We investigated whether dendritic cells (DCs) play a role in favoring granular lymphocyte (GL) proliferation in patients with lymphoproliferative disease of granular lymphocytes (LDGL). The presence of in vivo circulating DCs was studied in 11 patients (5 CD3+ and 6 CD3- LDGL). Autologous immature (iDCs) and mature (mDCs) DCs generated in vitro were studied for stimulatory activity on cell proliferation of CD3+ and CD3- GLs. The topographic organization of GLs and DCs was also studied in bone marrow (BM) biopsies. Peripheral blood (PB) CD3- GLs from patients showed significant proliferative activity in the presence of iDCs and mDCs. Conversely, monoclonal CD3+ GLs were unresponsive to autologous and allogeneic PB DCs. Analysis of BM biopsies demonstrated a topographic distribution of DCs and GLs that indicates contact between the 2 cell types. On functional assays, DCs obtained from BM were more efficient than PB DCs in stimulating CD3- GLs, and surprisingly, a low but definite stimulatory effect was demonstrated also on CD3+ GLs. The putative contact between DCs and GLs in the BM and, more crucial, the proliferative response of discrete GL populations to DC stimulation suggest the presence of a specific antigen within BM DCs, providing evidence for a role of DCs in the pathogenesis of LDGL.
Introduction
Lymphoproliferative disease of granular lymphocytes (LDGL) is a heterogeneous disorder characterized by the chronic proliferation of granular lymphocytes (GLs).1-4 Immunologic classification of this disease distinguishes a CD3+ form, which is more common, and a CD3- natural killer (NK) type variant. Proliferating GLs in patients with LDGL largely belong to the CD3+ CD16+ T-cell subset (85%), usually expressing monoclonal rearrangements of the T-cell receptor (TCR).1-4 The cases sustained by CD3- NK cells are less frequent (15%) and are mostly represented by polyclonal proliferations, characterized by a restriction of the killer immunoglobulin-like receptor (KIR) family antigen expression.5 Recent studies concerning the pathogenesis of LDGL suggest a first step characterized by the proliferation of GLs, largely sustained by interleukin 2 (IL-2).6 Further stabilization of cell proliferation is suggested to be related to the production of other cytokines, in particular IL-15, mostly released by cells belonging to the monocytic/macrophagic lineage,7 including dendritic cells (DCs). DCs are professional antigen-presenting cells that play a critical role in T-cell activation; recent data indicate that DCs are also involved in the activation of normal NK cells.8-10 The DCs in peripheral tissues are immature but capable of active antigen uptake through regulated endocytosis and phagocytosis. After exposure to microorganisms DCs become mature and express high levels of costimulatory and adhesion molecules that favor T-cell stimulation.
Although their direct involvement has never been demonstrated, DCs are thought to play a role in several pathogenetic steps during the establishment of LDGL. At first, because proliferating cells in LDGL belong to the cytotoxic arm of immune response,1,3,11,12 GLs would have to establish contact with DCs to be primed against their specific target. The exact nature of the target is not known; however, the relationship with viral infections as inciting events has been suggested by different groups.13-16 The putative agent is likely to act as a burst antigen to start a cytotoxic immune response, and for this reason many different viruses have been proposed as etiologic agents in the pathogenesis of this disease.14,16,17 Another crucial step of the potential role for DCs in the pathogenesis of LDGL might involve the maintenance of GL proliferation, and IL-15 has been reported to be a crucial cytokine involved in this regard.7 Together with IL-15, other potentially crucial cytokines such as IL-12 and IL-18,18 which are produced by DCs, might play a role in the pathogenesis of disease. Data obtained from in vitro studies using human cells7 and in vivo studies in animal models19 support these conclusions.
Taking all these considerations together, a potential role for DCs in the pathogenesis of GL proliferation can be suggested. Several issues however still remain elusive; in particular, because pathologic GLs typically circulate in peripheral blood, it is highly unlikely that a cell-to-cell contact might take place between DCs and GLs at this site. No information has been reported as to whether and where DCs and GLs might come into contact.
Using flow cytometry analysis and functional assays, in this study we investigated whether professional antigen-presenting cells, as DCs, can be demonstrated to play a role in favoring GL proliferation in patients with LDGL. We finally evaluated the phenotype and function of DCs collected in the bone marrow of our patients, where pathologic GLs could be detected, and the chance of a cell-to-cell contact between DCs and GLs is much more likely to take place.
