Abstract
In adult Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL), minimal residual disease (MRD) after stem cell transplantation (SCT) is associated with a relapse probability exceeding 90%. Starting imatinib in the setting of MRD may decrease this high relapse rate. In this prospective multicenter study, 27 Ph+ ALL patients received imatinib upon detection of MRD after SCT. Bcr-abl transcripts became undetectable in 14 (52%) of 27 patients, after a median of 1.5 months (0.9-3.7 months) (earlyCRmol). All patients who achieved an earlyCRmol remained in remission for the duration of imatinib treatment; 3 patients relapsed after imatinib was discontinued. Failure to achieve polymerase chain reaction (PCR) negativity shortly after starting imatinib predicted relapse, which occurred in 12 (92%) of 13 patients after a median of 3 months. Disease-free survival (DFS) in earlyCRmol patients is 91% ± 9% and 54% ± 21% after 12 and 24 months, respectively, compared with 8% ± 7% after 12 months in patients remaining MRD+ (P < .001). In conclusion, approximately half of patients with Ph+ ALL receiving imatinib for MRD positivity after SCT experience prolonged DFS, which can be anticipated by the rapid achievement of a molecular complete remission (CR). Continued detection of bcr-abl transcripts after 2 to 3 months on imatinib identifies patients who will ultimately experience relapse and in whom additional or alternative antileukemic treatment should be initiated.
Introduction
The prognosis of adult patients with Philadelphia chromosome (Ph)/bcrabl+ acute lymphoblastic leukemia (ALL) who are treated with chemotherapy alone is poor, with a less than 10% probability of long-term survival.1-5 Allogeneic stem cell transplantation (SCT) in first complete remission (CR1) is therefore considered the treatment of choice in adult Ph+ ALL, with 27%-46% long-term disease-free survival (DFS).6-11 Approximately 30% of patients undergoing allogeneic SCT in CR1 eventually relapse, however, making this the most frequent cause of treatment failure. Allogeneic SCT in second or third remission or as salvage therapy for refractory disease achieves long-term DFS in only a small minority of patients; if hematologic relapse occurs after allogeneic SCT, further treatment is rarely successful.12-15
The Abelson (ABL) tyrosine kinase inhibitor imatinib (Glivec, formerly STI571; Novartis, Basel, Switzerland) has pronounced but brief antileukemic activity in patients with advanced Ph+ ALL, including those failing SCT. However, sustained molecular remissions were almost never observed.14-16 Acquired resistance to imatinib is frequently caused by point mutations in bcr-abl that inactivate imatinib.17,18 More recently, we have shown that these mutations may predate imatinib therapy in at least some patients with advanced Ph+ ALL.19 The probability that such a mutation is present or develops by chance can be expected to be substantially lower in the setting of a low leukemic cell burden compared with overt hematologic relapse. Conceptually, imatinib should therefore be more effective if started early after SCT when the leukemic cell burden is still small (ie, in the setting of minimal residual disease [MRD]).
Reverse transcription–polymerase chain reaction (RT-PCR) is a sensitive method for detecting low-level bcr-abl transcripts to assess MRD in Ph+ ALL.20,21 The largest study reported to date that examined the relevance of MRD in Ph+ ALL demonstrated that detection of bcr-abl transcripts on at least one occasion after SCT was associated with a profoundly increased risk of relapse.22 In that study, only a small subset of the adult patients who were MRD+ by qualitative RT-PCR experienced prolonged DFS. This has been corroborated by several other reports demonstrating that detection of MRD after SCT was predictive of imminent relapse.23-26
We therefore initiated a prospective clinical trial to assess whether imatinib is a safe and effective therapy in patients with Ph+ ALL and molecular evidence of recurrent leukemia after allogeneic or autologous SCT.
