Abstract
Apoptosis is an intrinsic cell death program that plays critical roles in tissue homeostasis, especially in organs where high rates of daily cell production are offset by rapid cell turnover. The hematopoietic system provides numerous examples attesting to the importance of cell death mechanisms for achieving homeostatic control. Much has been learned about the mechanisms of apoptosis of lymphoid and hematopoietic cells since the seminal observation in 1980 that glucocorticoids induce DNA fragmentation and apoptosis of thymocytes and the demonstration in 1990 that depriving colony-stimulating factors from factor-dependent hematopoietic cells causes programmed cell death. From an understanding of the core components of the apoptosis machinery at the molecular and structural levels, many potential new therapies for leukemia and lymphoma are emerging. In this review, we introduce some of the drug discovery targets thus far identified within the core apoptotic machinery and describe some of the progress to date toward translating our growing knowledge about these targets into new therapies for cancer and leukemia.
Apoptosis inducers and effectors
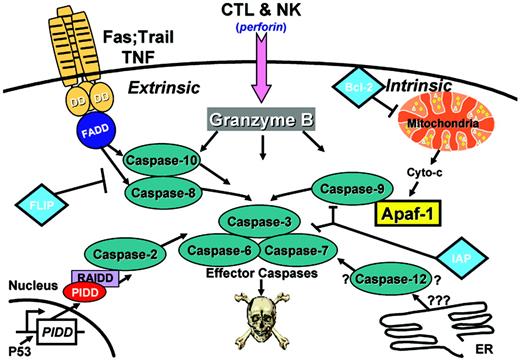
Apoptosis is caused by the activation of intracellular proteases, known as caspases.1 The human genome encodes 11 or 12 caspases, depending on certain hereditary polymorphisms.2 Numerous cellular targets of caspases have been identified, which in aggregate produce the characteristic morphology we call “apoptosis” when cleaved. Several pathways for triggering caspase activation exist, though 2 have been elucidated in great detail and have been the center of much attention in recent years. These 2 pathways for apoptosis are commonly referred to as the intrinsic and extrinsic pathways (Figure 1).3
The intrinsic pathway centers on mitochondria as initiators of cell death. Multiple signals converge on mitochondria, including DNA damage, microtubule disruption, and growth-factor deprivation, causing these organelles to release cytochrome c (cyt c) and other apoptogenic proteins into cytosol. In the cytosol, cyt c binds the caspase-activating protein, apoptotic protease-activating factor 1 (Apaf1), triggering its oligomerization into a hepatmeric complex that binds procaspase-9, forming a multiprotein structure known as the “apoptosome.”4 Physical binding of Apaf1 to procaspase-9 is mediated by their caspase recruitment domains (CARDs), through homotypic CARD-CARD binding. Activation of apoptosome-associated cell death protease caspase-9 then initiates a proteolytic cascade, where activated caspase-9 cleaves and activates downstream effector proteases, such as procaspase-3.
In contrast, the extrinsic apoptotic pathway relies on tumor necrosis factor (TNF) family death receptors for triggering apoptosis. A subgroup of the TNF family receptors contains a cytosolic death domain, which enables their intracellular interaction with downstream adapter proteins, linking these receptors to specific caspases. On ligand binding, TNF family receptors containing cytosolic death domains cluster in membranes, recruiting caspase-binding adaptor proteins, including the bipartite adapter Fasassociating protein with death domain (FADD) that contains both a death domain (DD) and a death effector domain (DED).5 The DED of FADD binds DED-containing procaspases (eg caspases-8 and -10), forming a “death-inducing signaling complex” (DISC) and resulting in caspase activation by an “induced proximity” mechanism.1
A delicate balance between proapoptotic and antiapoptotic regulators of apoptosis pathways is at play on a continual basis, ensuring the survival of long-lived cells and the proper turnover of short-lived cells in a variety of tissues, including the bone marrow, thymus, and peripheral lymphoid tissues. However, imbalances in this delicate dance of proapoptotic and antiapoptotic proteins occur in disease scenarios, including cancer, where the scales tip in favor of antiapoptotic proteins, endowing cells with a selective survival advantage that promotes neoplasia and malignancy.
Drug discovery strategies directed at core components of the apoptosis machinery
The apoptosis-blocking proteins that thwart signaling through specific apoptosis pathways provide targets for possible drug discovery, as do agents that stimulate proapoptotic proteins within these cell death pathways. Theoretically, all drug targets can be addressed in multiple ways, modulating the activity of the target at the levels of the DNA (gene expression), mRNA, or protein. A summary of some of the more promising strategies for altering the activity of apoptosis genes and proteins for cancer therapy is provided, according to the apoptosis pathways they modulate.
Intrinsic pathway
B-cell lymphoma-2 (Bcl-2) family proteins are the most prominent of the intrinsic pathway targets for cancer drug discovery. Although the human genome contains 25 members of this gene family, only 6 are antiapoptotic and thus represent logical targets for therapy: Bcl-2, Bcl-XL, Mcl-1, Bcl-W, Bfl-1, and Bcl-B. Overexpression of several of these antiapoptotic Bcl-2 family proteins has been documented in various hematopoietic malignancies, though data are most extensive for the founding member of the family Bcl-2 (for reviews, see Kitada et al6 and Kitada and Reed7 ). For example, elevated Bcl-2 protein as a result of t(14;18) translocations involving the BCL2 gene occurs in 80% to 90% of low-grade follicular non-Hodgkin lymphomas (NHLs).6 Approximately one third of diffuse large cell lymphomas (DLCLs) have pathologically elevated Bcl-2 (often in association with t(14;18) translocations or gene amplification), correlating with shorter patient survival when treated with combination chemotherapy, unless supplemented with anti-CD20 antibody (rituximab).8 Most chronic lymphocytic leukemias (CLLs) contain elevated Bcl-2, associated with hypomethylation of the BCL2 gene.9 In addition, elevated Mcl-1 protein expression occurs in nearly half of CLL B cells, correlating with failure to achieve complete remission after treatment with chemotherapy (for a review, see Kitada and Reed7 ). In some cases, increased Mcl-1 protein expression is associated with mutations in the promoter of the MCL1 gene.10 In contrast to genetic alterations that activate antiapoptotic genes such as BCL2 and MCL1, leukemias and lymphomas with microsatellite instability commonly develop inactivating mutations in proapoptotic gene BAX.11
Caspase-activation pathways. Additional pathways also exist, including (1) an apoptosis pathway induced by CTL and NK cells, in which serine protease Granzyme B is introduced into target cells; (2) an endoplasmic reticulum (ER) stress pathway, which involves caspase 12; and (3) a p53-induced pathway mediated by p53-inducible death domain (PIDD), which binds adaptor protein ICH-1/cell death protein-3 (CED-3) homologous protein with a death domain (RAIDD), an activator of caspase 2.
