The discovery of purine nucleoside phosphorylase (PNP) deficiency and T lymphocytopenia suggested that inhibition of this enzyme could serve as a therapeutic target. Inhibitors of PNP failed until structure-based synthesis of immucillin-H (BCX-1777, forodesine), a transition-state analog of PNP. The picomolar potency for PNP, T cell-selective cytotoxicity, and animal studies provided the rationale for use of forodesine in T-cell malignancies. Five patients were treated with an intravenous infusion of forodesine (40 mg/m2) on day 1; treatment continued on day 2; forodesine was administered every 12 hours for an additional 8 doses. Plasma and cellular pharmacokinetics and pharmaco-dynamics were investigated. Median peak level of forodesine (5.4 μM) was achieved at the end of infusion. This level was sufficient to increase plasma 2′-deoxyguanosine (dGuo) concentrations in all patients. Intracellular deoxyguanosine triphosphate (dGTP) increased by 2- to 40-fold in 4 of 5 patients (8 of 9 courses) and correlated with antileukemia activity in 4 patients. However, objective responses were not observed. This was the first clinical study in humans to demonstrate the plasma pharmacokinetics and the pharmacodynamic effectiveness of the PNP inhibitor, forodesine; however, regrowth of leukemia cells in the blood and marrow after course 1 suggested that a different therapeutic schedule should be considered for future studies.
Introduction
The enzyme purine nucleoside phosphorylase (PNP) is responsible for phosphorolysis of 2′-deoxyguanosine (dGuo) to the guanine nucleobase and 2′-deoxyribose-1-phosphate.1 X-ray crystallographic analyses suggested that the mammalian enzyme is a trimeric structure that accepts only 6-oxopurine nucleosides such as dGuo and inosine, but not 2′-deoxyadenosine or the pyrimidine 2′-deoxynucleosides as substrates.2 This selectivity is different from that observed with prokaryotic PNP.3 The exocyclic O6 of the base forms a hydrogen bond to the amino acid (Asn243) of the enzyme and provides the substrate specificity of the mammalian PNP.4 In addition to this selectivity, the substrate preference of human and bovine PNP is high, with Km values between 10 and 40 μM for inosine and dGuo, which results in high phosphorolysis efficiency (Vmax/Km = 2.56 for dGuo).1,5-7
Giblett et al8 discovered that the rare genetic deficiency of this enzyme, due to mutations in the gene encoding for PNP, causes profound T-cell lymphopenia.8,9 In addition, T cell-mediated immunity is affected in these patients.10-12 Initial studies in pediatric patients with PNP deficiency revealed that there was an increase in the level of plasma dGuo.12-14 Serum dGuo was maintained between 2 and 20 μM in these patients, compared with undetectable levels in healthy controls.15 T-cell specificity was due to the inherently greater phosphorylation of dGuo and slower catabolism of the phosphorylated dGTP in T cells. This, in turn, leads to deoxyguanosine triphosphate (dGTP)-directed inhibition of DNA synthesis and cell death.12-14,16-18 This knowledge provided the rationale for using PNP as a target for development of therapeutics that would be selective to T cells. Attempts to achieve high levels of plasma dGuo by intravenous infusion of dGuo were hampered by its rapid degradation resulting from the high specific activity of PNP, ubiquitous in large body organs such as liver, spleen, kidney, and circulating lymphocytes and erythrocytes in blood.9,19 Hence, pharmacologic inhibition of PNP would be required to increase plasma dGuo concentration.
Several agents have been shown to inhibit PNP,20 and pharmacokinetic studies have demonstrated that more than 95% continuous inhibition of PNP is required to achieve significant reduction in T-cell levels.21 Acyclovir, a potent inhibitor of herpes simplex virus replication, also inhibits PNP, albeit to a lesser extent. The low inhibitory potency of this agent (eg, Ki of 90 μM) makes it unsuitable for clinical use.22 Similarly, allopurinol, 6-mercaptopurine, and 6-methoxypurines inhibit PNP, but only at very high drug concentrations.23 C-8-substituted analogs such as 8-iodoguanosine and 8-aminoguanosine have been used as inhibitors of PNP.23 Studies in cell lines clearly demonstrated that 8-aminoguanosine inhibited PNP activity and resulted in T cell-selective cytotoxicity.24 Additional PNP inhibitors include analogs of dGuo such as 8-amino-9-(2-thienylmethyl)guanine (PD119229)25 and analogs of deazaguanine.26,27 However, the inhibitory activity was not as potent as that observed with N7 substituted congeners.4 A series of analogs with N7 substitution were found to be highly potent in inhibiting enzymatic activity of PNP. BCX-34 (peldesine) had a 50% inhibitory concentration (IC50) of 30 nM; however, when used in clinical trials to treat patients with psoriasis and cutaneous T-cell lymphomas, there was no significant clinical activity. Enzymatic studies indicated that BCX-34 had a rapid off rate and could not inhibit PNP sufficiently to elevate plasma dGuo to levels necessary for T-cell suppression.20,28
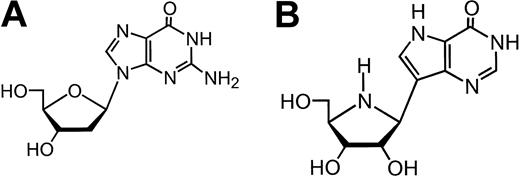