Patients, materials, and methods
Patients
All cases were characterized by a chronic peripheral blood lymphocytosis (lasting more than 6 months) sustained by at least 2000 GL/mm3.2 Five patients were affected by CD3+ LDGL and through molecular analysis were found to be monoclonally rearranged for TCR β and/or γ genes. Six patients were affected by CD3- LDGL. Six healthy subjects were studied as controls. Approval was obtained from the Ethical Committee of Padua University for these studies. Informed consent was obtained from each patient with LDGL and control subject.
Monoclonal antibodies
The commercially available fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) used belong to the Becton Dickinson (Sunnyvale, CA) and Immunotech (Marseille, France) series and included anti-CD3, anti-CD4, anti-CD19, anti-CD57, anti-CD5, anti-CD8, anti-CD16, and anti-CD56. Anti-KIR EB6 and GL183, anti-NCR (natural cytotoxicity receptor) NKp46 (BAB281), NKp30 (AZZ20), and NKp44 (Z231) were kindly provided by Dr A. Moretta (Genova, Italy). Flow cytometry analysis of DCs was performed with anti-CD1a, anti-CD40, anti-CD86, anti-CD83, anti-HLA-DR (human leukocyte antigen-DR), and anti-CCR7 (chemokine receptor 7) and with the Blood Dendritic Cell Enumeration kit (Miltenyi Biotec, Bergisch Gladbach, Germany). This kit combines the positivity for 3 different mAbs (blood dendritic cell antigen [BDCA]-1, BDCA-2, and BDCA-3) and can recognize 3 types of DCs: myeloid DC type 1 and type 2 (MDC1 and MDC2) and plasmacytoid DCs (PDCs).20 MDC1 were defined as cells expressing BDCA-1 (CD1c), lineage negative (Lin-), CD11c+; MDC2s were defined as cells expressing BDCA-3 at high intensity, Lin-, and PDCs were defined as cells expressing BDCA-2, Lin-, CD11c-.20
Cells were scored using a fluorescence-activated cell scanner (FACScan) analyzer (Becton Dickinson, Franklin Lakes, NJ), and data were processed using CELLQuest (Becton Dickinson).
Isolation of GLs from patients with LDGL
Purified (85%-95% CD3-CD16+) NK-type GL cells were obtained from peripheral blood mononuclear cells (PBMCs) by magnetic cell sorting (MACS; Miltenyi Biotec) using a 2-step protocol, as previously reported.7 Briefly, PBMCs were first incubated with purified anti-CD3, anti-CD14, and anti-CD19 mAbs (Becton Dickinson) for 20 minutes followed by negative selection with goat antimouse microbeads (Miltenyi Biotec). Purified (85%-95% CD16+CD3+) T-type GLs were obtained using purified anti-CD4, anti-CD14, and anti-CD19 mAbs (Becton Dickinson) for 20 minutes followed by negative selection with goat antimouse microbeads (Miltenyi Biotec). After collection, NK and T GLs were frozen in 90% fetal calf serum (FCS), 10% dimethyl sulfoxide (DMSO) until further use (usually for 5 or 6 days). Prior to use in experiments with cultured DCs, purified GLs were gently thawed, washed, and resuspended in RPMI 1640/10% FCS medium (RPMIc).
Generation of DCs
DCs were generated from PBMCs or bone marrow mononuclear cells, obtained using anti-CD14 MicroBeads, and MiniMACS separator (Miltenyi Biotec). CD14+ (10 × 104) resuspended in 100 μL was plated in 96-well flat-bottomed plates with RPMIc, plus recombinant human interleukin-4 (rhIL-4; Genzyme, Cambridge, MA) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Genzyme) added to a final concentration of 500 and 800 U/mL, respectively. On day 3, fresh complete RPMI and cytokines were added. To obtain mature DCs, fresh RPMI containing tumor necrosis factor α (TNF-α) (100 U/mL), GM-CSF (800 U/mL), and IL-4 (500 U/mL) were added on day 5 of incubation.
Proliferation assay
NK and T GLs from patients (gently thawed, washed, and resuspended in medium RPMI) at a concentration of 10 × 104 cells/mL were cultured with immature (at 3 days of culture) or mature DCs (at 5 days of culture, together with TNF-α) in 96-well flat-bottomed plates for 72 hours at 37°C in a 5% CO2 atmosphere. Each experiment was carried out in triplicate. The DC/GL ratio was chosen according to preliminary experiments in which different DC/GL ratios were tested, and a 1:1 ratio was demonstrated to provide the best response. For the last 18 hours of culture, the plates were pulsed with 1 μCi/well (0.037 MBq/well) 3H-thymidine; the cells then were harvested, and the radioactivity was measured with a β-counter. The results are expressed as a stimulation index (SI), according to the following formula: SI = cpm with stimulus × 100/cpm without stimulus.