Patients, materials, and methods
Eligibility
Patients at least 15 years of age who had received an allogeneic or autologous SCT for Ph+ ALL or lymphoid blast crisis (lyBC) of chronic myeloid leukemia (CML) were eligible if they had molecular evidence of recurring leukemia based on RT-PCR of bcr-abl transcripts. There were no restrictions regarding disease status at the time of SCT, type of transplant, conditioning regimen, or use of donor lymphocyte infusions (DLIs). Entry criteria included sufficient recovery of absolute neutrophil counts (ANC;
≥1 × 109/L) and platelet counts (≥50 × 109/L), absence of life-threatening or uncontrolled graft-versus-host disease (GvHD) or infections, Eastern Cooperative Oncology Group (ECOG) performance status 0 through 2, and adequate organ function, as previously described.14,15 All patients gave written informed consent to participate in the study, and the study was reviewed and approved by a recognized ethics review committee at each trial center. The study was performed in accordance with the Declaration of Helsinki (as amended in Tokyo, Venice, and Hong Kong).
Study design and treatment
Imatinib was initiated at a daily oral dose of 400 mg, which could be escalated by protocol-defined guidelines to 600 mg and subsequently to 800 mg daily in patients who remained bcr-abl+. Bone marrow aspirations for cytology and MRD analysis were scheduled after the first 28 days of imatinib treatment and subsequently after every 6 to 8 weeks during the first year of therapy, unless clinically otherwise indicated. Imatinib administration was scheduled for 1 year from first documentation of PCR negativity. DLIs were given at the investigator's discretion. Treatment was interrupted or reduced in response to nonhematologic or hematologic toxicity according to previously reported guidelines.14
Assessment of safety and efficacy
Clinical and laboratory evaluations were performed prior to and during treatment essentially as previously described.14,15 MRD analysis was performed centrally in the study's reference laboratory (Frankfurt) using quantitative (q) real-time PCR (Taqman; Perkin Elmer Applied Biosciences, Weiterstadt, Germany) analysis of peripheral blood (PB) and bone marrow (BM) samples as previously described.26,27 A molecular CR (CRmol) required PCR negativity in both PB and BM by q real-time-PCR of sufficient sensitivity, with confirmation by nested PCR. A CRmol was considered to be sustained if PCR negativity was documented over a time period of at least 3 months. Time to disease progression (TTP) was defined as the time from starting imatinib to reappearance of leukemic blasts in bone marrow and/or peripheral blood by conventional morphologic criteria or the occurrence of extramedullary disease. DFS was measured from start of imatinib until relapse or death from any cause; overall survival (OS) was measured from start of imatinib until death.