Schramm's group used another strategy to design more potent PNP inhibitors by identification of the transition-state structure stabilized by the target enzyme.29-31 Geometric and electrostatic properties of the transition state of substrate were used as an atomic blueprint to design chemically stable isologues to act as analogs. Using inosine as a substrate for transition-state analysis, a series of 9-deazanucleoside analogs, termed immucillins, was designed to mimic the transition state.32,33 The immucillins have a carbon-carbon linkage between a cyclic amine moiety that replaces ribose, and either 9-deaza-hypoxanthine or 9-deaza-guanine (immucillin H and immucillin G, respectively). These analogs inhibited PNP with high potency; the Ki values were in the 20 to 80 pM range for human and bovine enzyme.34 In vitro studies using cell lines established that immucillin H (BCX-1777, forodesine; Figure 1) resulted in T cell-selective cytotoxicity that was mediated by the intracellular accumulation of dGTP from exogenous dGuo.35,36 In vivo investigations in murine model systems further established utility and effectiveness of this agent in T-cell lysis.37
Based on these observations, a phase 1 clinical trial was designed to test the hypothesis that forodesine would inhibit PNP in vivo, resulting in biochemical sequelae that would be an effective treatment strategy for T-cell malignancies.38 To serve this purpose, patients with previously treated T-cell malignancies such as T-cell lymphoblastic lymphoma (T-LL), T-cell acute lymphoblastic leukemia (T-ALL), and T-cell prolymphocytic leukemia (T-PLL) were entered into this protocol, the first study of forodesine in humans. The present investigation reports the clinical outcomes as correlated with pharmacokinetics of forodesine during therapy, pharmacodynamic end points of dGuo accumulation in plasma as a result of PNP inhibition, and its conversion to dGTP in T-cell leukemia cells.
Patients and methods
Study group
Patients were eligible if they had previously treated relapsed or refractory T-LL, T-ALL, or T-PLL with a performance status 3 or better and adequate organ function with total bilirubin 1.5 mg/dL or less and creatinine 1.5 mg/dL or less (or if creatinine clearance was at least 45 mL/min). All patients were informed of the investigational nature of this protocol in accordance with institutional policies. An Institutional Review Board-approved informed consent form was signed by all patients for both clinical and pharmacology studies; approval was obtained according to the Declaration of Helsinki.
Therapy and statistical design
Forodesine was infused over 30 minutes on the first day. For days 2 to 5, the drug was administered twice daily at 12-hour intervals. The twice daily dosing schema of forodesine was chosen based on results of preclinical studies in primates suggesting a t½ for forodesine of approximately 3 hours and a t½ for plasma dGuo of 12 seconds.39 The starting dose of forodesine (40 mg/m2) was based on data from preclinical studies in primates regarding activity and toxicity, suggesting maximum daily exposure in primates of 20 mg/kg/d (equivalent to 800 mg/m2/d). Forodesine infusions were separated from citrated blood products by at least 30 minutes owing to unexplained deaths in preclinical animal studies when forodesine was given with citrate as the buffer vehicle. Additional courses of forodesine were allowed if stable disease or hematologic improvement was observed after course 1. Courses were repeated every 21 to 28 days. Intrapatient dose escalation was allowed for subsequent courses with 50% increments by protocol design if no clinically relevant drug toxicity was observed with the prior course.
The phase 1 statistical design was a continual reassessment method (CRM), with cohorts of 3 patients chosen for evaluation of toxicity frequency.40 The maximum tolerated dose (MTD) was projected to be the dose level associated with the probability of grade 3 or greater nonhematologic toxicity closest to 30%. The CRM was implemented by computerized entry of the toxicity profile observed with each patient evaluated at the 21-day time point from the start of forodesine. Toxicity was graded according to the National Cancer Institute Expanded Common Toxicity Criteria, version 2.0.
Response criteria
For T-ALL and T-PLL, complete remission (CR) was defined as 5% or fewer leukemia cells in a normocellular or hypercellular marrow with absolute neutrophil count (ANC) equal to or more than 1.0 × 109/L and platelet (PLT) count equal to or more than 100 × 109/L. Complete resolution of extramedullary disease was required. Partial response was defined with hematologic parameters as for CR, but with residual marrow disease 6% to 25% allowed and at least 50% reduction in extramedullary disease required. Hematologic improvement was defined as achievement of any of the following parameters: (1) increase in ANC by 50% and to greater than 109/L if below this level prior to therapy, (2) increase in hemoglobin level by 2 g/dL if below 10 g/dL prior to therapy or decrease in transfusion requirements by at least 50%, (3) increase in PLT count by 50% and to greater than 50 × 109/L if below this level prior to therapy or achievement of transfusion independence, (4) reduction in bone marrow disease to 5% or less, and (5) reduction in lymphadenopathy, hepatomegaly, or splenomegaly by at least 50%. For T-LL, the same criteria for CR applied; however, partial response was defined as at least 50% reduction in nodal and mediastinal disease with clearance of bone marrow disease, if present. Stable disease was defined as no significant change, whereas progression was defined as at least 25% increase in disease burden.