Immunohistologic studies
Immunohistochemistry was performed on B5-fixed, decalcified, paraffin-embedded bone marrow biopsy specimens after deparaffinization and rehydration by sequential double staining. In brief, the slides were pretreated by steaming for 30 minutes while immersed in citrate buffer (1 mM/L, pH 6.0) After antigen retrieval the slides were rinsed with water and then treated with methanolic peroxide to block endogenous peroxidase activity. The slides were then rinsed, and the primary antibody was applied. The following primary antibodies were used: CD56 (clone 564; diluted 1:50; NovoCastra, Newcastle, United Kingdom), CD57 (clone NK-1; diluted 1:100; NovoCastra) for staining of GLs (indicated as cocktail I); S100 polyclonal Ab (diluted 1:4000; Dako, Hamburg, Germany), CD1a (clone NTB1; diluted 1:50; NovoCastra), CD83 (1H4b; diluted 1:40; NovoCastra) for staining dendritic cells (indicated as cocktail II). Incubations were performed at room temperature. Sequential double staining was done in each case using cocktail I/cocktail II, the first incubation being revealed with Envision Peroxidase and nitroblue tetrazolium (NBT) chromogen as substrate, the second incubation being revealed with Envision alkaline phosphatase anti-alkaline phosphatase (APAAP) and 3-amino 9-ethylcarbazole (AEC) plus chromogen as substrate. Sections were counterstained with hematoxylin. Controls consisted of reactive lymph nodes, and negative bone marrow biopsies were performed for staging in patients with Hodgkin disease. The analysis was performed using a Leica DML B2 microscope (Wetzlar, Germany), equipped with Leica 10×/0.25 numeric aperture (NA) N PLAN, 20×/0.40 NA N PLAN, and 40×/0.65 NA N PLAN objectives. Pictures were taken using a Leica DFC camera equipped with IRFAN/View Image Viewer software.
Statistical analysis
Data are expressed as mean plus or minus SEM, and comparison between values was made using the Cochran-Cox analysis. A value of P less than .05 was accepted as significant.
Results
Circulating DCs in patients with CD3+ and CD3- LDGL
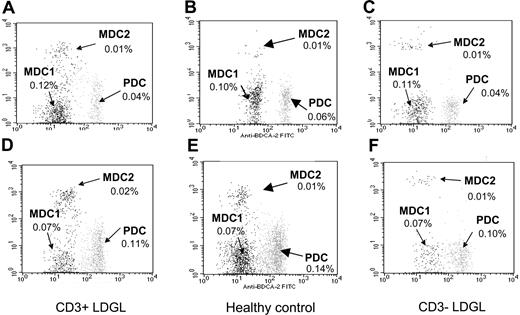
Using the combination of BDCA-1, BDCA-2, and BDCA-3 mAbs the percentage of circulating DCs was investigated in healthy individuals and in patients with LDGL. In patients with CD3+ (n = 5) and CD3- (n = 6) LDGL, the percentage of circulating DCs (ie, the sum of MDC1, MDC2, and PDC) was consistent (0.23 ± 0.01 and 0.21 ± 0.03, respectively) and not significantly different from the number of circulating DCs demonstrated in healthy controls (n = 6) (0.29 ± 0.02) (Figure 1A-C). The slight increase was related to the higher number of circulating DC1s in healthy individuals as compared with patients with LDGL.
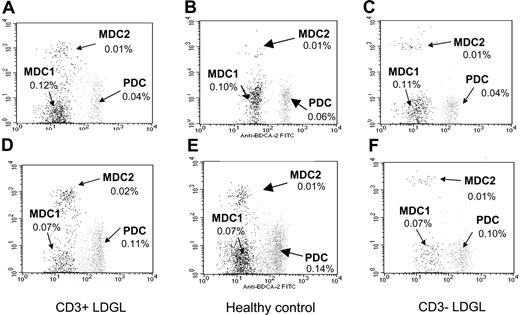
Analysis of circulating dendritic cells in patients and control subjects. The combination of 3 different mAbs was used to enumerate MDC1, MDC2, and PC dendritic cells. Data from a representative patient with CD3- LDGL of 6 cases studied, a patient with CD3+ LDGL of 5 cases studied, and a healthy control subject of 6 cases analyzed are shown. As compared with PB DCs (A-C), BM-derived DCs (D-F) were characterized by a higher percentage of PC DCs and a lower number of MDC1s. This trend was evident both in control subjects and in the patients studied.