Caspase-activation pathways. Additional pathways also exist, including (1) an apoptosis pathway induced by CTL and NK cells, in which serine protease Granzyme B is introduced into target cells; (2) an endoplasmic reticulum (ER) stress pathway, which involves caspase 12; and (3) a p53-induced pathway mediated by p53-inducible death domain (PIDD), which binds adaptor protein ICH-1/cell death protein-3 (CED-3) homologous protein with a death domain (RAIDD), an activator of caspase 2.
Attempts to overcome the cytoprotective effects of Bcl-2 or related antiapoptotic proteins in cancer and leukemia include 3 strategies: (1) shutting off gene transcription; (2) inducing mRNA degradation with antisense oligonucleotides; and (3) directly attacking the proteins with small-molecule drugs. A fourth strategy attempts to invoke endogenous antagonists of antiapoptotic Bcl-2 family proteins.
Drugs regulating BCL2 gene expression. Some synthetic retinoids reduce levels of BCL-2 or BCL-XL mRNA in leukemia cells, suggesting a potential explanation for the proapoptotic effect of these agents already approved for clinical use.12 Compounds that inhibit histone deactylases (HDACs), chromatin-modifying enzymes, also reduce the expression of BCL2, BCLX, or MCL1 at a transcriptional level in some leukemia cell lines and primary cultured myeloma and leukemia cells.13-15 Clinical trials of HDAC inhibitors are progressing, with hints of activity documented for lymphoma and some solid tumors.16 Peroxisome-proliferator-activated receptor γ (PPARγ)–modulating drugs also reduce expression of BCL2 or other antiapoptotic BCL2 family genes, at least in preclinical studies of human leukemia cell lines.17
Drugs attacking BCL-2 mRNA. Antisense oligodeoxynucleotides (ODNs) targeting the BCL-2 mRNA are undergoing clinical evaluation, with phase-3 trials underway or recently completed for relapsed or refractory CLL, acute myeloid leukemia (AML), and myeloma.18 The molecules in clinical trials are composed of nuclease-resistant phosphorothioates, which hybridize via Watson-Crick base pairing to the first 18 nucleotides within the open reading frame of BCL-2 mRNAs and induce RNaseH-mediated degradation.19 This DNA-based drug (G3139; oblimersen sodium) has been delivered in combination with cytotoxic chemotherapy, in an attempt to exploit the chemosensitizing properties of Bcl-2–based therapies. The first reported phase 1 trial of BCL2 antisense therapy showed promising activity against chemorefractory NHL.20 Uncontrolled phase 2 data also suggest that this BCL2 antisense molecule (G3139; oblimersen sodium) has promising bioactivity against B-cell malignancies and adult AML. A recently completed trial of oblimersen sodium for advanced melanoma (in combination with dacarbazine) demonstrated improved response rates and time to progression, but failed to meet its primary end point of prolonging survival. However, G3139 showed no benefit in myeloma.
Demonstration of a correlation between clinical responses and antisense-mediated knock-down of BCL-2 mRNA or protein levels in patients undergoing treatment with G3139 has been documented in some but not all studies, raising questions about the mechanism of this novel targeted therapy.21,22 However, it has been argued that cells with successful antisense-mediated reductions in BCL-2 mRNA are difficult to recover from patients, because of their rapid in vivo clearance induced by apoptosis. The Bcl-2 protein also exhibits a slow degradation rate (∼12-36 hours), suggesting that prolong suppression of mRNA accumulation is necessary to produce a decline in Bcl-2 protein.23 A proinflammatory effect of G3139, seen particularly at higher doses, suggests an additional nonantisense bioactivity, which may or may not be relevant to tumor responses. In this regard, cytosine-phosphodiester-guanine (CpG) motifs in synthetic DNA molecules trigger inflammatory responses by engaging Toll-like receptor-9 (TLR9), tapping into a mechanism of innate immunity intended to sense the presence of unmethylated bacterial DNA.24 Interestingly, mismatched ODNs containing CpG motifs reduce BCL2 expression and promote apoptosis of prostate cancer cell lines through an antisense-independent mechanism.25 Nevertheless, tumor xenograft studies of G3139 in immunocompromised mice provide strong support for an antisense-based mechanism, whereby sequence-specific reductions in BCL2 expression either directly trigger tumor apoptosis or sensitize malignant cells to traditional cytotoxic anticancer drugs.26-28
Aside from G3139, no nucleic acid–based inhibitors of Bcl-2 or its antiapoptotic relatives are presently in clinical testing, but preclinical studies have been reported for Bcl-XL.29 Dual Bcl-2/Bcl-XL-suppressing antisense oligonucleotides may overcome some of the potential problems of redundancy caused by simultaneous overexpression of multiple antiapoptotic members of the Bcl-2 family in malignant cells.30
