 5 × 104 CD34+ cells were incubated with ATO at various concentrations in 96-well round-bottom cell culture plates for 24 hours, 0.0185 MBq (0.5 μCi) 3H-thymidine was added for the last 18 hours of incubation, and radioactivity was measured using a scintillation counter.
Results
NF-κB activity in primary MDS cells
We evaluated NF-κB activity in bone marrow cells from 25 patients with MDS who had not received therapy in the recent past; all experiments were performed with fresh samples. Results are summarized in Table 1 and illustrated in Figure 1. (For reference purposes, both French-American-British [FAB] and World Health Organization [WHO] classifications are shown in Table 1.)
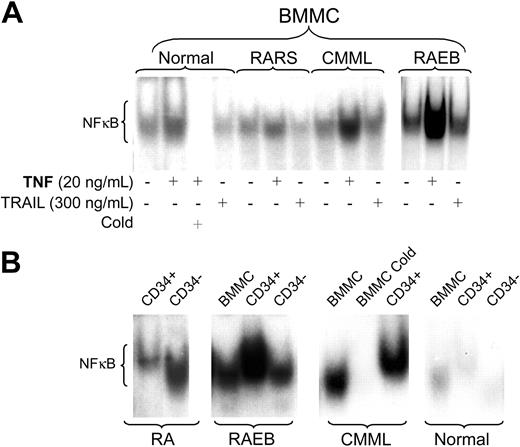
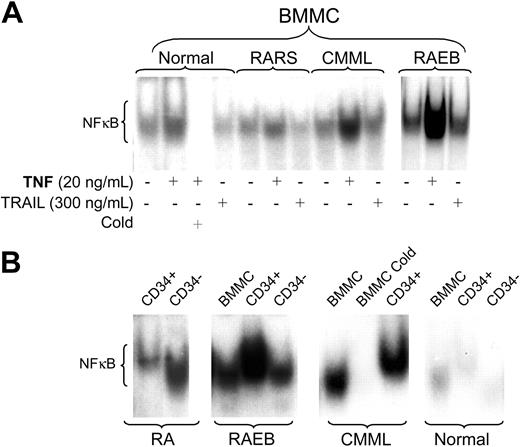
The levels of constitutive NF-κB DNA binding activity as determined by EMSA varied dependent upon the MDS disease category. BMMCs from patients with advanced MDS (including refractory anemia with excess blasts [RAEB] and RAEB in transformation [RAEB-T] by FAB criteria) showed significantly higher baseline levels of NF-κB nuclear translocation than with low-risk MDS, including refractory anemia (RA) and RA with ringed sideroblasts (RARS) (Table 1; P = .008). In low-risk MDS, the pattern of NF-κB activity in unfractioned BMMCs resembled that in normal marrow controls (Figure 1A). The patient in this group with the highest NF-κB activity in unfractioned BMMCs was a patient with a RA/5q-syndrome (patient 4). In 6 patients (2 RA; 2 RAEB; 2 CMML), sufficient cell numbers were available for EMSA of nuclear extracts from purified CD34+ precursors. In early-stage/low-risk MDS, CD34+ precursors consistently showed low NF-κB activity, but higher than observed in normal CD34+ precursors. In contrast, high NF-κB activity was observed in CD34+ cells from patients with RAEB (Figure 1B). Supershift experiments confirmed the presence of the p65 subunit of the NF-κB family in the nuclear extracts studied (data not shown).
NF-κB activities in marrow cells from patients with CMML were more variable (Table 1); however, activity overall correlated with the proportion of blasts. To characterize more narrowly differences between “dysplastic” and “proliferative” presentations of CMML, additional studies will be required.
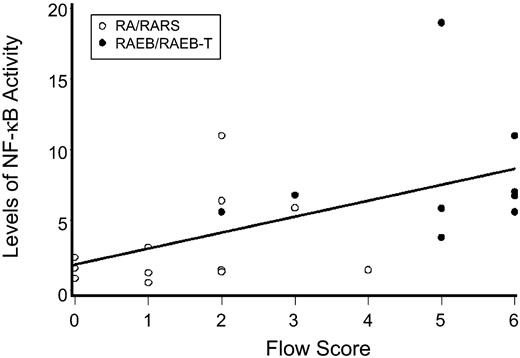
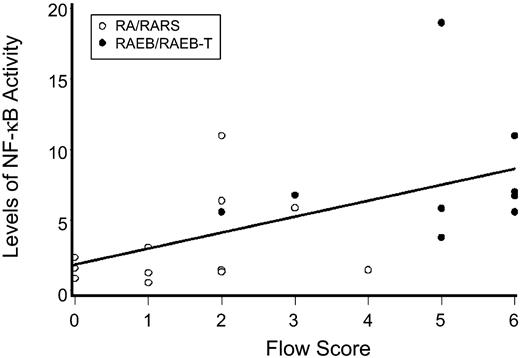
As we had previously shown a correlation between disease risk and flow cytometric aberrancies on MDS marrow cells,34 we correlated the levels of nuclear NF-κB activity with flow cytometric scores. As shown in Figure 2, there was a positive correlation between the severity of the flow score and the level of NF-κB activity (r = 0.55; 95% confidence interval [CI], 0.36 to 0.74; P = .01).