Analysis of circulating dendritic cells in patients and control subjects. The combination of 3 different mAbs was used to enumerate MDC1, MDC2, and PC dendritic cells. Data from a representative patient with CD3- LDGL of 6 cases studied, a patient with CD3+ LDGL of 5 cases studied, and a healthy control subject of 6 cases analyzed are shown. As compared with PB DCs (A-C), BM-derived DCs (D-F) were characterized by a higher percentage of PC DCs and a lower number of MDC1s. This trend was evident both in control subjects and in the patients studied.
The phenotype of DCs was also investigated in samples obtained from BM of patients and healthy control subjects (Figure 1D-F). Both patients and control individuals showed higher numbers of PC DCs in bone marrow as compared with PB samples. However, considering DCs obtained from each specific district, that is, peripheral blood and bone marrow, differences were not found to be statistically significant between patients with CD3+ and CD3- LDGL and control subjects.
Considering DCs generated in vitro from PB monocytes, phenotypic analysis showed no significant differences between DCs generated from patients and control subjects. As expected, mDCs clearly expressed CD83 and CCR7 antigens, which were not detectable on iDCs (not shown). Antigen expression of iDCs and mDCs generated from bone marrow cells did not differ from that of DCs obtained from peripheral blood. Both iDCs and mDCs were included within the BDCA-1+ cell subset, that is, MDC1 subset (data not shown).
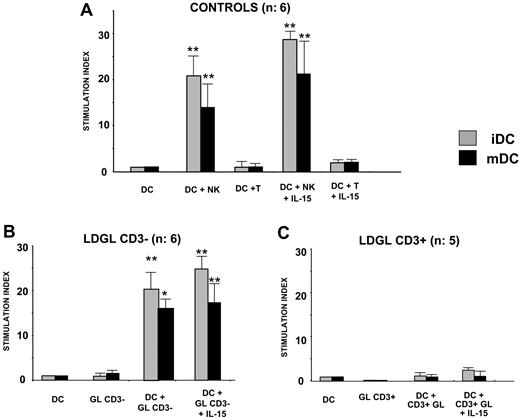
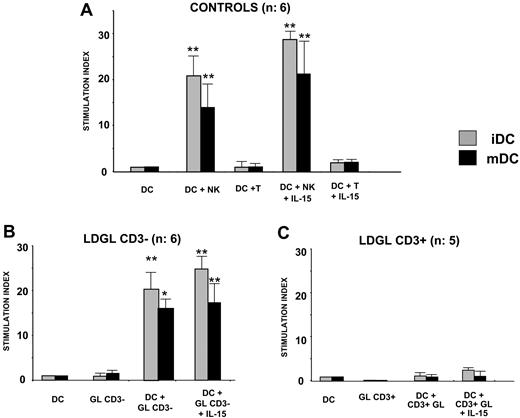
Proliferative activity induced by autologous DCs on normal NK cells, CD3- GL, and CD3+ GL from patients with LDGL. (A) Autologous DCs (n = 6) were able to induce significant proliferation in normal NK cells, whereas no proliferative activity was induced in T lymphocytes (not shown). (B) Proliferative activity induced by DCs in patients with CD3- LDGL (n = 6) was consistent with healthy control subjects (ratio DC/NK cells, 1:1). Immature DCs were able to induce a higher proliferation of CD3- GLs as compared with mature DCs. The addition of IL-15 showed a slight additive effect on proliferation. (C) No proliferative effect was induced by autologous DCs in CD3+ GLs (n = 5). Data represent mean ± SEM. *P < .05; **P < .1.
Proliferative activity induced by autologous DCs on normal NK cells, CD3- GL, and CD3+ GL from patients with LDGL. (A) Autologous DCs (n = 6) were able to induce significant proliferation in normal NK cells, whereas no proliferative activity was induced in T lymphocytes (not shown). (B) Proliferative activity induced by DCs in patients with CD3- LDGL (n = 6) was consistent with healthy control subjects (ratio DC/NK cells, 1:1). Immature DCs were able to induce a higher proliferation of CD3- GLs as compared with mature DCs. The addition of IL-15 showed a slight additive effect on proliferation. (C) No proliferative effect was induced by autologous DCs in CD3+ GLs (n = 5). Data represent mean ± SEM. *P < .05; **P < .1.