Statistical analysis
Differences in response rates by pretreatment characteristics among subgroups were analyzed by Fisher exact test. Time to progression, DFS, and OS curves were plotted according to the methods of Kaplan-Meier, with differences between patient groups analyzed by the log-rank test.
Results
Patients and time to progression
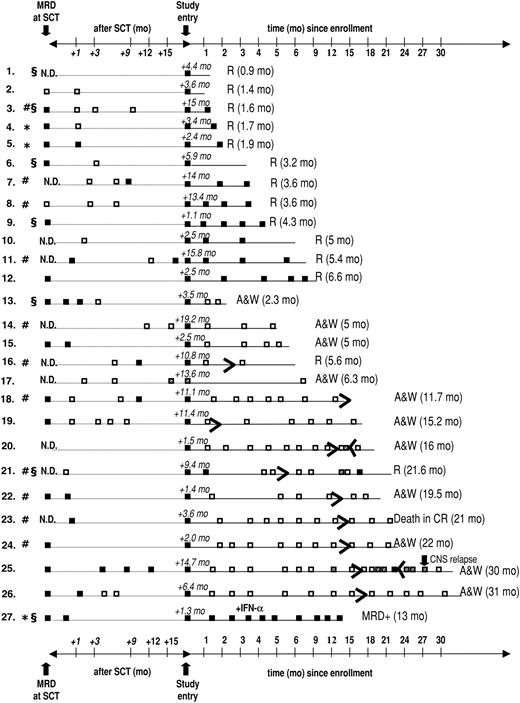
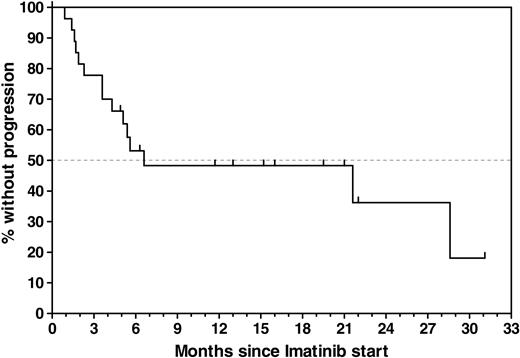
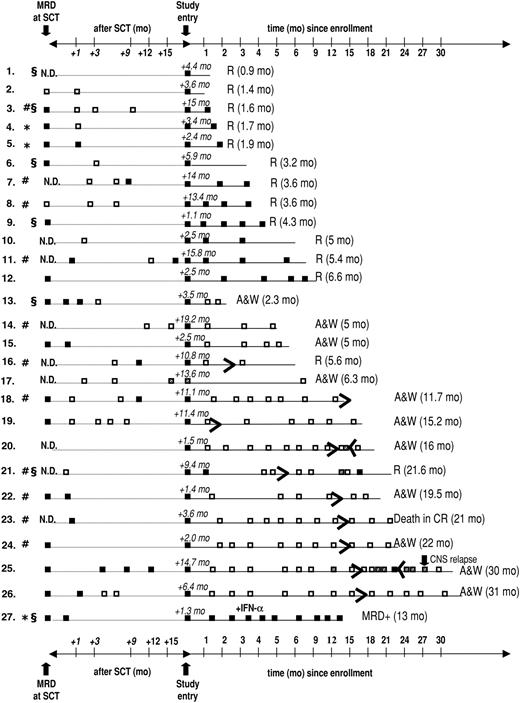
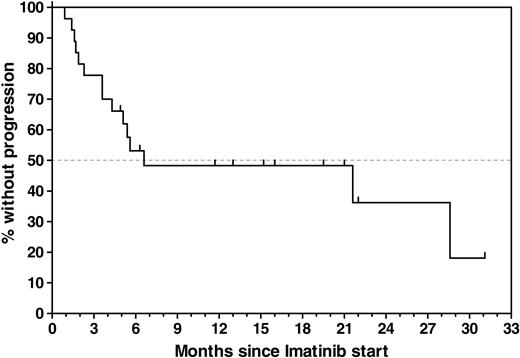
Twenty-seven patients who had previously undergone allogeneic (n = 24) or autologous (n = 3) SCT were enrolled, with a median age of 48 years (range, 16-63 years). Patient demographics and relevant transplantation-related data are provided in Table 1. MRD analyses that were performed within a 6-week interval before SCT are available in 17 (63%) of 27 patients, with a median ratio of bcr-abl to GAPDH of 8.67 × 10-5. During the time intervening between SCT and documented presence of bcr-abl transcripts on which study entry was based, 16 (59%) of the 27 patients were documented to be PCR– on at least 1 occasion; 2 of these patients had been PCR– before SCT, and 14 had converted to PCR negativity with SCT (Figure 1). In 11 patients, the detection of bcr-abl transcripts MRD that was used as the criterium for study entry was performed between 28 days and 49 days after SCT, coinciding with the first posttransplantation assessment. Overall, imatinib treatment was started a median of 4.4 months (range, 1.1-19.2 months) after SCT. The PCR positivity that led to study entry was first documented a median of 19 days (range, 1-257 days) before imatinib was initiated. After a median follow-up of 8.3 months (range, 0.9-31 months), 12 patients were alive in continuous CR (CCR) and 15 patients relapsed, resulting in an overall probability of relapse of 52% ± 10% during study treatment. Estimated median remission duration in all patients is 6.6 months (range, 0.9-31 months) (Figure 2).
Results of longitudinal MRD analysis in bone marrow performed at the time of (or within 6 weeks before) SCT, between SCT and study entry, and during imatinib therapy and follow-up. □ represents a negative result of quantitative RT-PCR; ▪, a positive PCR reaction; and ▦, a possible nonspecific result (ie, only 1 positive PCR reaction in duplicate samples or positivity exclusively in the nested PCR). R indicates relapse with the time from start of imatinib to relapse given in parentheses; * autologous SCT; #, patients with active GvHD; and §, SCT after dose-reduced conditioning; N.D., not done; A & W, alive and well.