Drugs attacking Bcl-2 proteins. Small-molecule inhibitors directly binding Bcl-2 or related antiapoptotic proteins have also entered clinical trials for cancer. The most advanced is a natural product, gossypol, whose ability to bind and inhibit Bcl-2 was unknown when initial clinical testing began.31 Gossypol is a compound found in cottonseeds originally used as an herbal medicine in China.32 Gossypol binds a hydrophobic pocket found on the surface of antiapoptotic Bcl-2 family proteins (Figure 2). This binding pocket represents a regulatory site, where endogenous antagonists dock onto Bcl-2 and related antiapoptotic proteins, negating their cytoprotective activity.33 The endogenous antagonists bind via a conserved 16 amino acid motif called a Bcl-2 homology-3 (BH3) domain. Proof of concept experiments using BH3 peptides have suggested that compounds docking at this regulatory site on Bcl-2 and Bcl-XL effectively promote apoptosis of lymphoma and leukemia cells in vivo in mice.34,35
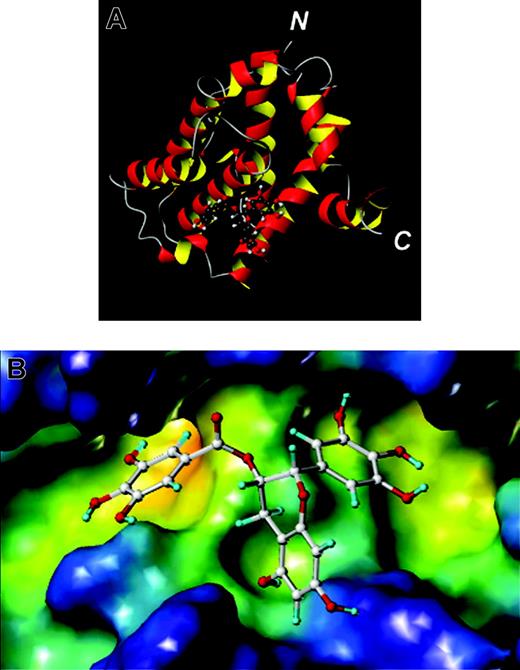
Bcl-XL with a chemical inhibitor obtained via docking studies. (A) Ribbon model of the NMR structure of Bcl-XL with the docked inhibitor epigallocatechin gallate (EGCG; ball-and-stick). (B) The BH3-binding pocket of Bcl-XL is shown and color coded according to cavity depth: blue, shallow; yellow, deep. The docked conformation of the compound EGCG is also shown (ball-and-stick). The figures were generated with MOLMOL (A) and MOLCAD (B) as implemented in Sybyl (Tripos, Saint Louis, MO). N indicates N-terminus; C, C-terminus.
Bcl-XL with a chemical inhibitor obtained via docking studies. (A) Ribbon model of the NMR structure of Bcl-XL with the docked inhibitor epigallocatechin gallate (EGCG; ball-and-stick). (B) The BH3-binding pocket of Bcl-XL is shown and color coded according to cavity depth: blue, shallow; yellow, deep. The docked conformation of the compound EGCG is also shown (ball-and-stick). The figures were generated with MOLMOL (A) and MOLCAD (B) as implemented in Sybyl (Tripos, Saint Louis, MO). N indicates N-terminus; C, C-terminus.
Gossypol interacts with the BH3-binding pockets of 4 antiapoptotic Bcl-2 family proteins tested to date, Bcl-2, Bcl-XL, Bcl-B, and Bfl-1, displacing BH3 peptides with an inhibitory concentration of 50% (IC50) of about 0.5 μM. A gossypol enantiomer may have superior activity compared with the naturally occurring racemer and is undergoing late steps of preclinical development in preparation for clinical trials.36 However, gossypol is a highly reactive compound, containing 2 aldehydes, probably explaining some of the toxicities originally seen in phase 1 trials of this natural product, in addition to producing unfavorable pharmacologic properties. For this reason, attempts to produce semisynthetic analogs that retain activity against Bcl-2 have begun. The most advanced is the compound apogossypol, in which the 2 aldehydes were eliminated.37
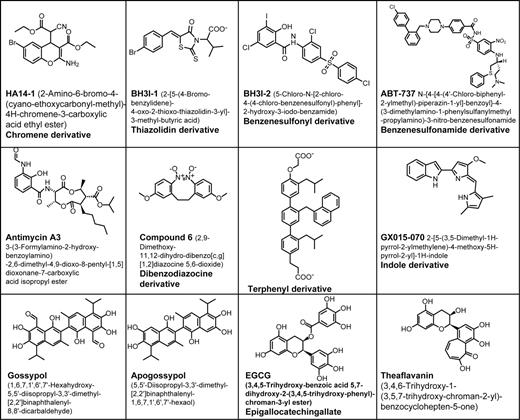
Bcl-2 antagonists. The structures of nonpeptidyl antagonists of Bcl-2 and Bcl-XL are shown.
Bcl-2 antagonists. The structures of nonpeptidyl antagonists of Bcl-2 and Bcl-XL are shown.
Several other chemical inhibitors of Bcl-2, Bcl-XL, and Mcl-1 have been reported, most of which are currently in preclinical evaluation, including: (1) HA14-1, a chromene derivative identified by a computational modeling approach38 ; (2) BH3I-1 and -2, a thiazolidin derivative and benzene sulfonyl derivative, respectfully, identified by screening using a BH3 peptide displacement assay39 ; (3) antimycin analogs, identified by computational modeling40 ; (4) certain theaflavins and epigallechatechins (EGCGs), natural products abundant in black and green tea, respectively, discovered by nuclear magnetic resonance (NMR)–based methods41 ; (5) ABT737, a synthetic small-molecule inhibitor produced by NMR-guided, structure-based drug design (Abbott Laboratories, North Chicago, IL),118 GX15-070 (Gemin X, Montreal, Canada), a synthetic broad-spectrum inhibitor of Bcl-2–family proteins, and others32 (Figure 3). Side-by-side comparisons of these chemical inhibitors of antiapoptotic Bcl-2 proteins have not been reported, but their approximate rank-order potency with respect to affinity for the BH3 pocket of Bcl-2 or Bcl-XL appears to be A-779024 > EGCG > theafavins > gossypol > apogossypol > HA14-1 and antimycin. Also, it is unclear which of the 6 antiapoptotic Bcl-2 family proteins are targeted by these compounds, though Bcl-XL and Bcl-2 define a minimal subset. Several issues will be encountered as these chemical antagonists of Bcl-2 move forward with their preclinical and clinical development, including compound stability and formulation, pharmacokinetics and metabolism, toxicity, and off-target mechanisms.