Effect of TNF-α and TRAIL on NF-κB activity
TNF-α and, to a lesser extent, TRAIL have been reported to trigger NF-κB activation,22,23,31,35 and both are up-regulated in MDS.5,7,36 The addition of exogenous TNF-α to MDS BMMCs enhanced NF-κB nuclear translocation above baseline levels in both normal and MDS BMMCs; however, the extent of NF-κB translocation differed. TRAIL, on the other hand, did not alter the constitutive NF-κB pattern (Figure 1A). These results suggest that the NF-κB pathway may be less relevant for TRAIL-induced apoptosis in this model, and are in agreement with data by others who showed that NF-κB activity did not correlate with responses to TRAIL in primary leukemic cells.37
NF-κB activity in marrow cells. (A) Bone marrow mononuclear cells (BMMCs) from healthy controls (shown are representative results from 1 of 3 marrows) and patients with MDS were treated with either TNF-α or TRAIL at the indicated concentrations for 20 hours. Nuclear extracts were isolated, and 32P NF-κB gel shift was performed. Unlabeled probe (Cold) was used for competitive inhibition. Shown are representative examples from patients with refractory anemia with ringed sideroblasts (RARS; tested were marrows from patients 10 and 11; shown are results from patient 10); chronic myelomonocytic leukemia (CMML; tested were patients 23 and 25; shown is patient 23) and RA with excess blasts (RAEB; tested were patients 13, 17, and 20; shown is patient 17). (B) EMSA for NF-κB using nuclear extracts from purified CD34+ and CD34- marrow cells compared with unfractioned BMMCs from primary MDS cells; equal amounts of protein were loaded for each patient (healthy controls and patients with CMML and RAEB are the same as in panel A; RA patients tested were 2, 5, and 7; shown are results in patient 7).
NF-κB activity in marrow cells. (A) Bone marrow mononuclear cells (BMMCs) from healthy controls (shown are representative results from 1 of 3 marrows) and patients with MDS were treated with either TNF-α or TRAIL at the indicated concentrations for 20 hours. Nuclear extracts were isolated, and 32P NF-κB gel shift was performed. Unlabeled probe (Cold) was used for competitive inhibition. Shown are representative examples from patients with refractory anemia with ringed sideroblasts (RARS; tested were marrows from patients 10 and 11; shown are results from patient 10); chronic myelomonocytic leukemia (CMML; tested were patients 23 and 25; shown is patient 23) and RA with excess blasts (RAEB; tested were patients 13, 17, and 20; shown is patient 17). (B) EMSA for NF-κB using nuclear extracts from purified CD34+ and CD34- marrow cells compared with unfractioned BMMCs from primary MDS cells; equal amounts of protein were loaded for each patient (healthy controls and patients with CMML and RAEB are the same as in panel A; RA patients tested were 2, 5, and 7; shown are results in patient 7).
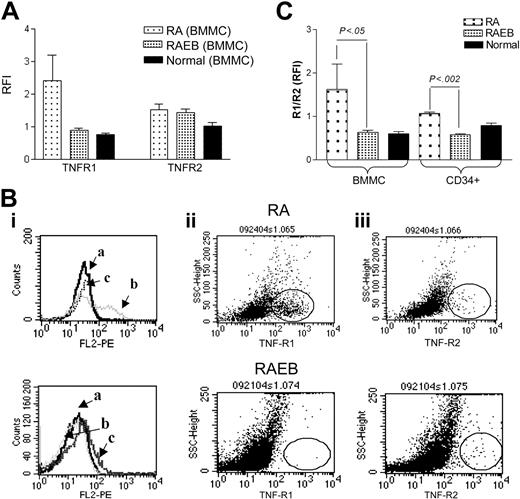
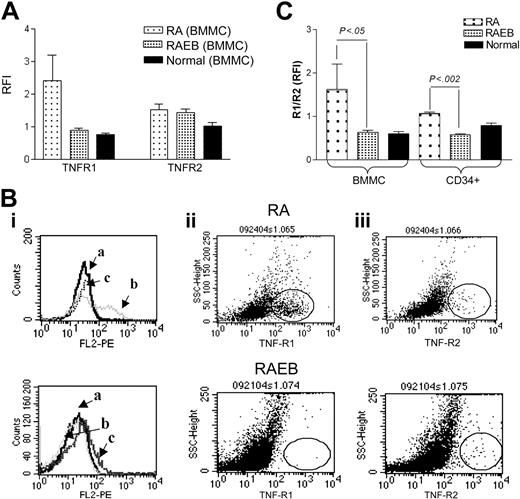
TNF-α signaling is mediated by 2 cell surface receptors, TNF R1 and R2. TNF R1 signals trigger not only proapoptotic (via caspase-8) but also antiapoptotic pathways (through NF-κB activation), while R2 lacks a cytoplasmic death domain and signals only through NF-κB. Since TNF R1- and R2-transmitted signals may lead to different end results, and evidence for altered TNF receptor expression in transformed cells has been reported,9,38,39 we determined the cell surface expression of TNF R1 and R2 in BMMCs from patients with MDS and healthy controls. TNF R1 was expressed at high levels among patients with early-stage disease (RA), while patients with advanced disease showed a pattern similar to that in normal BMMCs (Figure 3A-B). Furthermore, in CD34+ cells from patients with RA the ratio of R1 to R2 was significantly higher than in CD34+ cells from patients with RAEB (Figure 3C). The R1/R2 ratio in normal CD34+ cells lay in between RA and RAEB CD34+ cells; the values did not differ significantly from either. TNF R1 overexpression in early-stage MDS would be consistent with higher apoptotic rates, and a shift in favor of R2 may lead to predominantly antiapoptotic signals, mediated through NF-κB activation in advanced MDS.