Proliferative activity induced by PB autologous DCs in normal NK cells, CD3- GLs and CD3+ GLs from patients with LDGL
PB autologous DCs were able to induce significant proliferation in normal NK cells, whereas no proliferative activity was induced for T lymphocytes (Figure 2A). Proliferative activity induced by autologous DCs in patients with CD3- LDGL was consistent with normal control subjects (n = 6 cases). Immature DCs were able to induce a higher proliferation of CD3- GLs as compared with mDCs.
Proliferative activity induced by PB DCs obtained from control subjects and patients in the allogeneic setting
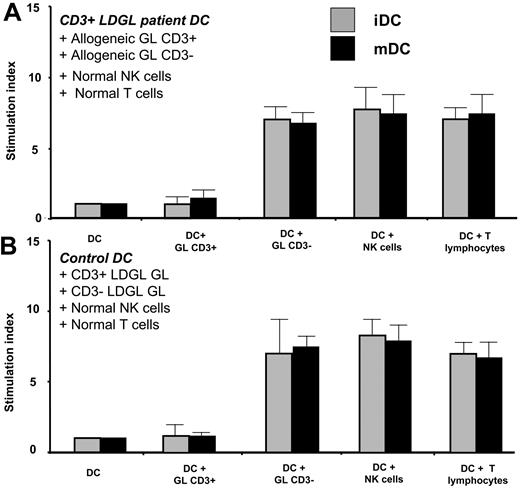
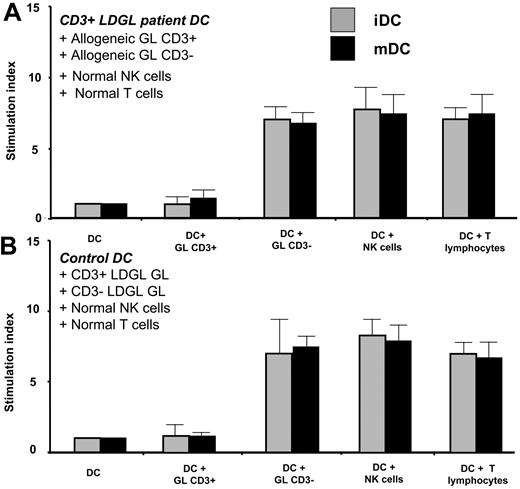
iDCs and mDCs were generated from patients with CD3+ LDGL and cultured with allogeneic CD3+ GLs, CD3- GLs, normal T and NK cells (Figure 3A). In addition, iDCs and mDCs generated from healthy control subjects were cultured with purified CD3+ or CD3- GL from patients with LDGL and also with normal T and NK cells (Figure 3B).
Proliferative activity induced by PB DCs obtained from control subjects and patients in allogeneic setting. iDCs and mDCs were generated from patients with CD3+ LDGL (n = 5) and cultured with allogeneic CD3+ GLs or allogeneic CD3- GLs, and normal NK cells or T lymphocytes (A). iDCs and mDCs generated from control subjects (n = 6) were cultured with purified CD3+ or CD3- GLs from patients with LDGL and normal allogeneic NK cells or T lymphocytes (B). DCs obtained from patients with CD3+ LDGL induced proliferation of allogeneic normal T and NK cells and CD3- GLs from patients with LDGL, whereas they were not able to stimulate allogeneic CD3+ GLs. Normal DCs stimulated allogeneic NK cells, T lymphocytes, and CD3- GLs from patients with LDGL but were not able to induce proliferation of allogeneic CD3+ GLs. Data represent mean ± SEM.
Proliferative activity induced by PB DCs obtained from control subjects and patients in allogeneic setting. iDCs and mDCs were generated from patients with CD3+ LDGL (n = 5) and cultured with allogeneic CD3+ GLs or allogeneic CD3- GLs, and normal NK cells or T lymphocytes (A). iDCs and mDCs generated from control subjects (n = 6) were cultured with purified CD3+ or CD3- GLs from patients with LDGL and normal allogeneic NK cells or T lymphocytes (B). DCs obtained from patients with CD3+ LDGL induced proliferation of allogeneic normal T and NK cells and CD3- GLs from patients with LDGL, whereas they were not able to stimulate allogeneic CD3+ GLs. Normal DCs stimulated allogeneic NK cells, T lymphocytes, and CD3- GLs from patients with LDGL but were not able to induce proliferation of allogeneic CD3+ GLs. Data represent mean ± SEM.