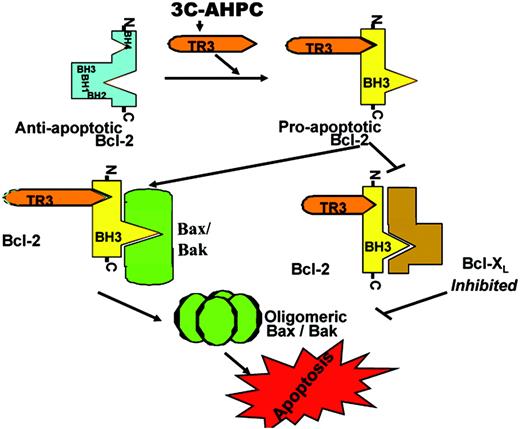
Activating endogenous antagonists of Bcl-2. A fourth route to addressing the problem of pathologic overexpression of Bcl-2 in malignant cells seeks to induce expression or activation of endogenous proteins that bind and negate the cytoprotective action of Bcl-2. For example, an orphan member of the retinoid/steroid family of nuclear receptors, TR3 (Nur77) translocates from nucleus to cytosol in response to certain cell death stimuli.42 In the cytosol, TR3 binds a regulatory domain within the Bcl-2 protein, thus accounting for accumulation of TR3 on mitochondria. TR3 induces a profound conformational change of Bcl-2, causing BH3 domain exposure and converting Bcl-2 from a protector to a killer.43 The BH3 domain of Bcl-2 binds proapoptotic Bcl-2 family members Bax and Bak, as well as antiapoptotic Bcl-2 family proteins, probably activating the proapoptotic while inactivating the antiapoptotic (Figure 4).
Recently, a class of compounds representing analogs of the retinoid 6-[3′-(1-adamantyl)-4′-hydroxyphenyl]-2-naphthalenecarboxylic acid (AHPN) has been identified that induces TR3 expression and translocation into the cytosol. The prototype compound, 3Cl-AHPC, lacks activity against retinoid receptors, though having evolved from attempts to synthesize highly selective retinoid ligands.44 3Cl-AHPC has demonstrated activity against cultured leukemia cells and in preclinical animal models of AML,45,46 retaining activity even against retinoid refractory leukemia cell lines. 3Cl-AHPC appears to induce phosphorylation of TR3, possibly via a Jun N-terminal kinase (JNK) pathway, correlating with its exodus from the nucleus.47 This compound may therefore provide a mechanism to invoke the TR3 pathway, converting Bcl-2 from a protector to a killer, and thus making Bcl-2 a liability rather than an advantage for malignant cells.
Opposing this TR3-mediated pathway for apoptosis is Akt/protein kinase B (PKB), which appears to nullify proapoptotic actions of TR3 by phosphorylation at serine 530.48,49 Thus, TR3 joins a long list of apoptosis-relevant substrates of Akt, which includes Bcl-2 antagonist BID, human caspase-9, proapoptotic kinase Ask1, p53 antagonist murine double-minute homologue (Mdm2), and others.50 Akt is activated downstream of several oncogenes of relevance to leukemia and lymphoma, including BCR/ABL of CML, Tcl 1 of T-cell CLL, NPM-ACK of anaplastic large cell lymphomas, FOP-FGFR1 of stem cell myeloproliferative disorder, LMP1 and LMP2A of Epstein-Barr virus, and the K1 protein of Karposi sarcoma–associated herpesvirus (KSHV) implicated in some lymphomas. It remains to be determined what effect the Tcl-1 oncoprotein has on nuclear export of TR3, but it may be of interest that this protein overexpressed in CLLs promotes nuclear targeting of Akt and regulates the transcriptional activity of TR3.48 Small wonder then that Akt has emerged as a prominent target for cancer drug discovery.51 Relatively selective but fairly weak inhibitors of Akt such as 1L-hydroxymethyl-chiro-inositol-2-(R)-2-methyl-3-O-octadecyl/carbonate) have been described, which has preclinical activity against multiple myeloma cells and sensitizes established myeloid leukemia cell lines to chemotherapeutic drugs, retinoids, radiation, and TNF-related apoptosis-inducing ligand (TRAIL).51 Interestingly, 3Cl-AHPC may also reduce Akt activity in tumor cells,52 further promoting the TR3 pathway for cell death. Also, heat shock protein 90 (HSP90) inhibitors such as 17-allylamino-17-demethoxygeldanamycin (17-AAG) destabilize and cause reductions in Akt levels.53
Model for TR3-mediated conversion from protector to killer. Bcl-2 can assume 2 conformational states, including an antiapoptotic conformation in which its BH3 domain is buried and a proapoptotic state in which its BH3 domain is exposed. The proapoptotic form can either activate proapoptotic proteins Bax and Bak or inactivate antiapoptotic proteins such as Bcl-XL.
Model for TR3-mediated conversion from protector to killer. Bcl-2 can assume 2 conformational states, including an antiapoptotic conformation in which its BH3 domain is buried and a proapoptotic state in which its BH3 domain is exposed. The proapoptotic form can either activate proapoptotic proteins Bax and Bak or inactivate antiapoptotic proteins such as Bcl-XL.