Levels of NF-κB activity as determined by EMSA for various FAB subtypes compared with marrow from healthy donors. Shown are results with nuclear extracts from bone marrow mononuclear cells (ie, the same population on which flow analysis was carried out). NF-κB activity was determined by densitometry using ImageQuant software. Results represent intensity ratios of patient samples over healthy controls against normal. The horizontal axis represents flow scores which were calculated as described in Table 1.34 The regression line illustrates correlations of flow scores and NF-κB activity (r = 0.55; 95% CI, 0.36 to 0.74; P = .01).
Levels of NF-κB activity as determined by EMSA for various FAB subtypes compared with marrow from healthy donors. Shown are results with nuclear extracts from bone marrow mononuclear cells (ie, the same population on which flow analysis was carried out). NF-κB activity was determined by densitometry using ImageQuant software. Results represent intensity ratios of patient samples over healthy controls against normal. The horizontal axis represents flow scores which were calculated as described in Table 1.34 The regression line illustrates correlations of flow scores and NF-κB activity (r = 0.55; 95% CI, 0.36 to 0.74; P = .01).
TNF-α receptor 1 (R1; p55) and R2 (p75) expression. (A) Flow cytometric analysis of membrane TNF R1 and R2 in BMMCs from patients with MDS (RA, n = 4; RAEB, n = 8) and healthy controls (n = 4). Results represent the mean ratios of fluorescence intensities (RFIs) between specific antibody and isotype control antibodies (total BMMCs). (B) Histograms and scattergrams showing TNF receptor patterns for isotype control (i), R1(ii), and R2 (iii) in 2 individual cases of MDS; of note is the prominence of R1 (ii) in RA, distinctly different from RAEB, where R2 (iii) is expressed at a level similar to R1 (ii). Fields with the scattergrams are circled to highlight differences in R1 and R2 expression between RA and RAEB. (C) Ratio of TNF R1 to R2 determined as in panel A in BMMCs and CD34+ cells from the same patients (comparison between RA and RAEB by unpaired t test), as well as healthy controls (n = 3).
TNF-α receptor 1 (R1; p55) and R2 (p75) expression. (A) Flow cytometric analysis of membrane TNF R1 and R2 in BMMCs from patients with MDS (RA, n = 4; RAEB, n = 8) and healthy controls (n = 4). Results represent the mean ratios of fluorescence intensities (RFIs) between specific antibody and isotype control antibodies (total BMMCs). (B) Histograms and scattergrams showing TNF receptor patterns for isotype control (i), R1(ii), and R2 (iii) in 2 individual cases of MDS; of note is the prominence of R1 (ii) in RA, distinctly different from RAEB, where R2 (iii) is expressed at a level similar to R1 (ii). Fields with the scattergrams are circled to highlight differences in R1 and R2 expression between RA and RAEB. (C) Ratio of TNF R1 to R2 determined as in panel A in BMMCs and CD34+ cells from the same patients (comparison between RA and RAEB by unpaired t test), as well as healthy controls (n = 3).
Inhibitory effect of ATO on NF-κB activation
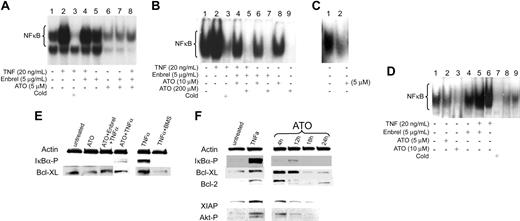
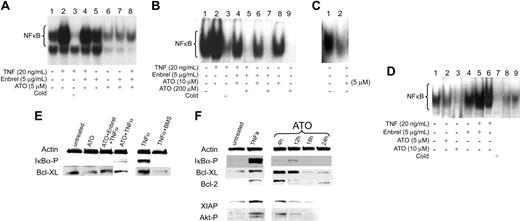
As stable “MDS cell lines” are lacking, we used leukemia-derived ML1 cells as a model to study the effects of ATO on NF-κB activation. ML1 showed constitutive NF-κB activation, and NF-κB nuclear translocation was enhanced in response to TNF-α (Figure 4A, lanes 1 and 2). In the presence of ATO (5-200 μM), nuclear translocation of NF-κB, as determined by EMSA, was reduced in normal marrow, ML1 cells, and primary MDS cells (Figure 4A-D), and no IκBα phosphorylation was detectable by Western blot. This pattern remained unchanged in the presence of TNF-α blockade by etanercept (Figure 4E). To determine if NF-κB inhibition in cells treated with ATO plus etanercept was modified by the neutralizing effects of etanercept on TNF-α-induced NF-κB activation, cells were exposed to etanercept and excess exogenous TNF-α. With exposure to TNF-α and etanercept (without ATO), there was only a minimal decrease in NF-κB nuclear translocation (Figure 4A, lane 4; and Figure 4D, lane 5), and phosphorylation of IκBα was lower when ML1 cells were treated with ATO + TNF-α in the presence of etanercept than with ATO and TNF-α only (Figure 4E). However, etanercept by itself did not enhance ATO-induced NF-κB inhibition (Figure 4A, lanes 6 and 7; Figure 4B, lanes 6 and 8). Thus, in this model NF-κB inhibition was primarily due to a direct effect of ATO, not further modified by TNF-α signals.