Drug and other chemicals
Forodesine for clinical use was provided by BioCryst Pharmaceuticals (Birmingham, AL). For quantitation of deoxynucleotides, deoxynucleotide triphosphates (dNTPs) were obtained from Amersham Biosciences (Piscataway, NJ) and were used as standards. [3H]-deoxyadenosine triphosphate (dATP) and [3H]-deoxythymidine triphosphate (dTTP) were purchased from Perkin Elmer Life Sciences (Boston, MA) and MP Biomedicals (Irvine, CA), respectively.
Samples for clinical pharmacology
Plasma, cellular, and urine pharmacokinetic studies were conducted in all patients. Blood sampling was performed before therapy; at the end of infusion (eoi) of dose 1 (30 minute); and 1, 2, 3, and 4 hours, between 6 and 10 hours, and then 24 hours after the start of first dose. Sampling on days 2 to 5 was performed prior to and at the eoi of doses 2, 4, 6, and 8 (first dose of the day). Collections were obtained via a separate intravenous line from that being used for forodesine infusions. Blood samples (3-10 mL) were obtained in green stopper Vacutainer tubes containing heparin and 50 μM BCX-3428 (BioCryst Pharmaceuticals) to inhibit PNP if not completely inhibited by forodesine. The tubes were placed immediately in an ice-water bath and transported to the laboratory.41 Urine samples were collected prior to drug infusion and during the first 48 hours of therapy while the patient was hospitalized. These samples were collected from 0 to 2 hours, 2 to 4 hours, 4 to 8 hours, 8 to 24 hours, and 24 to 48 hours after day 1 dosing of forodesine.
Plasma and urine pharmacology
The plasma was removed after centrifugation and stored at -70°C until high-pressure liquid chromatography (HPLC) analyses. Forodesine and inosine were determined by a validated liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) method. They were extracted using a Varian phenylboronic acid affinity solid phase extraction cartridge on a Zymark RapidTrace Workstation (Caliper Life Sciences, Hopkinton, MA). Chromatography was performed isocratically using a Zorbax SB C-3 column (Agilent Technologies, Lake Forrest, CA) with isocratic elution using 5 mM ammonium formate and 5% methanol, pH 3.0, in water on an Agilent 1100 HPLC system. Column effluent was analyzed by positive ion multiple reaction monitoring using a PE Sciex API 3000 MS/MS equipped with Turbo Ion Spray in positive ion mode. The concentrations of forodesine and inosine were then determined by weighted (1/×) quadratic regression analysis of peak areas produced from the standard curve spanning 5 to 1000 ng/mL for forodesine and 15 to 5000 ng/mL for inosine.
Plasma dGuo was quantitated by the LC/MS/MS procedure. The dGuo standard curve was validated from 1 ng/mL to 4000 ng/mL. dGuo was extracted from plasma using a Waters Oasis “HLB” affinity solid phase extraction cartridge using a Zymark RapidTrace Workstation. The extracts were analyzed using Zorbax SB C-3 reverse phase HPLC and multiple reaction monitoring method of mass spectrometry. A 0.5-minute gradient from 5% to 10% methanol in 0.1% acetic acid was used. Column effluent was analyzed by positive ion multiple reaction monitoring using a PE Sciex API 2000 MS/MS equipped with Turbo Ion Spray in positive ion mode. Urine forodesine, dGuo, and inosine concentrations were determined by the LC/MS/MS methods described for plasma.
Cellular pharmacology
Cell pellets from blood samples were diluted with phosphate-buffered saline and leukemia cells were isolated by Ficoll-Hypaque density gradient step-gradient centrifugation procedures.41 A Coulter channelyzer (Coulter Electronics, Hialeah, FL) was used to determine cell number and the mean cell volume. The nucleotides in the leukemia cells were extracted by 60% methanol as described,42 and the DNA polymerase assay as modified by Sherman and Fyfe43 was used to quantitate dNTPs in the cell extracts.42
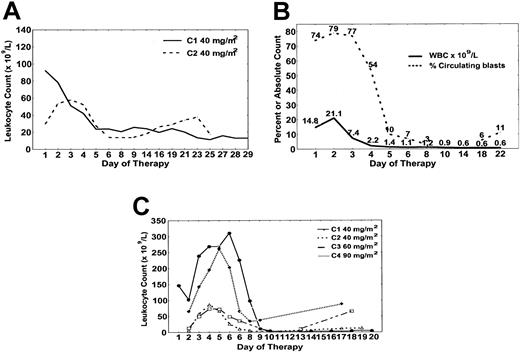
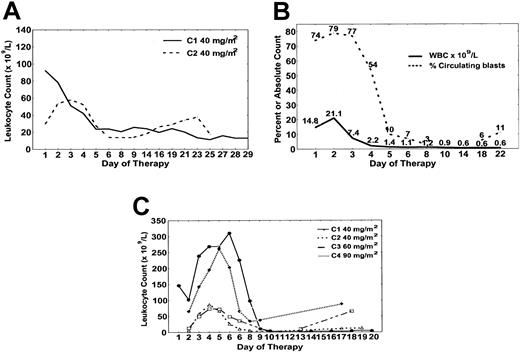
Hematologic response to forodesine. (A) Patient no. 2 had decrease in absolute prolymphocyte count with course 1 (solid) and course 2 (dashed) in the time course shown. (B) Patient no. 4 cleared circulating T cells by day 10. (C) Patient no. 5 had cytoreduction with each course of forodesine with absolute prolymphocyte counts plotted for course 1 (•), course 2 (▵), course 3 (□), course 4 (♦).