DCs from patients with CD3+ LDGL induced proliferation of allogeneic T and NK cells, and also CD3- GLs, whereas they were not able to stimulate allogeneic CD3+ GLs. Normal DCs stimulated allogeneic CD3- GLs, but they were unable to induce the proliferation of allogeneic CD3+ GLs.
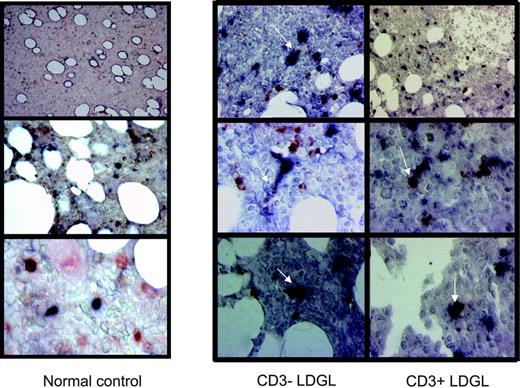
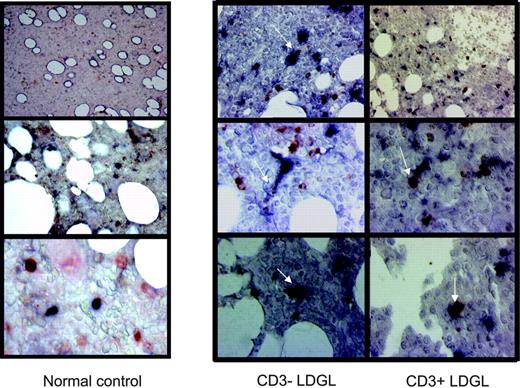
Immunohistologic analysis of DCs and GLs in patients with CD3+ and CD3- LDGL. Immunoperoxidase staining for CD56 and CD57 (red) and CD1a and CD83 (black) in bone marrow biopsies from control and representative patients with CD3- and CD3+ with LDGL. An evident contact between the 2 cell types can be seen in patients with LDGL indicated by arrows. However, GLs and DCs were clearly separated in healthy control subjects (left).
Immunohistologic analysis of DCs and GLs in patients with CD3+ and CD3- LDGL. Immunoperoxidase staining for CD56 and CD57 (red) and CD1a and CD83 (black) in bone marrow biopsies from control and representative patients with CD3- and CD3+ with LDGL. An evident contact between the 2 cell types can be seen in patients with LDGL indicated by arrows. However, GLs and DCs were clearly separated in healthy control subjects (left).
Immunohistologic analysis of DCs and GLs in patients with CD3+ (n = 5) and CD3- (n = 6) LDGL
Because bone marrow is a site of potential contact between DCs and GLs in vivo, we investigated whether a cell-to-cell contact takes place between these 2 cell types in bone marrow biopsies in patients with LDGL. A detectable infiltrate of GLs (ranging from 5% to 35%, interstitial type) was demonstrated in all patients studied. The distribution of GLs in bone marrow samples obtained from healthy individuals showed a scattered pattern of CD56 and CD57 cells without any topographic contiguity with DCs (Figure 4).
Clearly different and strongly suggestive of cell-to-cell contact was the pattern detected in patients with both CD3+ and CD3- LDGL. As shown in Figure 4, a very evident association is detectable between DCs and GLs. This picture is highly suggestive of in vivo cross talk between the 2 cell types.
Comparison between PB- and BM-derived autologous DCs in CD3- GLs and CD3+ GLs from patients with LDGL
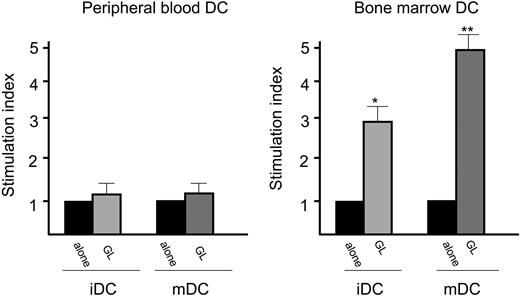
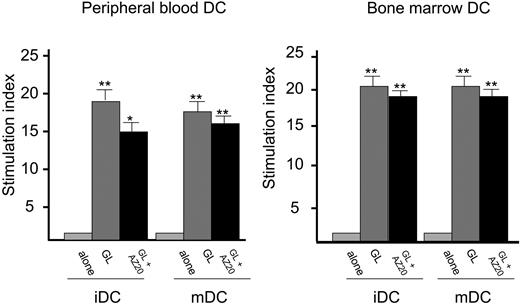
A comparative analysis was performed to evaluate the proliferative activity induced by DCs generated from bone marrow and peripheral blood. In patients with CD3- LDGL both PB- and BM-generated DCs were able to stimulate the growth of NK GLs, with slight but not significant differences (Figure 5). Considering patients with CD3+ LDGL, we could demonstrate a clear difference because DCs generated from BM were significantly more effective in inducing proliferation of autologous CD3+ GLs (Figure 6) as compared with DCs obtained from PB. This result strongly suggests that primed DCs were obtained from the BM microenvironment.