The concept of invoking endogenous antagonists of Bcl-2 is not novel. Antiapoptotic Bcl-2 family proteins are normally held in check by endogenous proteins that contain a BH3 domain, especially the so-called BH3-only proteins (BOPs). Several BOPs are linked to pathways that explain how currently available cytotoxic drugs trigger apoptosis, including: (1) the genes encoding Puma, Noxa, and Bid, whose expression is directly induced by p53 in response to DNA damage; (2) Bim proteins, which are normally sequestered on microtubules; and (3) Bax, which is held in an inactive conformation by the regulatory subunit (Ku70) of the DNA-dependent kinase, becoming released during DNA damage responses.54,55 Unfortunately, defects in pathways involving BOPs and pathologic overexpression of Bcl-2 all too frequently create roadblocks to apoptosis that must be overcome by alternative means. The observation that commonly used cytotoxic anticancer drugs are capable of modulating endogenous pathways impinging on Bcl-2 underscores why Bcl-2–targeted therapies are often synergistic with conventional cytotoxic agents. Knowledge of the molecular details of mechanisms by which traditional cytotoxic anticancer drugs affect Bcl-2 family proteins thus may help in selecting optimal combination therapies for clinical trials of Bcl-2 antagonists. Underscoring the importance of this knowledge base are recent studies suggesting that alkylating agents induce cell death via necrosis rather than apoptosis, independent of Bcl-2/Bax family proteins.56 It is therefore unfortunate that many early clinical trials of Bcl-2 antagonists (eg, G3139) have involved combination with alkylating agents, which may not provide a synergistic combination. New targets for drug discovery are also emerging from studies of these endogenous pathways, such as compounds that enhance wild-type p53 by blocking interactions with Mdm2 (Table 1).57
Extrinsic pathway
The extrinsic pathway is activated by TNF family ligands that engage TNF family death receptors, resulting in activation of caspases and apoptosis. Interest has emerged in therapeutics that kill cancer cells via the extrinsic pathway, particularly because chemorefractory cells often have defects in their intrinsic pathway, given the predominant reliance of cytotoxic drugs and x-irradiation on the mitochondrial cell death pathway.58 In this regard, the TNF cytokine family consists of 18 members in humans, with 29 counterreceptors.2,59 Some of these TNF family receptors transduce signals predominantly for cell survival, particularly those that bind intracellular tumor receptor–associated factor (TRAF) family adapter proteins that link to downstream protein kinases. Blocking these receptors represents a potentially attractive strategy for a variety of lymphoid malignancies, but will not be discussed here. Other members of the TNF family directly trigger apoptosis, particularly those that contain DDs within their cytosolic tails (n = 8 in humans). Of these receptors and their corresponding ligands, 4 receptors (3 ligands) have been studied in detail for use as cancer therapeutics, namely, TNF (TNFR1), FasL (Fas), and TRAIL (DR4; DR5). Strategies for exploiting apoptosis-inducing TNF family ligands and receptors for cancer therapy focus mostly on: (1) recombinant ligands (biologicals), expressing only the extracellular domain of these type 2 integral membrane proteins, or (2) agonistic monoclonal antibodies that bind the receptors and trigger apoptosis. In addition, however, emerging knowledge about intracellular modulators of apoptosis pathways used by TNF family death receptors has suggested opportunities for engaging the extrinsic pathway at other levels, improving sensitivity of malignant cells to biologicals that activate the extrinsic pathway.
TNF family ligands and receptor-targeting antibodies. More than 3 decades ago, TNF was reported to selectively kill tumor but not normal cells, raising hopes that this cytokine might be exploited for cancer treatment.60 Unfortunately, the proinflammatory actions of TNF precluded its systemic administration and squelched efforts to apply it clinically. Then, we knew little about how TNF transduces signals into cells making it difficult to propose alternative strategies. Today, we have an in-depth knowledge of the pathways that TNF and related cytokines activate, and from this knowledge has come several potential targets for drug discovery. We also understand far better how TNF family cytokines regulate pathways that control apoptosis, and we can begin to envision ways of exploiting that knowledge for sensitizing tumors to these cytokines that immune cells use for battling cancer.
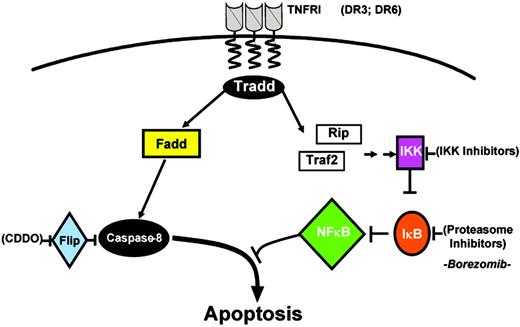
Binding of TNF to its cellular receptor, TNFR1, triggers 2 parallel signaling pathways (Figure 5). These pathways bifurcate at the adapter protein TNF receptor–associated death domain (TRADD). One of these pathways results in activation of caspase family proteases, triggering apoptosis. The other parallel pathway triggers activation of nuclear factor κB (NF-κB) family transcription factors. NF-κB influences the expression of many target genes involved in host defenses and immune regulation, but also genes that suppress apoptosis. As a result, this NF-κB pathway nullifies the caspase pathway,61 in addition to producing untoward inflammatory side effects.
Dual pathway activation by TNF. TNFR1 (as well as DR3 and DR6) recruits tissue necrosis factor–receptor associated death domain (TRADD), which mediates interactions with 2 opposing pathways. TRADD binds FADD, linking to a caspase activation pathway, and binds Rip (74-kDa protein) and tral2, which link to an NF-κB induction pathway. Targets for drug discovery are indicated. See “Extrinsic pathway” for details.
Dual pathway activation by TNF. TNFR1 (as well as DR3 and DR6) recruits tissue necrosis factor–receptor associated death domain (TRADD), which mediates interactions with 2 opposing pathways. TRADD binds FADD, linking to a caspase activation pathway, and binds Rip (74-kDa protein) and tral2, which link to an NF-κB induction pathway. Targets for drug discovery are indicated. See “Extrinsic pathway” for details.
The TNF family cytokines Fas ligand (FasL) and TRAIL (Apo2 ligand) trigger activation of the caspase pathway without concomitant induction of NF-κB. These death ligands are expressed on cytolytic T cells (CTLs), natural killer (NK) cells, and other types of immune cells and are used as weapons for eradication of virus-infected and transformed cells.59,62,63 Various studies using mice with ablation of genes encoding these death ligands or their receptors, as well as neutralizing antibodies and Fc-fusion proteins, have provided evidence that FasL and TRAIL play important roles in tumor suppression by cellular immune mechanisms. For example, FasL is important for CTL-mediated killing of some tumor targets, and TRAIL is critical for NK cell–mediated tumor suppression.64,65
The absence of proinflammatory effects of FasL and TRAIL has raised hopes that they might be successfully applied for cancer therapy, where TNF failed due to toxicity. Whereas agonistic antibodies that trigger the Fas (CD95) are unfortunately highly toxic to liver,66 TRAIL and agonistic antibodies that bind TRAIL receptors appear to be well tolerated in vivo. Indeed, phase 1 clinical trials have recently been completed using an agonistic monoclonal antibody ETR1 (Human Genome Sciences, Rockville, MD), directed against TRAIL receptor-1 (TRAIL-R1; DR4), revealing little toxicity.67 In mouse xenograft models using selected tumor human cell lines, TRAIL and agonistic antibodies directed against TRAIL receptors exhibit potent antitumor activity and often synergize with chemotherapy,68 raising hopes of using these biologic agents as a novel approach to cancer treatment and thereby mimicking some of the effector mechanisms used by the immune system in its defense against transformed cells.