Hematologic response to forodesine. (A) Patient no. 2 had decrease in absolute prolymphocyte count with course 1 (solid) and course 2 (dashed) in the time course shown. (B) Patient no. 4 cleared circulating T cells by day 10. (C) Patient no. 5 had cytoreduction with each course of forodesine with absolute prolymphocyte counts plotted for course 1 (•), course 2 (▵), course 3 (□), course 4 (♦).
Calculations and statistical analysis
Noncompartmental analysis was used to determine the pharmacokinetic parameters from the plasma concentration-time data. Urine excretion rate curves were generated for forodesine and inosine using noncompartmental analysis. Rate of drug or biomarker was plotted against the midpoint time of urine collection. The half-life (t½z, terminal elimination t½) could not be determined due to the small number of data points. The cumulative amount excreted in the first 24 hours (ie, milligrams) was determined for each patient where possible. Linear or rectangular hyperbola analyses for r values and P values for time-dependent accumulation of dGTP pharmacology were obtained using Prism software (GraphPad Software, San Diego, CA).
Results
Study group
Five patients with relapsed or refractory T-cell malignancies were treated with forodesine; prior therapy and patient characteristics are detailed in Table 1. Three patients had T-PLL and 2 had T-ALL. Forodesine (40 mg/m2) was administered according to protocol in all 5 patients. Patient nos. 2 and 5 received additional courses of forodesine, the latter with dose escalation (Tables 2, 3). After the first 5 patients were enrolled, review of the clinical and pharmacology data suggested that an alternative dosing schedule of forodesine should be considered, and enrollment in the phase 1 portion of the study ceased, although the MTD had not been reached.
Clinical outcomes
Overall, no objective responses were observed (Table 2). Three patients (nos. 1, 2, and 5) had stable disease after one course of forodesine; additional courses of forodesine were administered in the latter 2 patients. Patient no. 4 had initial reduction in tumor burden with regrowth of the leukemia, whereas patient no. 3 had progressive disease.
Patient no. 1 had a minor (25%) reduction in peripheral adenopathy during forodesine therapy and decrease in bone marrow involvement (from 94% to 12%) identified on day 21 (Tables 1, 2). For patient no. 2, response to course 1 of forodesine was observed with reduction in white blood cell (WBC) count from 121.1 × 109/L to 33.8 × 109/L by day 6, with stability until approximately day 28 when proliferation recurred (Figure 2A). Cytoreduction was again observed with course 2 of forodesine 40 mg/m2 with reduction in WBC count from 81.7 × 109/L to 46.7 × 109/L with stability thereafter (Figure 2A). The patient discontinued therapy owing to recurrence of neurologic toxicities at approximately day 21 of course 2. Patient no. 3 developed a progressive increase in WBC count (from 87.1 × 109/L to 200.5 × 109/L) by day 10, with a proportional increase in lymphocytes and decrease in absolute prolymphocytes (Table 2). Patient no. 4 had clearance of circulating blasts by day 10 and absence of detectable bone marrow disease on day 14. However, day 21 bone marrow aspiration revealed regrowth of T-ALL with persistent cytopenias (Table 2 and Figure 2B). For patient no. 5, the WBC count decreased from 150.6 × 109/L to 17.2 × 109/L by day 21 of course 1 of forodesine. No change in lymphadenopathy or splenomegaly was observed. Figure 2C details the pattern of change in the absolute prolymphocyte count with each subsequent cycle of forodesine (40 mg/m2, 60 mg/m2, 90 mg/m2), characterized by initial increase in the WBC count during days of forodesine infusion, with gradual nadir by day 10 to 14 of each cycle, and increase thereafter until developing rapidly progressive disease day 21 of course 4.
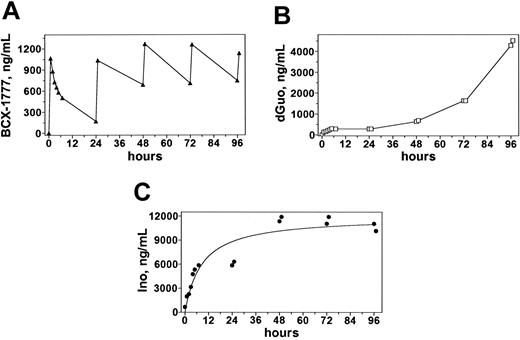
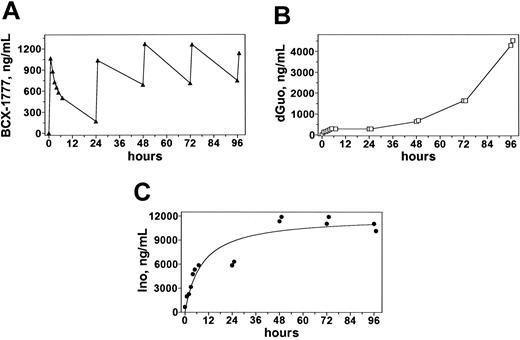
Plasma pharmacokinetics during and after infusions of 40 mg/m2 forodesine. Blood samples obtained from patient no. 5 were processed and analyzed on HPLC to quantitate concentrations of forodesine (A), dGuo (B), and inosine (C).