Effect of anti-KIR mAb and anti-NCR on proliferative activity induced by DCs
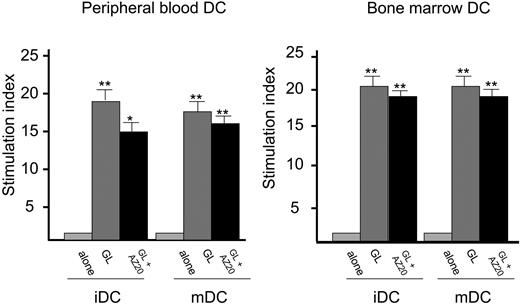
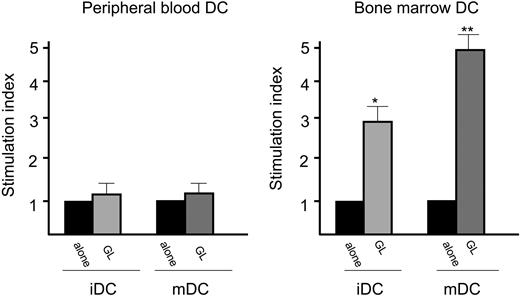
The addition of specific anti-KIR mAb in patients with CD3- LDGL and restricted KIR expression (2 of 6 cases) was unable to modify CD3- GL proliferation (not shown). The addition of the anti-NCR NKp30 mAb (AZ20 mAb), which has been demonstrated to be involved in the cytotoxicity mediated by NK cells against DCs, did not significantly modify proliferation of GLs induced by DCs (Figure 5).
Proliferative activity induced by autologous peripheral blood and bone marrow DCs in patients with CD3- LDGL. Data from PB and BM of 5 patients are shown. DCs were cultured with GL at a 1:1 ratio. Anti-NKp30 (AZ20) mAb was added together with autologous GLs at the concentration of 1 μg/mL. *P < .05; **P < .01 as compared with the baseline value. Data represent mean ± SEM.
Proliferative activity induced by autologous peripheral blood and bone marrow DCs in patients with CD3- LDGL. Data from PB and BM of 5 patients are shown. DCs were cultured with GL at a 1:1 ratio. Anti-NKp30 (AZ20) mAb was added together with autologous GLs at the concentration of 1 μg/mL. *P < .05; **P < .01 as compared with the baseline value. Data represent mean ± SEM.
Discussion
In this study we demonstrated that DCs generated from PB monocytes were able to stimulate the proliferation of GLs in patients with NK-type LDGL. This stimulating effect was not detectable in patients with CD3+ LDGL using autologous DCs generated in the same experimental conditions. Dendritic cells obtained from patients with LDGL were able to stimulate normal NK and T cells, indicating that they were fully functional. Interestingly, a significant proliferation was demonstrated when the DCs generated from BM of patients with CD3+ LDGL were used instead of those obtained in PB. Our data clearly indicate that DCs obtained from BM of patients were functionally different from those obtained from PB.
Our results demonstrated that DCs generated from PB monocytes are largely included in the subset of myeloid DC type 1, with myeloid type 2 and plasmacytoid representing the less relevant populations. This distribution reflects the relevant populations of in vivo-circulating DCs demonstrated in patients with LDGL and in healthy individuals. In both healthy control subjects and patients with LDGL, the amounts of DCs were nearly superimposable, indicating that an imbalance of circulating DCs was not a relevant feature in this disorder. Although different subsets of DCs have been reported to be involved in a variety of clinical conditions,21,22 this is not the case in LDGL. An alternative interpretation might be that peripheral blood is not suitable to obtain information on the role of DCs in LDGL. Indeed, a relevant issue that remains to be addressed concerns the fact that if a cross talk between DCs and GLs occurs, how and where does this take place? The possibility that DCs and GLs interact in peripheral blood is very unlikely, so we looked at the interaction between these cells at other body sites where these cell types have a higher chance of coming into contact with each other. Recently, it has been reported that NK cells and DCs can be demonstrated in tight contact within the T-cell zone of lymph nodes.23 However, lymphadenopathy is rare in patients with LDGL.3,24 On the contrary, the bone marrow has been frequently demonstrated to represent a privileged site of GL infiltration, both in CD3+25 and in CD3- LDGL cases. Accordingly, we focused our attention on whether a cell-to-cell contact might be demonstrated in the bone marrow biopsies of patients with LDGL, and we provide evidence that DCs and GLs of patients are in very close topographic distribution in the bone marrow microenvironment. More strikingly, this pattern was very characteristic of patients with LDGL and has never been found in healthy individuals.