Results of longitudinal MRD analysis in bone marrow performed at the time of (or within 6 weeks before) SCT, between SCT and study entry, and during imatinib therapy and follow-up. □ represents a negative result of quantitative RT-PCR; ▪, a positive PCR reaction; and ▦, a possible nonspecific result (ie, only 1 positive PCR reaction in duplicate samples or positivity exclusively in the nested PCR). R indicates relapse with the time from start of imatinib to relapse given in parentheses; * autologous SCT; #, patients with active GvHD; and §, SCT after dose-reduced conditioning; N.D., not done; A & W, alive and well.
Molecular response to imatinib
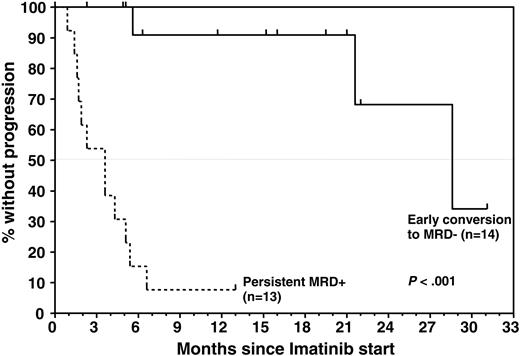
Fourteen of (52%) 27 patients achieved a molecular remission as defined by a decrease of bcr-abl transcripts below the detection threshold of both quantitative and nested RT-PCR, previously shown to have a sensitivity of approximately 1 bcr-abl+ cell in 105-106 cells.26,27 These results are depicted graphically in Figure 1. Molecular remissions were attained soon after imatinib was started: the majority of patients became bcr-abl– within 2 months (n = 9) or during the third month (n = 3) of treatment (earlyCRmol); the median time to PCR negativity was 1.5 months (range, 0.9-3.7 months). Of the 16 patients entered because of molecular relapse (ie, reappearance of bcr-abl transcripts after SCT), 8 (50%) patients rapidly became PCR– while the other 8 patients remained bcr-abl+ and went on to relapse. The rate of conversion to PCR negativity in the subset of patients with molecular relapse is thus essentially identical to that of the patients as a whole. Conversely, of the 14 patients who became PCR– on imatinib, 8 had been enrolled upon demonstration of molecular relapse after transplantation, while 6 (43%) patients had not been documented to be MRD– at any time prior to study entry (Figure 1). Of note, 13 of the 14 patients who achieved an earlyCRmol received imatinib at a daily dose of 400 mg, and these patients remained PCR– throughout imatinib treatment. Conversely, the probability that a patient with MRD persisting after 3 months of Imatinib would still achieve a CRmol was lower than 10%. This failure to convert to PCR negativity was not affected by dose escalation to 600 mg/d (n = 4) or 800 mg/d (n = 1).
Time to progression (TTP) calculated from the start of imatinib treatment for all 27 patients. Estimated median TTP for all patients was 6.6 months (range, 0.9-31 months). The estimated progression-free survival rate for all patients was 48% ±10% at 6.6 months without censoring at the time of imatinib discontinuation.
Time to progression (TTP) calculated from the start of imatinib treatment for all 27 patients. Estimated median TTP for all patients was 6.6 months (range, 0.9-31 months). The estimated progression-free survival rate for all patients was 48% ±10% at 6.6 months without censoring at the time of imatinib discontinuation.
Treatment outcome in relation to molecular response
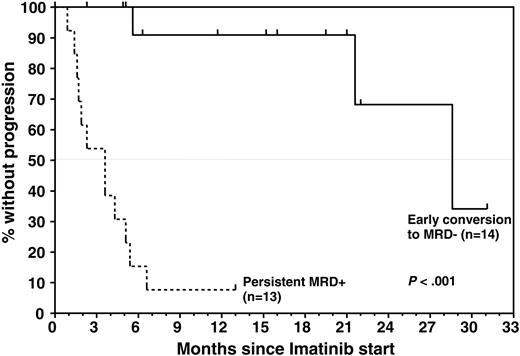
Achievement of an earlyCRmol was highly predictive of a favorable treatment outcome, whereas MRD that was still detectable 2 to 3 months after starting imatinib heralded almost certain relapse (Figures 1 and 3). Eleven of 14 patients who had achieved a CRmol were in ongoing molecular remission after a median follow-up of 15.6 months (range, 2-31 months). None of the earlyCRmol patients relapsed while on imatinib. Three patients in this group experienced bone marrow (n = 2) or isolated central nervous system (CNS) relapse 3.3 months, 16.4 months, and 13.6 months after discontinuation of imatinib. The 2 patients experiencing bone marrow relapse had discontinued imatinib prematurely, after 2.3 and 5.2 months.