Plasma pharmacokinetics during and after infusions of 40 mg/m2 forodesine. Blood samples obtained from patient no. 5 were processed and analyzed on HPLC to quantitate concentrations of forodesine (A), dGuo (B), and inosine (C).
Toxicity
Details of the toxicities observed with forodesine are provided in Table 3. The most common toxicity observed was grade 3-4 neutropenia, associated with infection or fever of unknown origin (or both) in 2 cases. Grade 1-2 headaches, hypocalcemia, and nausea/vomiting were observed during days of infusion of forodesine. Autonomic dysfunction (orthostasis) was observed as a late event (approximately day 21 or later) in 2 patients; this was attributed to forodesine in patient no. 2 and to prior vincristine treatment in patient no. 5.
Plasma pharmacology
The typical pharmacokinetic profile of forodesine revealed that the peak level of forodesine was achieved at the end of the infusion (Figure 3A). The forodesine elimination was slow; median t½ was 11 hours. At 24 hours, a detectable level of the parent drug was observed. Assessments at the end of infusions 2, 4, 6, and 8 indicated that there was similar accumulation of the drug after subsequent infusions. The median concentration of the parent drug in the 5 patients after the first infusion was 1440 ng/mL or 5.4 mM (Table 4).
The concentration of dGuo increased throughout the treatment period, reaching a maximum of 20 μM at the end of the last infusion (Figure 3B). For T-PLL patients nos. 2, 3, and 5 the dGuo levels peaked at the end of the last infusion, whereas that was achieved prior to completion of treatment for T-ALL patients nos. 1 and 4 (Table 5). The dGuo Cmax values ranged from 701.5 ng/mL (2.6 μM) to 9155 ng/mL (34 μM) for the patients treated with 40 mg/m2 and increased with dose escalation of forodesine from 4245 ng/mL (16 μM) for course 2 of 40 mg/m2 to 9835 ng/mL (37 μM) for course 4 of 90 mg/m2 treatment (Table 5; patient no. 5). There was a rapid increase in plasma inosine concentrations after the initial dose of forodesine that was maintained throughout the treatment periods (Figure 3C) for all patients except patient no. 4 whose pattern of inosine response mirrored the dGuo response.
Urine pharmacology
The summary of the forodesine urine excretion data after the initial infusion of forodesine and up to the next 24 hours is provided in Table 6. The maximum rate of excretion ranged from 3.7 mg/h to 10.7 mg/h for the 6-hour mid-time point. Three hours was peak time for the median maximum excretion rate. The majority (54%-73%) of the forodesine was cleared by the kidneys (Table 6). The cumulative amount excreted did not plateau and the total forodesine excreted over 24 hours underestimated the total forodesine excreted via the kidneys. The excretion rate for dGuo was similar to that of forodesine, whereas the inosine excretion rate was significantly greater (Table 7). Unlike forodesine, the excretion rates for both dGuo and inosine remained the same throughout the 24-hour period.
Cellular pharmacology
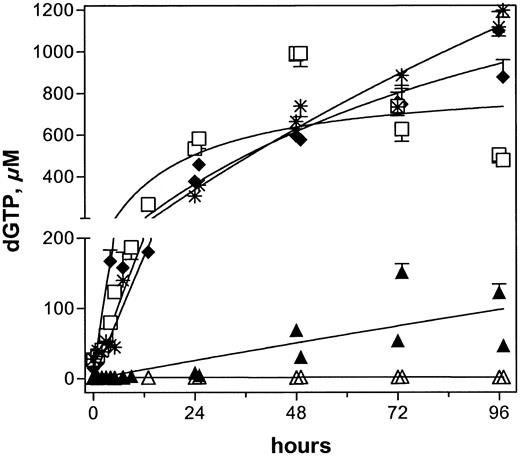
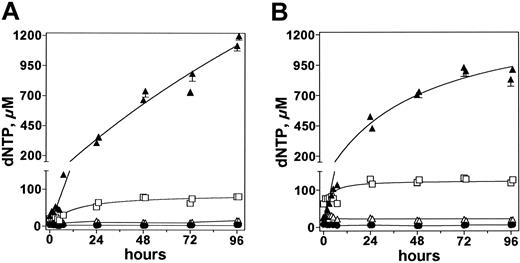
An increase in dGuo concentration in plasma suggested that there was inhibition of PNP in all patients. When analyzed for a concomitant increase in intracellular dGTP levels, except for lymphoblasts from patient no. 3, all had a gradual increase in dGTP (Figure 4). The endogenous pretreatment dGTP concentrations varied substantially, with a median of 15 μM (range, 1-28 μM; n = 5). Blasts from patients nos. 1 and 3 had only 1 μM and 1.5 μM endogenous dGTP levels, respectively.