Inhibitory effects of ATO and etanercept (Enbrel) on NF-κB. 5 × 106 ML1 cells (A), BMMCs from a patient with RAEB (tested were marrows from patients 14, 15, 19, and 20; shown are results from patient 14) (B), and a patient with CMML (tested were patients 22 and 24; shown is patient 22) (C), and from a healthy donor (tested were 3) (D) were exposed to ATO in the presence of etanercept for 1 hour, after which TNF-α was added at the indicated concentration for 20 hours. Nuclear extracts were isolated, and 32P NF-κB gel shift was performed. A 50-fold molar excess of unlabeled probe (Cold) was used for competitive inhibition. (E) ML1 cells were exposed to ATO, etanercept, and BMS345541 (in this example at concentrations of 10 μM, 5 μg/mL, and 5 μM, respectively), for 1 hour, after which TNF-α (20 ng/mL) was added; cells were harvested 1 hour later. Phosphorylated IKBα and Bcl-XL were determined by Western blot (shown are results from 1 of 3 independent experiments). Actin served as protein control load. BMS345541, a specific inhibitor of IKBα kinase, was used here for comparison with the inhibitory effect of ATO on NF-κB. (F) ML1 cells were exposed to ATO (shown are results at 5 μM) for various time periods (4-24 hours). Results in untreated cells and cells exposed to TNF-α are shown for reference purpose (XIAP and Akt-P were not determined at 24 hours). Phosphorylated IKBα, Bcl-XL, Bcl-2, XIAP, and Akt-P were determined by Western blot. Results showed that ATO decreased NF-κB nuclear translocation and downstream antiapoptotic proteins under NF-κB control.
Inhibitory effects of ATO and etanercept (Enbrel) on NF-κB. 5 × 106 ML1 cells (A), BMMCs from a patient with RAEB (tested were marrows from patients 14, 15, 19, and 20; shown are results from patient 14) (B), and a patient with CMML (tested were patients 22 and 24; shown is patient 22) (C), and from a healthy donor (tested were 3) (D) were exposed to ATO in the presence of etanercept for 1 hour, after which TNF-α was added at the indicated concentration for 20 hours. Nuclear extracts were isolated, and 32P NF-κB gel shift was performed. A 50-fold molar excess of unlabeled probe (Cold) was used for competitive inhibition. (E) ML1 cells were exposed to ATO, etanercept, and BMS345541 (in this example at concentrations of 10 μM, 5 μg/mL, and 5 μM, respectively), for 1 hour, after which TNF-α (20 ng/mL) was added; cells were harvested 1 hour later. Phosphorylated IKBα and Bcl-XL were determined by Western blot (shown are results from 1 of 3 independent experiments). Actin served as protein control load. BMS345541, a specific inhibitor of IKBα kinase, was used here for comparison with the inhibitory effect of ATO on NF-κB. (F) ML1 cells were exposed to ATO (shown are results at 5 μM) for various time periods (4-24 hours). Results in untreated cells and cells exposed to TNF-α are shown for reference purpose (XIAP and Akt-P were not determined at 24 hours). Phosphorylated IKBα, Bcl-XL, Bcl-2, XIAP, and Akt-P were determined by Western blot. Results showed that ATO decreased NF-κB nuclear translocation and downstream antiapoptotic proteins under NF-κB control.
Recent reports suggest that the phosphatidylinositol (PI) 3-kinase/Akt pathway is involved in TNF-α-mediated NF-κB activation40,41 and in constitutive NF-κB activation in leukemic cells, apparently via activation of IκB kinases.42 Conversely, others have shown that Akt can be regulated by NF-κB.43 In ML1 cells, ATO down-regulated Bcl-XL, Bcl-2, and XIAP, all molecules under NF-κB control, with potent antiapoptotic function (Figure 4F), and phosphorylation of Akt decreased, to a limited extent. These effects were time dependent and became most prominent after 18 hours. Bcl-XL in ML1 cells was also down-regulated in the presence of the specific IκB kinase inhibitor, BMS34554144 (Figure 4E), in agreement with results obtained with other specific pharmacologic antagonists of NF-κB.43 Thus, ATO had broad effects, not only on well-characterized antiapoptotic molecules, but also on alternative survival pathways such as PI3 kinase/Akt.
Effects of ATO, etanercept, and TNF-α on apoptosis in normal marrow, MDS marrow, and ML1 cells. 1 × 106 mononuclear cells/mL were exposed to ATO in the presence of etanercept for 1 hour, and TNF-α was added at the indicated concentrations for 20 hours. Results shown here were obtained with ATO at 5μM, etanercept at 5μg/mL, and TNF-α at 20 ng/mL. Apoptosis was determined by Annexin V and propidium iodide staining. Values are corrected for background apoptosis as described in “Materials and methods.” (A) Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. Differences between normal and MDS marrows were significant (*P < .01; **P < .05; ***P < .002; unpaired t test). (B) CD34+ cells from a healthy control and 2 MDS patients (CMML, RA) were exposed to ATO as described for panel A (without etanercept and TNF-α). (C) CD34+ cells from healthy controls (normal bone marrow [NBM]) and MDS patients (RAEB) were exposed to ATO for 20 hours, and thymidine incorporation was determined to evaluate cell proliferation. As expected with CD34+ cells, spontaneous thymidine uptake was low, but it was further reduced by treatment with ATO or ATO + etanercept.
Effects of ATO, etanercept, and TNF-α on apoptosis in normal marrow, MDS marrow, and ML1 cells. 1 × 106 mononuclear cells/mL were exposed to ATO in the presence of etanercept for 1 hour, and TNF-α was added at the indicated concentrations for 20 hours. Results shown here were obtained with ATO at 5μM, etanercept at 5μg/mL, and TNF-α at 20 ng/mL. Apoptosis was determined by Annexin V and propidium iodide staining. Values are corrected for background apoptosis as described in “Materials and methods.” (A) Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. Differences between normal and MDS marrows were significant (*P < .01; **P < .05; ***P < .002; unpaired t test). (B) CD34+ cells from a healthy control and 2 MDS patients (CMML, RA) were exposed to ATO as described for panel A (without etanercept and TNF-α). (C) CD34+ cells from healthy controls (normal bone marrow [NBM]) and MDS patients (RAEB) were exposed to ATO for 20 hours, and thymidine incorporation was determined to evaluate cell proliferation. As expected with CD34+ cells, spontaneous thymidine uptake was low, but it was further reduced by treatment with ATO or ATO + etanercept.