Twelve of 13 patients who did not achieve an earlyCRmol relapsed while imatinib treatment was still ongoing. Only 1 patient who remained MRD+ is still in hematologic CR more than a year since starting imatinib; this patient underwent autologous stem cell transplantation before study entry and subsequently received low-dose interferon-α in addition to imatinib.
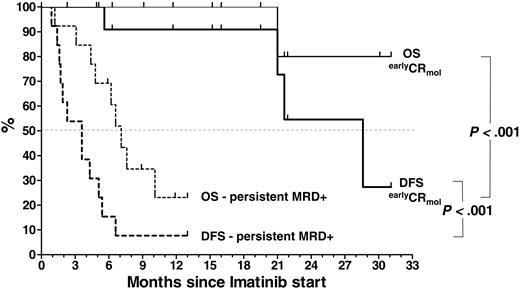
Median remission duration from start of imatinib is 3.6 months (range, 0.9-6.6 months) in the MRD+ group and 28.6 months in the earlyCRmol group. The probability of DFS and OS after 1 year are 91% ± 9% and 100% in the earlyCRmol group and 8% ± 7% and 23% ± 13% at 13 months in the MRD+ patients, respectively (P < .001 for DFS and P < .001 for OS) (Figure 4). DFS and OS are 54.5% ± 21% and 80% ± 18% at 2 years in the earlyCRmol patients, while only 1 patient in the MRD+ group (receiving additional interferon-α) has survived beyond 1 year (13 months).
Treatment outcome after imatinib discontinuation
At the time of last follow-up, 10 patients had discontinued imatinib. Three of these patients experienced molecular relapse, 2 of whom had discontinued imatinib prematurely (Figure 1). Imatinib was resumed in 2 patients (patient nos. 20 and 25), both of whom again converted to PCR negativity after 1.5 months and 6 months, respectively. One patient (patient no. 21) refused renewed imatinib treatment and relapsed within 2 months of documented molecular relapse. Six of the 10 patients are alive in ongoing molecular CR off imatinib, and 1 patient died of a stroke in CRmol.
Disease history and response to imatinib
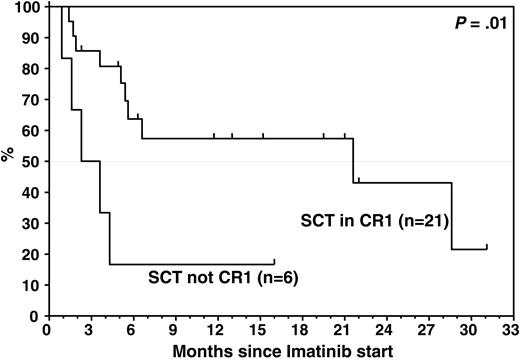
When we assessed the earlyCRmol rate and DFS in relation to the disease status at the time of SCT, the response to MRD-triggered imatinib was significantly inferior in patients who did not receive transplants in CR: none of the 5 patients who had received transplants in first or second relapse (n = 3) or with refractory disease (n = 2) achieved a CRmol, in contrast to 13 (62%) of 21 patients who underwent transplantation in CR1. The MRD levels at the time of SCT did not differ significantly between patients who underwent transplantation in CR1 versus beyond CR1. Median time to relapse on imatinib was 3 months in patients who underwent transplantation with more advanced disease, compared with 21.6 months when SCT was performed in CR1 (P = .01; Figure 5).