After infusion of forodesine on day 1, the circulating leukemia cells had similar increases in dGTP concentration in patients nos. 2, 4, and 5. The pretreatment levels of 15, 27, and 28 μM increased by 5- to 10-fold within the first 8 hours and by 10- to 20-fold within 24 hours after start of therapy, and with the additional infusions, there was a gradual increase in dGTP levels; the highest concentrations were about 1 mM. Patient no. 1 started with 1 μM dGTP, which increased by 3- and 8-fold at 8 and 24 hours and continued during the 5 days of therapy. In contrast to the other 4 patients, leukemia cells from patient no. 3 showed no significant change in cellular dGTP levels.
Leukemia cells obtained from patients nos. 2 to 5 were analyzed for other deoxynucleotides. As with dGTP, there was a gradual increase in dATP levels for the first 24 hours after start of therapy and by 46 hours a plateau was achieved. For illustration purposes, data in one patient (no. 5) are presented (Figure 5). In contrast to dATP levels, deoxycytidine triphosphate (cCTP) and dTTP levels remained unchanged throughout the 5 days of therapy, suggesting the effect was only on purine deoxynucleotides (Figure 5). The endogenous concentrations of dATP were 31 and 66 μM in patients nos. 2 and 4, respectively. As in patient no. 5, dATP increased in these 2 patients without any effect on pyrimidine deoxynucleotides. For patient no. 3, there was no increase in intracellular dATP level (1 μM at the start of therapy), similar to the absence of increase in intracellular dGTP concentration.
To test whether the perturbation of purine deoxynucleotide pool was maintained from one course to the next, 16 peripheral blood samples from patients nos. 2 and 5 were collected during course 1 and course 2. In both cases, the pattern of dGTP and dATP increase was similar from one course to the next (Figures 5A-B), suggesting that circulating leukemia cells maintain the phosphorylation and elimination characteristics and result in similar levels of increase in dGTP and dATP with retreatment. Again, pyrimidine deoxynucleotide pools were unperturbed after forodesine administration in each course.
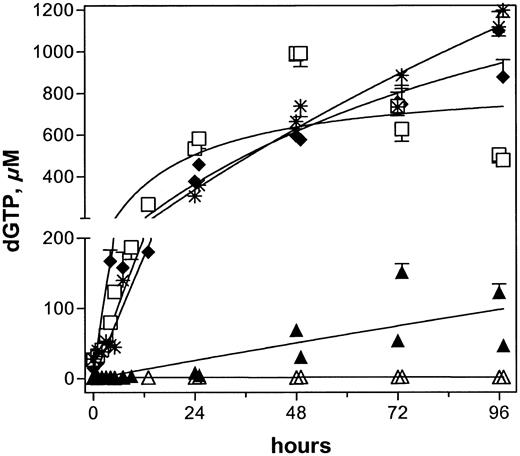
Accumulation of dGTP in all 5 patients after infusions of 40 mg/m2 forodesine. Peripheral blood samples were collected and leukemia cells were isolated to extract dNTPs. Intracellular levels of dGTP were quantitated and plotted for patient nos. 1 (▴),2(♦),3(▵),4(□), and 5 (*).
Accumulation of dGTP in all 5 patients after infusions of 40 mg/m2 forodesine. Peripheral blood samples were collected and leukemia cells were isolated to extract dNTPs. Intracellular levels of dGTP were quantitated and plotted for patient nos. 1 (▴),2(♦),3(▵),4(□), and 5 (*).
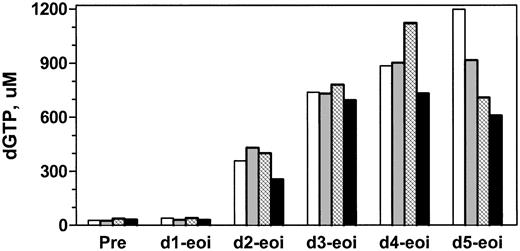
To investigate whether there was a dose-response relationship, pharmacology samples were collected from patient no. 5 after 40, 40, 60, and 90 mg/m2 forodesine. Sixteen samples per course were collected to compare the accumulation of dGTP, dATP, dCTP, and dTTP. The starting level of dGTP was similar in each course: 28, 25, 37, and 32 μM, respectively. Increasing the dose from 40 to 60 to 90 mg/m2 did not further augment intracellular dGTP accumulation in these prolymphocytes (Figure 6) even though there was a proportional increase in dGuo in plasma (Table 4).
Discussion
The purpose of this phase 1 trial was to determine the maximum tolerated dose of BCX-1777 (a transition-state analog inhibitor of PNP) and to relate pharmacodynamics of the drug to the administered dose. Because prior investigations of another PNP inhibitor (BCX-34 or peldesine21 ) had failed to achieve this objective, this study had both clinical and biologic end points (sufficient drug to maintain increased levels of plasma dGuo and intracellular dGTP in malignant T cells without excessive toxicity). Pharmacokinetic and pharmacodynamic parameters were used to determine the maximum biologically effective dose.
