Abstract
Cell-cell contact is required for efficient transmission of human T-lymphotropic virus type 1 (HTLV-1). An HTLV-1–infected cell polarizes its microtubule-organizing center (MTOC) toward the cell-cell junction; HTLV-1 core (Gag) complexes and the HTLV-1 genome accumulate at the point of contact and are then transferred to the uninfected cell. However, the mechanisms involved in this cytoskeletal polarization and transport of HTLV-1 complexes are unknown. Here, we tested the hypothesis that engagement of a specific T-cell surface ligand is synergistic with HTLV-1 infection in causing polarization of the MTOC to the cell contact region. We show that antibodies to intercellular adhesion molecule-1 (ICAM-1; CD54) caused MTOC polarization at a higher frequency in HTLV-1–infected cells. ICAM-1 is upregulated on HTLV-1–infected cells, and, in turn, ICAM-1 on the cell surface upregulates HTLV-1 gene expression. We propose that a positive feedback loop involving ICAM-1 and HTLV-1 Tax protein facilitates the formation of the virologic synapse and contributes to the T-cell tropism of HTLV-1. In contrast, MTOC polarization induced in T cells by antibodies to CD3 or CD28 was significantly inhibited by HTLV-1 infection.
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus that infects 10 to 20 million people worldwide. The majority (95%) of HTLV-1–infected individuals remain asymptomatic, but about 3% develop adult T-cell leukemia or lymphoma and another 3% are affected by inflammatory disorders, of which HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) is the most commonly recognized.1
HTLV-1 transmission between individuals depends on cell-cell contact,1 because virtually no cell-free infectious virions are produced during natural infection. Igakura et al2 showed that cell contact rapidly induces an HTLV-1–infected T cell to orient its microtubule organizing center (MTOC) toward the cell-cell junction. HTLV-1 core (Gag) complexes and the HTLV-1 genome accumulate at the cell-cell junction. The structure of the resulting cell-cell contact has been termed a virologic (or viral) synapse (VS)2 since it resembles the immunologic synapse (IS), the specialized contact made between a lymphocyte and an antigen-presenting cell.3 A synapse is an organized structure that allows signaling and directional protein transfer between 2 cells. Identification of the mechanisms by which HTLV-1 induces formation of the VS will serve to elucidate how the virus persists and spreads, and it may provide new targets for interruption of HTLV-1 propagation.
The IS and the VS share at least 2 structural features. First, the T cell's MTOC is oriented toward the cell-cell junction. Second, the lymphocyte function-associated antigen-1 (LFA-1)–associated molecule talin is organized in ring-shaped microdomains or patches in the cell contact area.2 However, there is a crucial difference between the VS and the IS: in the IS, the MTOC is oriented toward the synapse inside the T cell that has recognized its cognate antigen,4 whereas in the VS the MTOC is polarized toward the synapse inside the HTLV-1–infected cell. Therefore, while formation of the IS is initiated by signaling through the T-cell receptor,5 the critical factor that drives VS formation is HTLV-1 infection, irrespective of the antigen specificity of the T cells involved.2 It was concluded that at least 2 signals are necessary to trigger VS formation: one from HTLV-1 infection of the T cell and the second from contact with another cell. The aim of this study was to identify T-cell surface molecules involved in triggering the polarization of the microtubule cytoskeleton that is characteristic of the HTLV-1–associated virologic synapse. We adopted a simple antibody-coated bead-cell conjugate formation assay that others have used previously to dissect the mechanisms involved in T-cell activation.6-10 Latex beads were coated with antibodies to T-cell surface molecules known to be involved in T-cell activation, such as CD2 and CD3, LFA-1 (CD11a and CD18), and CD28,11,12 and also molecules known to be up-regulated on HTLV-1–infected T cells, such as intercellular adhesion molecule-1 (ICAM-1) and CD25.13-15 The results were corroborated by 2 independent types of experiment, one using soluble cyclic peptides that block the interaction between ICAM-1 and LFA-1, the other using cell lines with a nonexpressing or constitutively activated LFA1 gene. Evidence from all 3 types of experiments indicates that engagement of ICAM-1 on the surface of the HTLV-1–infected cell is sufficient to account for the signal from cell contact that triggers the observed preferential polarization of the microtubule cytoskeleton in the HTLV-1 virologic synapse.
Patients, materials, and methods
Patients and cells
The HTLV-1–immortalized cell line MS9 was a gift from Dr David Derse, National Cancer Institute, Rockville, MD. MS9 cells were derived by coculture of phorbol-12-myristate-13-acetate–activated human peripheral blood mononuclear cells (PBMCs) with DBS-FRhL (clone B5) cells that were infected with the HTLV-1 molecular clone, pHTLV-X1MT.16 MS9 cells were cultured in RPMI 1640 (Sigma-Aldrich, Dorset, United Kingdom) supplemented with 2 mM glutamine (Invitrogen, Paisley, United Kingdom), 100 IU/mL penicillin (Invitrogen), 100 IU/mL streptomycin (Invitrogen), 20% heat-inactivated fetal calf serum (FCS; PAA Laboratories, Somerset, United Kingdom), and 100 U/mL recombinant interleukin 2 (IL-2; Sigma-Aldrich).