Time to progression by molecular response to imatinib, which was determined by longitudinal analysis of MRD levels throughout study treatment. All patients had detectable bcr-abl transcripts at study entry. Median TTP in patients who were found to convert to PCR negativity soon after initiation of imatinib was significantly longer than in patients who remained PCR positive (28.6 months versus 3.6 months; P < .001). Estimated progression-free survival rate for MRD responders was 91% ± 9% at 12 months and 68% ± 21% at 24 months compared with 8% ± 7% at 13 months for nonresponders.
Time to progression by molecular response to imatinib, which was determined by longitudinal analysis of MRD levels throughout study treatment. All patients had detectable bcr-abl transcripts at study entry. Median TTP in patients who were found to convert to PCR negativity soon after initiation of imatinib was significantly longer than in patients who remained PCR positive (28.6 months versus 3.6 months; P < .001). Estimated progression-free survival rate for MRD responders was 91% ± 9% at 12 months and 68% ± 21% at 24 months compared with 8% ± 7% at 13 months for nonresponders.
DFS and OS by MRD response to imatinib treatment, determined by longitudinal analysis of bcr-abl transcript levels after study entry, at which all patients were PCR positive. Median DFS and OS in patients achieving early PCR negativity was significantly superior to that in patients who remained MRD+ after initiation of imatinib (28.6 months versus 3.6 months, P < .001; and median not yet reached versus 7.1 months, P < .001, respectively). The estimated DFS and OS rates in the MRD– group were 54.5% ± 21% and 80% ± 18% at 24 months compared with 8% ± 7% and 23% ± 13% in the MRD+ group, respectively. The event at month 21 is due to a death in CR.
DFS and OS by MRD response to imatinib treatment, determined by longitudinal analysis of bcr-abl transcript levels after study entry, at which all patients were PCR positive. Median DFS and OS in patients achieving early PCR negativity was significantly superior to that in patients who remained MRD+ after initiation of imatinib (28.6 months versus 3.6 months, P < .001; and median not yet reached versus 7.1 months, P < .001, respectively). The estimated DFS and OS rates in the MRD– group were 54.5% ± 21% and 80% ± 18% at 24 months compared with 8% ± 7% and 23% ± 13% in the MRD+ group, respectively. The event at month 21 is due to a death in CR.
The results of pretransplantation salvage therapy with imatinib for relapsed (n = 4) or refractory (n = 5) Ph+ ALL were likewise predictive of the molecular response to MRD-triggered imatinib in this study: whereas 4 of the 5 patients who responded to imatinib salvage prior to SCT achieved an earlyCRmol during imatinib therapy after SCT, none of the 4 patients who failed imatinib salvage prior to SCT subsequently attained PCR negativity at any time during study treatment (P = .04).
Of the 9 patients who had never received imatinib prior to transplanation, 6 (67%) achieved rapid PCR negativity, which was not significantly different from the 44% complete molecular response rate among the whole group of patients who had received prior imatinib (n = 18) (P = .42) or the 44% conversion to PCR negativity in the subset of patients (n = 9) who received imatinib as an element of primary treatment rather than salvage therapy (P = .6).
Response in relation to patient and disease characteristics
We also examined whether other disease characteristics and transplantation-associated variables known to be associated with outcome after SCT had an impact on molecular response to imatinib and treatment outcome. An assessment of the tumor load present at start of imatinib, using bcr-abl transcript levels as a surrogate marker, revealed lower median bcr-abl/GAPDH ratios at study entry in patients who subsequently achieved an earlyCRmol (4.82 × 10–7; range, 3.68 × 10–5 – 1.25 × 10–8) than in patients who remained MRD+ (1.45 × 10–5; range, 4.2 × 10–3 – 1.29 × 10–6), although this was not statistically significant. However, 13 of the 20 patients with transcript levels below 10–4 by quantitative RT-PCR attained a molecular remission, whereas none of the 5 patients with a bcr-abl/GAPDH ratio above 10–4 became PCR negative (P = .015).
TTP by remission status at SCT. Median TTP in patients beyond CR1 at SCT was significantly inferior to that in patients who received transplants in CR1 (3 months versus 21.6 months; P = .01).
TTP by remission status at SCT. Median TTP in patients beyond CR1 at SCT was significantly inferior to that in patients who received transplants in CR1 (3 months versus 21.6 months; P = .01).