Intracellular modifiers of extrinsic pathway—drug targets. Among the prominent modulators of sensitivity to TNF family death receptors are NF-κB family transcription factors and FLICE (Fas-associating protein with death-domain–like interleukin-1β–converting enzyme)–inhibitory protein FLIP, a DED-containing protein that binds the apical proteases in the extrinsic pathway, procaspases-8 and -10.69 Both of these drug targets have garnered the attention of the researchers seeking ways of increasing sensitivity of malignant cells to TNF-family cytokines.
NF-κB. Abnormal elevations in NF-κB activity occur in many tumors, including many lymphoid malignancies. In fact, the first NF-κB family member identified, c-REL, is the cellular counterpart of a viral transforming gene, v-rel, discovered in the avian leukosis virus. The importance of NF-κB for suppression of TNF-induced apoptosis is well established,70 and several antiapoptotic genes are among the direct transcriptional targets of Rel family proteins, including the genes encoding c-FLIP, inhibitor of apoptosis protein-2 (cIAP2), Bcl-XL, and Bfl-1. Consequently, interfering with signal transduction events that generate active NF-κB has emerged as an attractive strategy for sensitizing cancer cells to TNF and related cytokines.
Studies of signaling events responsible for NF-κB activation by TNF have revealed several candidate targets potentially amenable to small-molecule drug discovery. For example, though alternative pathways exist, an evolutionarily conserved pathway for NF-κB activation has been elucidated involving various upstream kinases funneling signals into a pivotal kinase complex that consists of 2 homologous serine kinases, inhibitor of NF-κB kinase (IKKα) and IKKβ, and a scaffold protein, IKKγ (NF-κB essential modulator [NEMO]).70 This IKK complex is responsible for phosphorylating endogenous inhibitors of NF-κB, the IκB proteins, thus targeting them for ubiquitination and subsequent proteasome-dependent degradation. Degradation of IκB releases NF-κB, allowing translocation into the nucleus.71 Thus, the protein kinases involved in this pathway have emerged as promising drug targets, as well as the proteasome71,72 (Figure 5).
Chemical inhibitors of IKKs have been described, which display proapoptotic activity against cultured malignant cells, including BMS-345541,73 Bay 11-7082,74 SC-514,75 sulfasalazine,76 silibinin, a dietary flavonoid,77 pyrolidine dithiocarbamate,78 natural product wedelolactone, semisynthetic derivatives of B-carboline natural products,79 and others. The specificity of these inhibitors is questionable, but toxicology studies will determine whether they might nevertheless be clinically useful. Proteasome inhibitors include bortezomib, recently approved for treatment of refractory myeloma and in clinical testing for several types of cancer and leukemia80,81 (Millennium Pharmaceuticals, Cambridge, MA). Bortezomib (PS-341) inhibits the protease activity of the proteasome.81,82 Pharmacologic inhibition of proteasome activity suppresses IκB degradation, but also has effects on stability of many cellular proteins, and thus the antitumor activity of proteasome inhibitors may only partly be related to NF-κB activity.
FLIP. Many leukemias and lymphomas exhibit intrinsic resistance to TNF, TRAIL, and FasL, despite expressing the necessary cell-surface receptors.83 Multiple antagonists of the extrinsic pathway have been identified, including several DED-containing proteins that compete for binding to the adapter proteins or procaspases that participate in TNF family death receptor signaling.84 Among these, FLIP has received the most attention for its role in producing Fas- and TRAIL-resistant states in tumor cells,85,86 including Reed-Steinberg cells of Hodgkin disease, EBV+ Burkitt lymphomas, KSHV (HHV8)–associated B-cell lymphomas, and other types of B-cell NHLs.
The c-FLIP protein is highly similar in sequence to procaspase-8 and -10, containing tandem DEDs, followed by a pseudo-caspase domain lacking enzymatic activity. Two isoforms of c-FLIP are produced from a single gene, including the long form as described and a shorter isoform that consists only of DED domains. The shorter FLIP thus resembles analogous proteins encoded in genomes of animal viruses. FLIP-S is exclusively antiapoptotic, whereas FLIP-L can be either proapoptotic or antiapoptotic, depending on its levels of expression relative to procaspase-8 and -10.87 FLIP proteins form complexes with procaspase-8 and -10, preventing their effective activation, as well as competing for binding to adapter proteins required for caspase recruitment to death receptor complexes.69,86 Overexpression of FLIP occurs commonly in a variety of B-cell malignancies, and some AMLs and CLLs. FLIP promotes tumor progression in immunocompetent mouse models of lymphoma, implying in vivo selection for high FLIP expression as a result of confrontations of neoplastic cells with the immune system.