Apoptosis induced by ATO in the presence of etanercept
In ML1 cells and in primary MDS marrow cells, ATO induced apoptosis in a dose-dependent fashion (2-10μM). ATO-induced apoptosis was significantly higher in MDS cells than in cells from healthy controls (Figure 5A). The addition of the soluble TNF receptor, etanercept (5 to 10μg/mL), did not significantly change apoptosis even in the presence of exogenous TNF-α, in disagreement with a report by others that TNF-α blockade interfered with ATO cytotoxicity.45 In purified CD34+ cells from primary marrow samples, the rate of ATO-induced apoptosis was higher than in unfractionated BMMCs (Figure 5B). Furthermore, ATO inhibited proliferation to a greater extent in CD34+ cells from MDS marrow than in normal marrow CD34+ cells; there was a suggestion that the addition of etanercept further decreased proliferation in MDS, but not in normal CD34+ cells (Figure 5C).
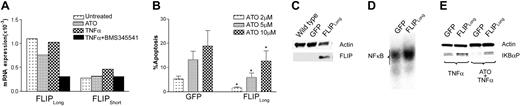
Effect of ATO treatment on expression the NF-κB-dependent gene, FLIP. (A) ML1 cells were exposed to ATO and the IκB kinase inhibitor BMS 345541 for 2 hours, and TNF-α was added for 1 hour. RNA was extracted, and FLIP mRNA levels were determined using quantitative real-time PCR. Values for each gene were normalized to expression levels of β2-microglobulin. Message levels for FLIPLong were substantially higher than for FLIPShort, resulting in a high FLIPLong/FLIPShort ratio, similar to that seen in advanced MDS. Inhibition of IKBα kinase by BMS345541 predictably blocked FLIP message. ATO reduced FLIPLong message (as expected, due to NF-κB inhibition), and TNF-α enhanced FLIPShort mRNA above the level of untreated controls. What factors are responsible for differences in the responses of the 2 splice variants is currently not clear. Shown are results achieved with ATO at 5μM, TNF-α at 20 ng/mL, and BMS345541 at 5μM. (B) ML1 cells transduced to overexpress FLIPLong were treated as indicated and evaluated for apoptosis by Annexin V and PI staining, GFP-tranduced cells served as control. Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. FLIPLong overexpression reduced the rate of apoptosis in response to ATO at all doses tested (*P < .05; paired t test). Values are corrected for background apoptosis as described in “Materials and methods.” (C) ML1 wild-type cells, ML1 cells transduced with GFP control vector (GFP) and with FLIPLong (FLIPLong), respectively, were assessed for FLIPLong expression by Western blot. (D) Spontaneous NF-κB binding as determined by 32P NF-κB gel shift of nuclear extracts from control-transduced (GFP) and FLIPLong-overexpressing (FLIPLong) ML1 cells. (E) GFP and FLIPLong cells were exposed to ATO for 1 hour, followed by TNF-α or TNF-α only and harvested 1 hour later. Phosphorylated IKBα (IKBα-P) was determined by Western blot. Results shown were obtained with ATO at 10 μM and TNF-α at 20 ng/mL. Thus, ATO down-regulated FLIP, but FLIPLong overexpression was associated with increased NF-κB activity and ATO resistance.
Effect of ATO treatment on expression the NF-κB-dependent gene, FLIP. (A) ML1 cells were exposed to ATO and the IκB kinase inhibitor BMS 345541 for 2 hours, and TNF-α was added for 1 hour. RNA was extracted, and FLIP mRNA levels were determined using quantitative real-time PCR. Values for each gene were normalized to expression levels of β2-microglobulin. Message levels for FLIPLong were substantially higher than for FLIPShort, resulting in a high FLIPLong/FLIPShort ratio, similar to that seen in advanced MDS. Inhibition of IKBα kinase by BMS345541 predictably blocked FLIP message. ATO reduced FLIPLong message (as expected, due to NF-κB inhibition), and TNF-α enhanced FLIPShort mRNA above the level of untreated controls. What factors are responsible for differences in the responses of the 2 splice variants is currently not clear. Shown are results achieved with ATO at 5μM, TNF-α at 20 ng/mL, and BMS345541 at 5μM. (B) ML1 cells transduced to overexpress FLIPLong were treated as indicated and evaluated for apoptosis by Annexin V and PI staining, GFP-tranduced cells served as control. Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. FLIPLong overexpression reduced the rate of apoptosis in response to ATO at all doses tested (*P < .05; paired t test). Values are corrected for background apoptosis as described in “Materials and methods.” (C) ML1 wild-type cells, ML1 cells transduced with GFP control vector (GFP) and with FLIPLong (FLIPLong), respectively, were assessed for FLIPLong expression by Western blot. (D) Spontaneous NF-κB binding as determined by 32P NF-κB gel shift of nuclear extracts from control-transduced (GFP) and FLIPLong-overexpressing (FLIPLong) ML1 cells. (E) GFP and FLIPLong cells were exposed to ATO for 1 hour, followed by TNF-α or TNF-α only and harvested 1 hour later. Phosphorylated IKBα (IKBα-P) was determined by Western blot. Results shown were obtained with ATO at 10 μM and TNF-α at 20 ng/mL. Thus, ATO down-regulated FLIP, but FLIPLong overexpression was associated with increased NF-κB activity and ATO resistance.