As an additional step, we generated DCs from bone marrow of patients to evaluate whether they were able to sustain the stimulation of autologous GLs. Whereas in patients with CD3- we could demonstrate only a slight increase of stimulatory activity, interestingly in patients with CD3+ a statistically significant stimulatory effect on GLs was demonstrated. Provided that CD3+ GLs of patients with LDGL are equipped with a monoclonal TCR, it is expected that they would grow only in the case of recognition of their specific antigen responsible for activation. As a matter of fact, many reports demonstrated that, although GLs presented a monoclonal rearrangement of TCR, they are still responsive to stimuli through TCR, suggesting that monoclonal cells are equipped with a fully functional TCR.26,27 The lack of specific antigen on in vitro-generated DCs was likely to be the reason for the absence of proliferation of clonal autologous or allogeneic GLs (not polyclonal normal T cells), because they could be stimulated only by DCs pulsed with specific antigen. According to these results we speculate that the putative-inciting agent responsible for GL proliferation might be located within BM dendritic cells, which can be viewed as cells pulsed in vivo and thus directly involved in the pathogenesis of disease.
Some NK receptors have been demonstrated to be involved in the cross talk between DCs and NK cells or NK-cytotoxic T lymphocytes (CTLs). Among them, NKp30 has been demonstrated to be the receptor involved in the lysis of DCs by NK cells,9 although no information is yet available on the role of this receptor in the control of cell proliferation. The addition of anti-NKp30 mAb in our experimental model was not associated with a significant reduction of proliferation. This result can also be viewed in the light of previously published data which demonstrated low in vivo expression of NCRs in patients with LDGL.6,28 Additional studies are needed to define the molecules involved in the cross talk between DCs and GLs in patients with LDGL.
Our data might also help explain some intriguing results reported in patients with CD3+ LDGL. We demonstrated that CD3+ GLs showing up-regulation of rafts, as evaluated by GM1 antigen expression, can be observed in PB of patients.29 These recently activated cells are characterized by high production of interferon-γ (IFN-γ) and a high percentage of cycling cells and are supposed to represent the renewing cell population, responsible for maintaining GL proliferation.29 It is argued that these recently activated cells might correspond to cells released from BM soon after activation by DCs. Studies are in progress to evaluate immune synapses in these GL/DC complexes for molecules involved in this process.
Proliferative activity induced by autologous peripheral blood and bone marrow DCs in patients with CD3+ LDGL. Data from PB (left) and BM (right) of 5 patients are shown in the figure. DCs were cultured with GLs at a 1:1 ratio. *P < .05; **P < .01. Data represent mean ± SEM.
Proliferative activity induced by autologous peripheral blood and bone marrow DCs in patients with CD3+ LDGL. Data from PB (left) and BM (right) of 5 patients are shown in the figure. DCs were cultured with GLs at a 1:1 ratio. *P < .05; **P < .01. Data represent mean ± SEM.
The evidence we provided that DCs obtained from BM of patients with LDGL are likely to be correlated with the induction of proliferative activity of GLs in these patients might help define the mechanisms involved in the pathogenesis of LDGL, thus making DCs a putative target for innovative therapy in the treatment of this disorder.
Prepublished online as Blood First Edition Paper, August 9, 2005; DOI 10.1182/blood-2005-05-1972.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Ministero dell'Istruzione dell'Università e della Ricerca (MIUR), the Consiglio Nazionale delle Ricerche (CNR), the Fondo per gli Investimenti della Ricerca di Base (FIRB), the Fondo Regionale Regione Veneto, and by grants from the Istituto Oncologico Veneto.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr A. Moretta (Genoa) for providing EB6, GL183, and AZ20 mAbs and Dr M. Vitale (Genoa) for critical revision of the manuscript. We are grateful to Dr Martin Donach for editing the manuscript.