Pharmacokinetic investigations of the parent drug in plasma showed that concentrations between 4 and 8 μM BCX-1777 were achieved with 40 mg/m2 dosing (Table 4). This starting dose was thus likely sufficient to achieve an effective inhibitory level of forodesine in plasma, given that the concentration needed to inhibit the human PNP enzyme is in the picomolar range.34 Previous pharmacokinetic investigations in primates indicated that the terminal elimination rate was less than 3 hours,39 prompting selection of a twice daily dosing schema for initial study. However, the observed peak level of forodesine (median 5.4 μM); and the long t½ (median 10 hours) in this study suggested that once daily dosing of 40 mg/m2 might be sufficient to provide adequate and maintained drug exposure for inhibition of PNP.
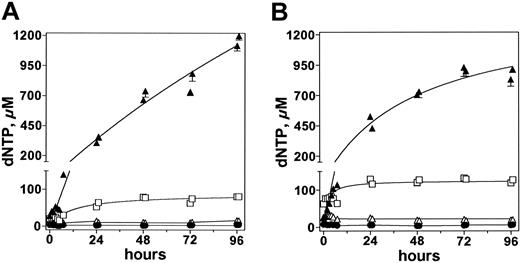
Comparison of deoxynucleotide level after forodesine therapy. Patient no. 5 received 2 courses of forodesine 40 mg/m2 day 1, then twice daily for 4 days. Samples were assayed after infusions 2, 4, 6, and 8. Intracellular concentrations of dCTP (•), dTTP (▵), dATP (□), and dGTP (▴) were quantitated by DNA polymerase assay and are plotted after the first (A) and second (B) courses.
Comparison of deoxynucleotide level after forodesine therapy. Patient no. 5 received 2 courses of forodesine 40 mg/m2 day 1, then twice daily for 4 days. Samples were assayed after infusions 2, 4, 6, and 8. Intracellular concentrations of dCTP (•), dTTP (▵), dATP (□), and dGTP (▴) were quantitated by DNA polymerase assay and are plotted after the first (A) and second (B) courses.
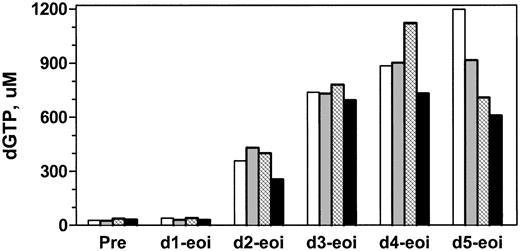
Accumulation of dGTP at the end of infusion with different courses of forodesine. Patient no. 5 was treated with 40, 40, 60, and 90 mg/m2/d during course 1 (white bars), course 2 (gray bars), course 3 (hatched bars), and course 4 (black bars) of therapy. Intracellular levels of dGTP were quantitated by DNA polymerase assay after first infusion on every day of therapy.
Accumulation of dGTP at the end of infusion with different courses of forodesine. Patient no. 5 was treated with 40, 40, 60, and 90 mg/m2/d during course 1 (white bars), course 2 (gray bars), course 3 (hatched bars), and course 4 (black bars) of therapy. Intracellular levels of dGTP were quantitated by DNA polymerase assay after first infusion on every day of therapy.
Forodesine is a potent inhibitor of PNP activity. In vitro studies showed Ki values ranging from 0.5 to 1.2 nM with purified murine, canine, and primate enzymes37 and 20 to 80 pM for purified human and bovine enzymes.34 Although the concentration of PNP in large body organs is not known, the high plasma concentrations of forodesine in the micromolar range and greater volume of distribution than total body water (Table 4) suggested that complete inhibition of PNP would occur. The kidneys appeared to be the major route of elimination of forodesine with clearance rates approximating calculated creatinine clearance. Hence, there was competition between binding to tissue PNP and renal elimination. Consistent with these observations, infusions of forodesine for 30 minutes resulted in a rapid increase of both plasma dGuo and inosine, and a single dose produced a sustainable 24-hour dGuo response (Figure 3 and Table 5).
With these high and maintained plasma levels of dGuo, it was expected that circulating T cells would also accumulate high levels of intracellular dGTP (Figure 4). In 3 patients (nos. 2, 4, 5), the leukemia cells accumulated concentrations of dGTP, which were 40- to 60-fold greater than pretreatment levels. Patient no. 1, on the other hand, had a 10-fold increase, whereas patient no. 3 had no augmentation in intracellular dGTP level. These data, albeit in a limited number of patients, strongly suggested heterogeneity regarding accumulation of dGTP, which is not directly related to plasma levels of dGuo. Patient no. 2 had a dGuo level of 4 μM with a 60-fold increase in dGTP, whereas patient no. 1 had a high level of dGuo (36 μM) with only a 10-fold increase in dGTP. No increase in dGTP level was observed in patient no. 3 despite a plasma dGuo concentration of 3 μM (Table 5). Similarly, intracellular accumulation of dGTP was not dependent on the excretion rate of dGuo; patients nos. 2, 4, and 5 with highest increases in dGTP had 5, 15, and 4 mg/h excretion of dGuo, respectively (Table 7). Hence, it appeared that the accumulation of dGTP was dependent on the inherent capability of circulating T cells to phosphorylate dGuo and maintain dGTP. This is in keeping with the observation that leukemia cells with higher levels of endogenous dGTP accumulated even greater levels of dGTP after forodesine was infused. For example, lymphoblasts or prolymphocytes from patients nos. 2, 4, and 5 started with intracellular dGTP at 15, 27, and 28 μM, respectively, and further increased dGTP to 40- to 60-fold. Thus, the initial cellular dGTP concentration may serve as an indicator of potential for dGTP augmentation on forodesine administration and, perhaps, predict clinical response.