At the time of enrollment, 11 patients (46%) were still receiving immunosuppressive therapy because of active GvHD (chronic n = 5, acute n = 3; Table 1) or because of the short time interval since SCT (n = 3). Immunosuppression was tapered and rapidly discontinued in 10 of these 11 patients, and 1 patient was still receiving a low dose of cyclosporin A (CSA) at the time of relapse. Seven (64%) of 11 patients with active GvHD at study entry achieved an earlyCRmol versus 7 (54%) of 13 patients without active GvHD (not significant) (Figure 1).
While disease-free survival did not differ significantly between patients with and without GvHD (P = .24), overall survival by Kaplan-Meier analysis was superior in patients with GvHD, with an estimated OS at 1 and 2 years of 87.5% ± 12% versus 40% ± 17% (P = .03 by log-rank test) and 58% ± 25% versus 40% ± 17%), respectively. Median OS was reached in the GvHD group after 31 months of follow-up and was reached after 7.6 months in the patient group without GvHD.
A total of 5 patients received immunotherapy (DLI) after transplantation, either prior to imatinib only (n = 1), prior to and after imatinib (n = 1), or only after imatinib (n = 3) (Tables S2 and S3, available on the Blood website; see the Supplemental Tables link at the top of the online article). There was no evidence of efficacy in any of the patients; specifically, none of the 4 patients who received DLIs during the study achieved PCR negativity, and all of them relapsed.
There was no significant difference regarding molecular response to imatinib, TTP, DFS, or OS with respect to age, sex, bcr-abl breakpoint, type of transplant (matched related donor/matched unrelated donor), use of a T-cell–depleted versus T-cell–replete graft (n = 4 versus n = 20), myeloablative versus dose-reduced conditioning (n = 20 versus n = 7) or autologous (n = 3) versus allogeneic SCT (n = 24). However, the small number of patients in most of these subgroups profoundly limits delineation of any significant differences in terms of response to imatinib, particularly as the patient's disease status at time of transplantation was heterogeneous (Table 1).
Tolerability/adverse events
Observed side effects were mild to moderate nausea, emesis, periorbital edema, and elevated liver enzymes. No grade IV hematologic toxicity was observed. No worsening of preexisting GvHD and no de novo GvHD occurred. Overall, 3 patients discontinued imatinib prematurely because of gastrointestinal discomfort (n = 1; after 5 months), edema and weight gain (n = 1; after 2.3 months), and patient decision (n = 1; after 1.4 months).
Discussion
Allogeneic hematopoietic SCT is the only approach considered curative in adult patients with Ph+ ALL,5-11,28 but relapses are frequent and are associated with a dismal prognosis.29-31 We previously showed that fewer than 10% of patients who received transplants who also received imatinib as single-agent salvage therapy for hematologic relapse experienced long-term DFS.14,15 In the present study, we demonstrate that earlier initiation of imatinib, triggered by molecular rather than hematologic evidence of leukemic recurrence, is associated with prolonged DFS in approximately 50% of patients. Moreover, we show that both short-term and long-term treatment outcomes can be anticipated with near certainty on the basis of bcr-abl transcript levels determined within the first 2 to 3 months of treatment. Specifically, patients failing to achieve a molecular remission within this short time period after starting imatinib (median, 1.5 months) are destined to relapse, whereas patients achieving an earlyCRmol have a high probability of a sustained molecular remission and long-term disease free survival. We observed no relapse in the earlyCRmol group while imatinib was still ongoing (P < .001). Given the median time to disease recurrence of only 3 months and the fact that 6 of 12 patients already relapsed during the third month of imatinib treatment, bcr-abl positivity that persists after 2 months of imatinib monotherapy in our experience mandates institution of additional antileukemic therapy. Conspicuously, the only patient in the MRD+ group who has not relapsed is receiving low-dose interferon-α in addition to imatinib, based on preliminary evidence that imatinib and interferon-α exert a synergistic antileukemic effect in Ph+ ALL.32,33
While the most effective strategies for treatment intensification remain to be determined, our data provide clear evidence that early MRD analysis provides critical information for guiding therapeutic intervention at the level of low leukemic cell burden. Such MRD-guided treatment decisions are reminiscent of acute promyelocytic leukemia, in which molecular recurrence constitutes an indication to intensify treatment, including possible transplantation.34,35