Although no compounds have been described that directly bind FLIPs, experimental agents have been reported that reduce FLIP protein expression. Among these are synthetic triterpenoids 2-cyano-3,12-dioxoolean-1,9-bien-28-oic acid (CDDO) and CDDO-methyl ester (CDDO-me), which induce ubiquitination and proteasome-dependent destruction of FLIPs in cultured cancer cells, sensitizing solid tumor lines to apoptosis induction by TNF, Fas, and TRAIL.88 Moreover, CDDO analogs exhibit single-agent activity in terms of inducing apoptosis in vitro of cultured leukemia cells, including chemorefractory CLLs, AMLs, and myelomas.89,90 The mechanism of CDDO and related compounds is under investigation, but most studies report caspase-8 activation as a proximal event, indicative of extrinsic pathway stimulation.89,91 As such, CDDO and its analogs may provide an option for bypassing blocks to apoptosis typically arising within the mitochondrial apoptosis pathway in chemorefractory tumors. CDDO and CDDO-me are undergoing final preclinical development steps in preparation for clinical trials. Additional possible routes to FLIP suppression include NF-κB pathway inhibitors and HDAC inhibitors.92
Convergence pathway
The intrinsic and extrinsic pathways for apoptosis converge on downstream effector caspases. Certain effector caspases are targets of suppression by an endogenous family of antiapoptotic proteins called inhibitor of apoptosis proteins (IAPs). IAPs contain one or more copies of a domain called the baculoviral IAP repeat (BIR).93 BIRs are sometimes accompanied by other domains, including really interesting new gene (RING) and ubiquiting-conjugating enzyme domains, endowing them with additional properties such as ability to target themselves and associated proteins for ubiquitation and proteasome-dependent degradation. The human genome encodes 8 IAP family members: X-linked inhibitor of apoptosis (XIAP), cIAP1, cIAP2, Naip, Survivin, ML-IAP (Livin; K-IAP), ILP2 (Ts-IAP), and Apollon (BRUCE-a protein [baculovirus integrin-associated protein repeat containing ubiquitin-conjugating enzyme]).2
Pathologic overexpression of IAPs has been documented in cancer and leukemia.94-96 The functional importance of IAPs for apoptosis suppression in cancers has been documented by antisense experiments, in which knocking-down expression of SURVIVIN, XIAP, or others induced apoptosis of tumor cell lines in culture or sensitized to apoptosis induced by anticancer drugs.97-100
Routes to small-molecule drug discovery have been revealed through knowledge of the structural and molecular details of how IAPs inhibit caspases, enlightened by insights gained from studies of endogenous inhibitors of IAPs. For instance, specific segments within IAP family proteins have been identified that bind certain caspases, providing structural information useful for generating compounds that disrupt these protein interactions, freeing caspases to induce apoptosis.101,102
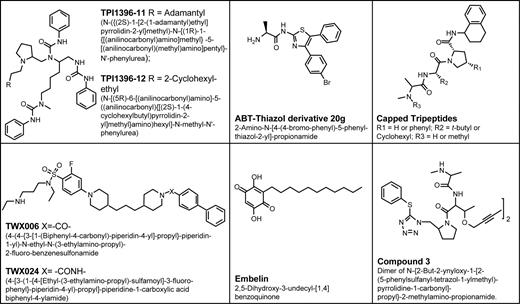
Compounds targeting IAP family proteins. Using an enzyme derepression assay in which XIAP-mediated suppression of caspase-3 is overcome by chemical compounds, 2 classes of XIAP antagonists were identified that target in the vicinity of the second BIR domain (BIR2), freeing caspase-3, including di/tri-phenylureas and benzenesulfonamide derivatives103,104 (Figure 6). The phenylureas exhibit broad-spectrum apoptosis-inducing activity against cultured tumor cell lines and primary leukemias, and suppress growth of tumor xenografts in mice without apparent toxicity to normal tissues.103 Importantly, these compounds induce apoptosis through a Bcl-2/Bcl-XL–independent mechanism,119 suggesting they may prove useful for chemorefractory cancers and leukemias.
Mimicking endogenous antagonists of IAPs defines another strategy for identifying chemical inhibitors. Endogenous proteins, second mitochondria-derived activator of caspases (SMAC) and OMI (HtrA2), bind and suppress IAPs, releasing caspases.101 A 4′mer peptide corresponding to the N-terminus of the mature SMAC protein is sufficient to bind IAPs and block their association with caspases, analogous to similar mechanisms previously defined in Drosophila where IAPs are suppressed by endogenous antagonists Reaper, Hid, Grim, and Sickle.105 By fusing membrane-penetrating peptides onto SMAC, OMI, Hid, or Reaper peptides, apoptosis of human cancer cell lines can be induced in culture and tumor growth suppression obtained in xenograft models in mice.106-108 These data thus provide proof-of-concept evidence that compounds mimicking effects of IAP-binding peptides could potentially be exploited as drugs for cancer treatment.
IAP antagonists. The structures of nonpeptidyl chemical inhibitors are shown.
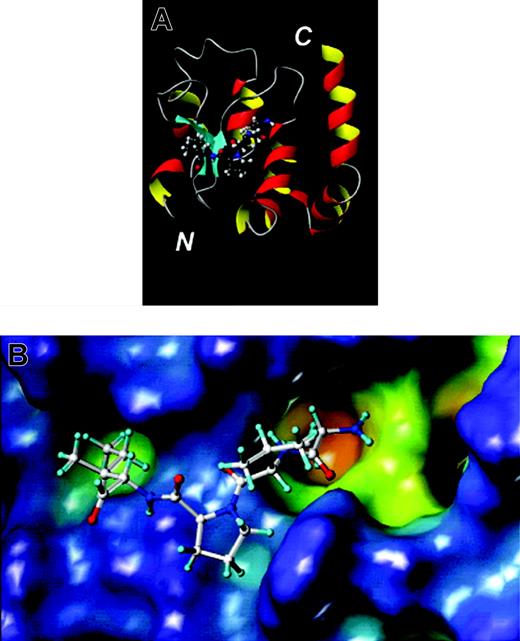
The 3-dimensional structure of the BIR3 domain of XIAP complexed with SMAC reveals the N-terminal 4 amino acids of mature SMAC bind in the same crevice normally occupied by the N-terminus of the small subunit of processed caspase-9, thus suggesting competition for binding101 (Figure 7). Consequently, compounds that mimic the SMAC 4′mer peptide should dislodge active caspase-9 from BIR3, promoting apoptosis. Several groups have initiated preclinical studies of SMAC mimics, though few details are available currently. Reported antagonists targeting the SMAC-binding site include natural product, embelin,109 various peptidiomimetics and nonpeptidic small molecules110-112 (Figure 6).