Effect of ATO treatment on antiapoptotic proteins under NF-κB control: reduction of ATO cytotoxicity in cells overexpressing FLIPLong
FLIP is an antiapoptotic molecule, which exists in the form of several splice variants. It interferes with receptor-mediated activation of caspase-8, induced by TNF-α or other proapoptotic ligands. FLIP transcription is regulated by NF-κB, and FLIP participates in an auto-amplification loop via interactions with RIP and TRAF2.11,12,46 mRNA levels of both FLIPLong and FLIPShort splice variants declined in ML1 cells treated with the IκBβ kinase inhibitor, BMS543541, in agreement with the control of FLIP by NF-κB. Similar effects were observed for FLIPLong when cells were exposed to ATO, suggesting that down-regulation of the FLIPLong splice variant was involved in ATO-induced apoptosis, while FLIPShort control may have different kinetics (Figure 6A).
We showed previously that the expression of FLIPLong and FLIPShort splice variants is dysregulated in MDS. Specifically, levels of FLIPLong increased and levels of FLIPShort decreased with progression of MDS from RA to RAEB (or more advanced disease), leading to an increase in the FLIPLong/FLIPShort ratio.10 Determination of mRNA levels for FLIPLong and FLIPShort in 3 samples in the present study from which high-quality RNA was available, showed a pattern identical to that in our original report,10 and the FLIPLong/FLIPShort ratio directly correlated with the levels of NF-κB activity (Table 2).
To further characterize the role of FLIP in this model, we overexpressed FLIPLong in ML1 cells using a lentiviral vector (Figure 6C). As shown in Figure 6B, following treatment with ATO (2-10 μM), FLIPLong-overexpressing ML1cells exhibited significantly lower rates of apoptosis than did cells transduced with a control vector, supporting a role for FLIP in the regulation of responses to ATO. FLIPLong-overexpressing ML1 cells showed higher levels of NF-κB activity than GFP control cells, as determined by EMSA (Figure 6D). Further, treatment of FLIPLong-overexpressing ML1 cells with ATO, followed by exposure to TNF-α, was still associated with IκBα phosphorylation (and, hence, NF-κB activation) rather than the expected NF-κB blockade seen in control cells (Figure 6E). These observations are consistent with the concept that the resistance to apoptosis mediated by FLIPLong overexpression was due not solely to its direct antiapoptotic function, but also an autocrine amplification loop, which contributes to further NF-κB activation.
Effect of ATO on cellular GSH content
Other mechanisms proposed for ATO-mediated cytotoxicity are the insertion of sulfhydryl groups into the mitochondrial membrane, and production of reactive oxygen species. Glutathione regulates intracellular redox status and provides antioxidant activity by cycling between its reduced (GSH) and oxidized (GSSG [oxidized GSH]) forms. GSH content in ML1 cells was measured after exposure to ATO. There was no significant change in GSH levels after 6 hours (30.5 ± 1.5 vs 34.5 ± 0.5 nmol/mg protein untreated and ATO-exposed, respectively), and 24 hours (33.1 ± 5.6 vs 34.1 ± 5.9 nmol/mg protein untreated and ATO-exposed, respectively). Also, there was no significant difference in GSH levels between wild-type, GFP control and ML1 cells overexpressing FLIPLong, that would have explained differences in apoptosis sensitivity (Figure 7A, insert), as shown by others.47,48 We depleted GSH from the cell lines by exposure to L-buthionine sulphoximine (BSO; 10-200 μM). A plateau of 50% to 60% GSH depletion was achieved at a BSO concentration of 50 μM or higher (Figure 7A). This level of depletion of GSH had no effect on cell viability. Finally, altering GSH levels by BSO treatment did not change the extent of apoptosis (Figure 7B). Thus, GSH depletion did not play a crucial role in this model, and manipulation of GSH by itself may not be useful to overcome FLIPLong-mediated resistance to apoptosis by therapeutically achievable levels of ATO.
Intracellular (reduced) GSH levels in ML1 cells. (A) GSH levels in ML1 cells transduced with control vector (GFP) or FLIPLong (FLIPLong) after 24 hours of exposure to L-buthionine sulphoximine (BSO) at concentrations of 10 to 200 μM. Test results were expressed as a proportion of baseline (set as 100%, GSH values in each determination were the average of 2 measurements). Shown in the insert is a comparison of GSH levels in wild-type (WT), GFP, and FLIPLong-transduced ML1 cells. (B) ML1 cells were exposed to BSO, at concentrations of 10 to 200 μM (shown are results with 100 μM) for 24 hours followed by treatment with ATO at 2 to 10 μM as in Figure 6B (shown are results at 5 μM) for 18 hours, and apoptosis was determined. Taken together the data showed that FLIPLong cells were more resistant to ATO than GFP-transduced cells. GSH depletion by BSO was not effective in enhancing ATO-induced apoptosis in either control-transduced GFP or in FLIPLong-overexpressing ML1 cells. Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicate.
Intracellular (reduced) GSH levels in ML1 cells. (A) GSH levels in ML1 cells transduced with control vector (GFP) or FLIPLong (FLIPLong) after 24 hours of exposure to L-buthionine sulphoximine (BSO) at concentrations of 10 to 200 μM. Test results were expressed as a proportion of baseline (set as 100%, GSH values in each determination were the average of 2 measurements). Shown in the insert is a comparison of GSH levels in wild-type (WT), GFP, and FLIPLong-transduced ML1 cells. (B) ML1 cells were exposed to BSO, at concentrations of 10 to 200 μM (shown are results with 100 μM) for 24 hours followed by treatment with ATO at 2 to 10 μM as in Figure 6B (shown are results at 5 μM) for 18 hours, and apoptosis was determined. Taken together the data showed that FLIPLong cells were more resistant to ATO than GFP-transduced cells. GSH depletion by BSO was not effective in enhancing ATO-induced apoptosis in either control-transduced GFP or in FLIPLong-overexpressing ML1 cells. Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicate.