Dose escalation of forodesine did not appear to increase intracellular dGTP further. By increasing the dose of forodesine from 40 to 60 or to 90 mg/m2, a corresponding increase in plasma dGuo from 15 to 20 to 37 μM, respectively, was observed; however, the extent of dGTP increase was not further augmented by these higher levels of dGuo (Figure 6). These data, although limited owing to evaluation in a single patient, suggested that dGTP accumulation was saturated at or below 15 μM dGuo in plasma.
In addition to increased intracellular dGTP levels, there was a less pronounced, yet significant, effect on dATP concentration (Figure 5). The mechanism for this increase is not known, but it was also observed in T-lineage cell lines when incubated with forodesine and exogenous dGuo in vitro.36 The human PNP lacks significant substrate potential for deoxyadenosine (dAdo). Therefore, inhibition of PNP activity on adenosine or dAdo, resulting in an increase in dAdo followed by intracellular phosphorylation to dATP, does not seem to be the route for this increase in dATP. It is more likely that dATP is generated intracellularly.
It is possible that this increase in dATP generated by forodesine therapy will contribute to the therapeutic applicability of forodesine for use in hematologic malignancies. Increased dATP levels are responsible for cell death when the dAdo deaminase inhibitor, deoxycoformycin, is used as a chemotherapeutic drug in T-lineage leukemias and B-cell chronic lymphocytic leukemia. Increase in dATP results in inhibition of ribonucleotide reductase (RNR),44 perturbation of dNTP pool (thereby inhibiting DNA synthesis), and cleavage of procaspase-9 to active caspase-9, which activates downstream pathways of apoptosis.45
It has been postulated that the mechanism by which PNP inhibition kills T cells is through an increase in dGTP followed by inhibition of reduction of cytidine diphosphate (CDP) and uridine diphosphate (UDP) to pyrimidine deoxynucleotides by RNR. In addition, as mentioned, dATP is a global inhibitor of RNR. Hence, the pyrimidine deoxynucleotide pool would be expected to decrease after forodesine treatment. However, data in the present trial clearly demonstrated that pyrimidine pools were not affected, suggesting (1) no effect on the activity of RNR, (2) activity of the enzyme may be too low in more indolent leukemias, or (3) pyrimidine deoxynucleotides were maintained by the salvage route.
In summary, although no objective clinical responses were observed, there was clear antileukemia activity with forodesine, which correlated with intracellular accumulation of dGTP (Figures 2 and 4). In the patient with progression of disease during therapy, no intracellular accumulation of dGTP was observed. In contrast, the other 4 patients had cytoreduction of disease, which correlated with marked increases in intracellular dGTP. In patients who received more than one course of forodesine, recurrent T-cell proliferation noted prior to subsequent courses was associated with prestudy levels of intracellular dGTP. Despite dose escalation of forodesine, no additional accumulation of dGTP was observed compared with the initial course, suggesting that duration of therapy may be of more clinical relevance than dose. Although cytoreduction data demonstrated a relationship between intracellular accumulation of dGTP/dATP and death of T-lineage cells; proliferation of the remaining population of T cells after therapy suggested that this effect was not maintained with the current dosing schema of forodesine. Thus, ongoing phase 2 clinical trials are exploring the efficacy of protracted single daily dosing of intravenous forodesine,46-48 while phase 1 studies with an oral formulation have just been initiated.
Prepublished online as Blood First Edition Paper, August 30, 2005; DOI 10.1182/blood-2005-03-1309.
Supported in part by grants CA57629 and CA81534 from the National Cancer Institute, Department of Health and Human Services.
J.M.K., L.H., and S.B. are employed by BioCryst Pharmaceuticals, Inc, whose product was studied in the present work.
V.G. participated in the design of the protocol, selection of pharmacokinetic and pharmacodynamic end points, and analyses of cellular pharmacodynamics, and wrote the manuscript. J.M.K. was involved with plasma and urine pharmacokinetic assay development, study design, and data analysis, and performed all pharmacokinetic analysis on plasma and urine using WinNonLin for forodesine, dGuo, and inosine, and wrote the plasma and urine pharmacology for the Food and Drug Administration report. W.P. participated in cellular pharmacokinetic analyses and had a major role in scientific writing of the data in the manuscript. M.A. performed all deoxynucleotide assays along with standard curves for all samples collected. L.H. was responsible for the bioanalytical assays and quality control audit of the bioanalytical data and also played a major role in development of the BioCryst plasma pharmacology assays used in the study. M.D. was responsible for obtaining and processing peripheral blood samples from all the patients. S.B. participated in analysis of plasma pharmacology and interpretation. J.D. is the research nurse who coordinated the clinical and research studies. W.G.W., S.F., and H.K. participated in the clinical trial. D.T. is the principal investigator of the clinical protocol, participated in study design of the protocol, wrote and ran the protocol, analyzed clinical outcome and toxicity, and wrote the clinical portion of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Brenita Tyler, who assisted Min Du in blood collection and processing of samples.