The 48% DFS rate that we observed with MRD-triggered imatinib in the present study is considerably higher than the 5% long-term DFS reported for imatinib treatment of Ph+ ALL patients relapsing after SCT.15 Conspicuously, patients with more advanced disease (ie, refractory or relapsed Ph+ ALL at the time of SCT) did not benefit from subsequent preemptive, MRD-triggered imatinib. Similarly, higher bcr-abl transcript levels were indicative of an inferior response. Taken together, these observations are consistent with a model in which additional genetic aberrations conferring imatinib resistance (eg, bcr-abl point mutations) accumulate with greater frequency in more advanced, genetically less stable leukemias. Interestingly, the median time interval between SCT and detection of bcr-abl transcripts was shorter in the cohort of patients failing to achieve a CRmol (median 3.4 months versus 6.2 months), indicating that an inferior response to imatinib was associated with more dynamic disease kinetics. These data imply that it may not be sufficient to implement future imatinib-based therapies on the basis of MRD monitoring, but that they should be initiated at the earliest possible time after SCT for Ph+ ALL, when the potential leukemic cell load is minimal.
The relative contributions of imatinib and immunologic mechanisms in inducing PCR negativity in our patients are uncertain, particularly as graft versus leukemia (GvL) is generally considered to have a more limited impact on clinical outcome in Ph+ ALL as opposed to Ph+ CML.5,13 The majority of patients in our study (16 of 27) were enrolled for molecular relapse after having achieved PCR negativity subsequent to SCT. In conjunction with the rapid response kinetics following institution of imatinib and our finding that the molecular response rate did not differ significantly between patients with and without GvHD, this argues for a causal relationship between imatinib treatment and ensuing molecular response, rather than a merely coincidental disappearance of bcr-abl transcripts resulting instead from GvL effects. Moreover, the risk of relapse is known to be dramatically increased in patients with molecularly detectable bcr-abl transcripts22-25,36 and belated disappearance of residual bcr-abl transcripts after SCT is rarely observed in Ph+ ALL. Taken together, it appears unlikely that the earlyCRmol observed in this study would have occurred independently of imatinib in the responding 50% of patients. This does not exclude the possibility that GvHD influenced the long-term clinical outcome. In fact, overall but not disease-free survival was significantly better in the group of patients who had evidence of GvHD at study entry. Interestingly, 3 patients who had achieved a molecular CR while on study again became PCR positive several months after they had discontinued imatinib; 2 of them restarted imatinib and again became PCR negative, and 1 patient refused imatinib and subsequently relapsed.
In conclusion, we demonstrate the ability of single-agent imatinib to induce sustained molecular remissions and contribute to prolonged DFS in a substantial proportion of patients with Ph+ ALL, contingent upon initiation in the setting of MRD and at an early time point in the clinical evolution of the leukemia. Our study establishes the importance of determining bcr-abl transcript levels for guiding imatinib-based posttransplantation therapy of Ph+ ALL by showing that short- and long-term treatment outcome can be predicted by the molecular remission status after 2 months of imatinib. Taken together, these data provide the basis for future treatment strategies for Ph+ ALL in which imatinib is initiated early after SCT and subsequent treatment decisions are based on molecular evidence of recurring leukemia rather than overt hematologic relapse.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2004-05-1746.
Supported by grants from the BMBF Competence Network “Acute Leukemias” Grant No. 01G19971, the German Genome Research Network (NGFN), the Wilhelm Sander Stiftung and the Adolf-Messer Foundation, Germany, and from Novartis Pharma AG, Nürnberg, Germany.
One of the authors (H.G.) is employed by a company (Novartis) whose product was studied in the present work. O.G.O. and D.H. received research support from Novartis and served as consultants.
This study was presented in part at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2003, San Diego, CA.37
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Brigitte Gehrke, Tamara Hirdes, Doreen Badowski, and Sandra Markovic for their excellent technical assistance.