Discussion
TNF-α and other proapoptotic cytokines play an important role in the pathophysiology of MDS.5,7,49 As MDS progresses, the rate of apoptosis in clonal cells declines. The mechanism responsible for disease progression is not known. Some reports have shown increased expression of antiapoptotic proteins of the Bcl-2 family with more advanced disease.50 More recently, Yamamoto et al evaluated the IAP family proteins in MDS and suggested a role for XIAP in the transforming process to overt leukemia.51 We have shown previously that FLIP is dysregulated in MDS. FLIPLong levels correlated negatively with apoptosis, and while FLIPLong protein levels were readily detectable in advanced disease, only low levels were present in early-stage MDS.10 As these antiapoptotic proteins are under NF-κB control, we hypothesized that this transcription factor may be a key regulator for disease progression. The status of NF-κB at a given disease stage in the marrow of patients with MDS, and its functional relevance are not well characterized.52 Bueso-Ramos et al studied NF-κB levels in mononuclear cells in a series of myeloid malignancies and showed low levels in 5 MDS cases studied, while high activity was detected in 47% of AML cases.53
The present results show an increase in NF-κB activation that correlated with MDS disease stage (ie, cells from patients with more advanced MDS had higher NF-κB activity). While in marrow from healthy donors NF-κB activity was comparable in CD34+ and CD34- subsets, significant changes were noted in MDS marrow. In early-stage MDS, NF-κB activity was lower in CD34+ than in CD34- cells, consistent with a high rate of apoptosis in early (CD34+) precursors at that disease stage. In more advanced MDS, however, NF-κB activity was higher in CD34+ than in CD34- cells. Such a pattern would be consistent with the up-regulation of FLIP, and other NF-κB-dependent antiapoptotic regulators (bcl-XL, bcl-2, XIAP), and resistance to apoptosis (in clonal MDS cells), as also observed in other disease states.16,18-21 While we were unable to show a strict positive correlation between NF-κB and mRNA of the 2 FLIP splice variants, the FLIPLong/FLIPShort ratio was low in marrows from early-stage MDS and increased with more advanced disease, in excellent agreement with the potent antiapoptotic activity of FLIPLong. Although FLIP has been shown to be under NF-κB control, FLIPLong and FLIPShort expression is differentially regulated and exhibits different kinetics,12 which may explain the differences at the mRNA and protein levels that we observed previously.10
The mechanisms involved in the activation of NF-κB in MDS are not well defined. Levels of TNF-α, a potent activator of NF-κB, are frequently up-regulated in MDS but do not strictly correlate with NF-κB activity.5,54 Further, TNF-α levels are more commonly elevated in early stage disease, where NF-κB activity is not enhanced, suggesting that an autocrine TNF-α stimulus is probably not the sole mechanism involved in the constitutive NF-κB activation.
Based on observations by Swanabori et al, who showed that the ratio of TNF R1 and R2 mRNA shifts in favor of R2 with more advance disease, we hypothesized that the R1/R2 ratio may be relevant for apoptosis (or resistance to it).9 Our data on TNF receptor R1 and R2 expression indeed corroborate Swanabori's findings at the protein level in both BMMCs and CD34+ MDS cells. Therefore, shifting the balance from R1 to R2 expression would activate NF-κB and downstream survival signals.
Since the regulation of apoptosis appears to be a central event in the pathophysiology of MDS, and since ATO, which inhibits NF-κB, has been shown to offer therapeutic benefit particularly in patients with early-stage MDS, we attempted to further characterize the effects of ATO on MDS marrow cells. Our data show that ATO treatment resulted in NF-κB inhibition and apoptosis, not only in ML1 cells, but also in normal and MDS-derived marrow cells. We had previously observed that blockade of TNF-α, which is up-regulated in many patients with MDS, improves hemopoiesis from MDS marrow,1,5 apparently by reducing apoptosis. Both ATO and TNF-α affect NF-κB activity, and the present data show that the addition of etanercept to neutralize TNF-α (originally with the intent of protecting nonclonal cells5 ) did not interfere with ATO-induced apoptosis and did not enhance proliferation in CD34+ MDS cells (Figure 5).
Associated with ATO-mediated NF-κB inhibition and induction of apoptosis was the down-regulation of NF-κB-dependent antiapoptotic genes, including FLIPLong, Bcl-xl, Bcl-2, and XIAP. As these molecules are dysregulated in MDS and up-regulation may contribute to MDS progression by mediating apoptosis resistance,50 these data suggest that ATO should be useful in the treatment of patients with MDS. However, studies on FLIPLong overexpression in ML1 cells showed a significant decrease in apoptosis in response to ATO, adding a note of caution. FLIP serves as a central regulator of apoptosis and exerts its antiapoptosis effects not only by inhibiting caspase-8, but also by modulating the NF-κB pathway via an autoamplification loop as shown here (Figure 6D) and in other reports.11,12,46 Thus, in patients with more advanced MDS and high levels of FLIP, ATO very likely would have to be combined with other agents capable of interfering with FLIP amplification in order to be therapeutically effective. Attempts to use GSH depletion as a way of enhancing ATO toxicity, as has been shown in monocytic and promyelocytic leukemic cell lines,47,55 were not successful in our model, suggesting cell type-dependent differential responses.