BIR domain and its inhibitory SMAC peptide derived from x-ray crystallography. (A) The x-ray structure of the third BIR domain of XIAP (ribbon drawing) in complex with the tetra-peptide AVPI derived from SMAC (ball-and-stick). (B) The surface of the third BIR domain of XIAP is shown and color coded according to cavity depth: blue, shallow; yellow, deep. The AVPI tetra-peptide is shown in ball-and-stick representation. The figures were generated with MOLMOL (A) and MOLCAD (B) as implemented in Sybyl (Tripos).
BIR domain and its inhibitory SMAC peptide derived from x-ray crystallography. (A) The x-ray structure of the third BIR domain of XIAP (ribbon drawing) in complex with the tetra-peptide AVPI derived from SMAC (ball-and-stick). (B) The surface of the third BIR domain of XIAP is shown and color coded according to cavity depth: blue, shallow; yellow, deep. The AVPI tetra-peptide is shown in ball-and-stick representation. The figures were generated with MOLMOL (A) and MOLCAD (B) as implemented in Sybyl (Tripos).
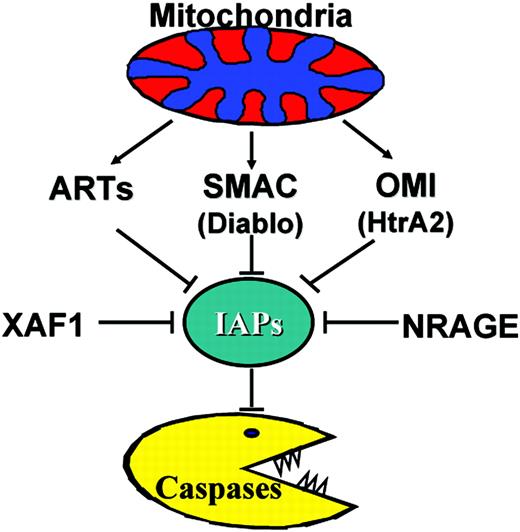
Besides SMAC and OMI, additional endogenous antagonists of IAPs have been described: H5/peanut-like protein 2 (PNUTL2)/CDCre12b (ARTS), x-linked inhibitor of apoptosis protein–associated factor 1 (XAF1), and neutrophin receptor–interacting melanoma antigen homolog (NRAGE)113-115 (Figure 8). Structural details of how these proteins interact with IAP targets are unknown, but could provide additional strategies for generation of 1small-molecule antagonists that work through non-SMAC mechanisms. Given that the reported phenylurea-based antagonists of XIAP target a non-SMAC site on XIAP,103 it will be interesting to determine whether those compounds mimic ARTS, NRAGE, or XAF1 in terms of their docking site on XIAP.
Finally, various indirect strategies for negating IAP activity are emerging. For example, phosphorylation of survivin on threonine 34 is required for cell division and suppression of apoptosis.116 The kinases predominantly responsible are Cdc2 or related cyclin-dependent kinases. Compounds inhibiting Cdc2 family kinases include flavopiridol (Sanofi Aventis, Cambridge, MA), currently in clinical trials.
Drugs inhibiting IAP family gene expression. Preclinical efforts to reduce expression using antisense-based drugs are well underway. Antisense ODNs targeting SURVIVIN mRNA have been evaluated,97 and designated for clinical development (ISIS 23722; LY21 81308), using second-generation antisense chemistry (ISIS Pharmaceuticals, Carlsbad, CA/Lilly, Indianapolis, IN). An antisense-based approach to Survivin is particularly attractive because the mechanism by which this protein suppresses caspases differs from most other IAPs,117 and a clear path forward for compound discovery remains uncertain based on protein structure data.33 In addition, a phosphorothioate-based antisense ODN targeting XIAP (AEG35156/GEM640) has been designated for human clinical trials (Agera Therapeutics, Montreal, Quebec, Canada). It remains to be determined whether a highly selective (antisense) versus broad-spectrum (small-molecule) approach to attacking IAPs will yield the optimal therapeutic index.
Endogenous antagonists of IAPs. The known mammalian inhibitors of IAPs are indicated. ARTS, SMAC, and OMI normally reside inside mitochondria. Only selected members of the IAP family are targeted by these endogenous antagonists.
Endogenous antagonists of IAPs. The known mammalian inhibitors of IAPs are indicated. ARTS, SMAC, and OMI normally reside inside mitochondria. Only selected members of the IAP family are targeted by these endogenous antagonists.
Conclusions
Emerging knowledge about molecular mechanism of apoptosis dysregulation in cancer and leukemia has revealed a plethora of potential drug discovery targets. Structural analysis of apoptosis proteins and studies of their biochemical mechanisms have suggested strategies for lead generation, resulting in numerous novel chemical entities with mechanism-based activity. Although providing encouraging proof-of-principle data that validate these targets, much work lies ahead in terms of optimizing the spectrum of activity of compounds that interact with multiple members of apoptosis protein families, improving their pharmacologic properties, establishing optimal formulations for stability and delivery, and elucidating dose-limiting toxicities. Many of the most logical targets for promoting apoptosis of cancer and leukemia cells are technically challenging, often involving disrupting protein interactions or altering gene expression, as opposed to traditional pharmaceuticals that attack enzymes. Modern techniques of structure-based drug optimization render this task feasible, but still challenging. Such targets require long-term commitments, often outstripping the usual drug discovery and development cycle time practiced at pharmaceutical companies. For those with the stamina, the rewards are likely to be great, creating a new era in cancer therapy where the intrinsic or acquired resistance of malignant cells to apoptosis can be pharmacologically reversed, reinstating natural pathways for cell suicide.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2004-07-2761.
J.C.R. discloses ownership of stock in ISIS Pharmaceuticals and Genta, and is on the Board of Directors of ISIS Pharmaceuticals.
We thank I. Pedersen and S. Kitada for helpful information; A. Godzik, X. Zhang, and G. Salvesen for suggestions about figures; and M. LaMie and J. Valois for manuscript preparation. Our apologies to the many authors whose work we could not cite due to manuscript length limitations.