In summary, the present study showed that NF-κB activity correlated with MDS disease stage and flow scores, which have been shown to be of prognostic relevance. ATO induced apoptosis in hemopoietic cells in general, and in marrow cells from patients with MDS in particular, by interfering with NF-κB and the transcription of NF-κB-dependent antiapoptotic proteins. However, constitutive NF-κB activity and dysregulated FLIP levels may confer resistance to ATO in advanced-stage MDS, and combined approaches are likely to be necessary to enhance responses to ATO.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-04-1424.
Supported by Public Health Service (PHS) grants CA87948, HL36444, and HL66947. D.M.B.K. was supported by a grant from Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brazil and National Cancer Institute (NCI)/Office of International Affairs.
D.K. designed and conducted most experiments and wrote the manuscript; V.L. carried out experiments on cellular redox potential; N.A. performed cell separations and assisted with Western blots; S. Seal generated the lentiviral constructs; B.S. provided patient samples and critically reviewed the data; and H.J.D. designed experiments, critically reviewed and analyzed results, and provided manuscript revisions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Loken and D. Wells for providing the flow scores; T. Gooley for his help with statistical analysis; E Bryant for her help with FISH studies, S. Tapscott and S. Nadler for their expertise and criticism, R. Cady for providing the arsenic trioxide, and B. Larson, H. Crawford, and S. Carbonneau for help with manuscript preparation.

![Figure 5. Effects of ATO, etanercept, and TNF-α on apoptosis in normal marrow, MDS marrow, and ML1 cells. 1 × 106 mononuclear cells/mL were exposed to ATO in the presence of etanercept for 1 hour, and TNF-α was added at the indicated concentrations for 20 hours. Results shown here were obtained with ATO at 5μM, etanercept at 5μg/mL, and TNF-α at 20 ng/mL. Apoptosis was determined by Annexin V and propidium iodide staining. Values are corrected for background apoptosis as described in “Materials and methods.” (A) Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. Differences between normal and MDS marrows were significant (*P < .01; **P < .05; ***P < .002; unpaired t test). (B) CD34+ cells from a healthy control and 2 MDS patients (CMML, RA) were exposed to ATO as described for panel A (without etanercept and TNF-α). (C) CD34+ cells from healthy controls (normal bone marrow [NBM]) and MDS patients (RAEB) were exposed to ATO for 20 hours, and thymidine incorporation was determined to evaluate cell proliferation. As expected with CD34+ cells, spontaneous thymidine uptake was low, but it was further reduced by treatment with ATO or ATO + etanercept.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-04-1424/2/m_zh80240587450005.jpeg?Expires=1765937680&Signature=ks~AY~yELBMl8fAViT1AeQIhcybPz6sRry1dSpMSCsYeLS0l~6PbK1~P-m0GZBDqcQ4P3ojdKW00cFDCWaGFKqAnCN0p8jZm8VmSeFrAPwSFNLdrmf02B4eDMV0B6~LEz5Aq8N~qFbMYSExcXk4Y-OKPFdKZgojnDE5mArLCBoL9PW7SiPLDFMghRvxp15t0mjCABMPNt0p6FF0HkE066ITTSOuV7SWsGC42svp9xoh3ZN~PDqGg7xftfdDjgihGNBQHctI4QddMlnSd-BSSyZstR2nCcF49esxLIhWowCW0rh~nfv2dpvIqBJ04a8vIfUmS3gGMya5APJjM2PSfcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. Effects of ATO, etanercept, and TNF-α on apoptosis in normal marrow, MDS marrow, and ML1 cells. 1 × 106 mononuclear cells/mL were exposed to ATO in the presence of etanercept for 1 hour, and TNF-α was added at the indicated concentrations for 20 hours. Results shown here were obtained with ATO at 5μM, etanercept at 5μg/mL, and TNF-α at 20 ng/mL. Apoptosis was determined by Annexin V and propidium iodide staining. Values are corrected for background apoptosis as described in “Materials and methods.” (A) Results represent the mean plus or minus SE of 3 independent experiments, each carried out in duplicates. Differences between normal and MDS marrows were significant (*P < .01; **P < .05; ***P < .002; unpaired t test). (B) CD34+ cells from a healthy control and 2 MDS patients (CMML, RA) were exposed to ATO as described for panel A (without etanercept and TNF-α). (C) CD34+ cells from healthy controls (normal bone marrow [NBM]) and MDS patients (RAEB) were exposed to ATO for 20 hours, and thymidine incorporation was determined to evaluate cell proliferation. As expected with CD34+ cells, spontaneous thymidine uptake was low, but it was further reduced by treatment with ATO or ATO + etanercept.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-04-1424/2/m_zh80240587450005.jpeg?Expires=1766001775&Signature=ORWVcVPk2Isx0KQbDuRQ7NAwCqUbhffQWqzAh1Wn48Na1Y5Li~oSDRiPuv-~7LL~OcFTSBO14jkYL8u0av25mU9f4-6K2kUpYCSUEg-o62gs4FhBYyQN6Tr72orJOMiU9S8zj5cqW7uMLTFs3E0mi8MMBB3g1Hiq3fAz49MFBYNEzopCqGv0sdWmUwTtcwx6Lab3GHsJ-A3AuoD4I3qOR5SvXXH2UGJFAVx3n-jUdIXZdJjIxSK-wAI7tbMM9a0kPo0xGI4OIM-Teambf5xRo~bESEsUKmKTcI4UiYs0MDS1g5IS0CtoEVBo7sn0IgRlHNOcgid9KpAFZ4ojAVWtWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