The Jβ2.7 cells were a gift from Dr Catarina Hioe, VA Medical Center Research Service, New York, NY. They were derived from Jurkat cells treated with ethyl methanesulfonate and selected for complete loss of cell surface LFA-1. The cells were then transfected with cDNA of the LFA-1 wild-type α chain subunit (CD11a) to restore LFA-1 cell surface expression (Jβ2.7/LFA-1 wt). A cell line expressing a constitutively active form of LFA-1 was created using a deletion mutant of the LFA-1 α subunit (Jβ2.7/LFA-1 Δ). A Jβ2.7 control line was generated by transfecting the vector alone (Jβ2.7/mock), which expressed no LFA-1 on the surface.17,18 These cell lines were cultured in RPMI 1640 (Sigma-Aldrich) supplemented with 2 mM glutamine (Invitrogen), 100 IU/mL penicillin (Invitrogen), 100 IU/mL streptomycin (Invitrogen), 20% heat-inactivated FCS (PAA Laboratories), and 3 μg/mL puromycin (Sigma-Aldrich).
PBMCs were obtained from uninfected laboratory controls and HTLV-1 seropositive HAM/TSP patients with a high proviral load attending the National Centre for Human Retrovirology at St Mary's Hospital, London. All patients gave informed consent. PBMCs were isolated via density gradient centrifugation on Histopaque-1077 (Sigma-Aldrich), washed twice with phosphate-buffered saline (PBS) and then once in PBS/10% FCS. CD4+ cells were isolated using indirect magnetic labeling with the CD4+ T Cell Isolation Kit from Miltenyi Biotech (Surrey, United Kingdom). The manufacturer's instructions were followed for negative selection of CD4+ T cells from PBMCs. This procedure yielded CD4+ cells at a purity of greater than 95%, ascertained by flow cytometry (data not shown). Before use, the isolated CD4+ T cells were cultured overnight, widely dispersed in 10-cm diameter tissue culture dishes (106 cells/mL), to allow spontaneous expression of HTLV-1 proteins.19 The culture medium used was RPMI 1640 (Sigma-Aldrich) supplemented with 2 mM glutamine (Invitrogen), 100 IU/mL penicillin (Invitrogen), 100 IU/mL streptomycin (Invitrogen), and 20% heat-inactivated FCS (PAA Laboratories).
Antibodies
The following antibodies were used for staining of cells for microscopy: the mouse immunoglobulin G2b (IgG2b) anti–HTLV-1 Gag p19-specific monoclonal antibody (mAb), clone GIN7,20 used at a concentration of 1 μg/mL; and the rat anti–tubulin α mAb (Chemicon Europe, Southampton, United Kingdom), used at a concentration of 3 μg/mL. Secondary antibodies for fluorescent staining with the anti-tubulin α and the anti-Gag p19 antibodies were Fluorescein (fluorescein isothiocyanate [FITC]) AffiniPure-labeled donkey anti–rat IgG (used at a concentration of 150 μg/mL; Stratech Scientific, Cambridgeshire, United Kingdom) and Alexa Fluor 568 goat anti–mouse IgG2b (used at a concentration of 2μg/mL; Molecular Probes Europe BV, Leiden, The Netherlands), respectively.
Antibodies used to coat the polystyrene latex beads were as follows: anti-CD54 (ICAM-1), clone HA58; anti-CD2, clones 4B2, CLB-T11/1 and 4B2, CLB-T11.2/1; and anti-CD28, clone CLB-CD28/1, 15E8, all mouse IgG1 antibodies (Research Diagnostics, Flanders, NJ); anti-CD3, clone HIT3a (mouse IgG2a) from Bioscience (Insight Biotechnology Ltd., Wembley, Middlesex, United Kingdom); anti-CD11a clone G25.2 (mouse IgG2a) from Becton Dickinson United Kingdom, Oxford, United Kingdom; anti-CD11a, clone mAb2421,22 and anti-CD54 (ICAM-1), clone 15.223,24 (both mouse IgG1 antibodies), gifts from Nancy Hogg, Cancer Research United Kingdom London Research Institute, London, United Kingdom; anti-CD11a, clone 38 (mouse IgG2a); anti-CD18 antibody, clone YFC118.3 (rat IgG2b), and anti-CD25, clone M-A251 (mouse IgG1) from Serotec, Oxford, United Kingdom; anti-CD54 (ICAM-1), clone 84H10 (mouse IgG1), anti-CD50 (ICAM-3), clone HP2/19 (mouse IgG2a); and anti-CD58 (LFA-3), clone AICD58 (mouse IgG2a), from Immunotech (Beckman Coulter United Kingdom, High Wycombe, United Kingdom). All isotype control antibodies were obtained from Serotec.
Antibody-coated latex beads
For each antibody, 80 × 106 surfactant-free sulfate white polystyrene latex 5-μm beads (Interfacial Dynamics, Portland, OR) were washed twice in 10 mL 0.025 M MES (2-[N-Morpholino] ethanesulfonic acid; buffer pH 6.1; Sigma-Aldrich). The beads were centrifuged for 20 minutes at 3000g and resuspended in 1 mL MES buffer in a 15-mL centrifuge tube. The respective antibody (100 μg) was added and incubated overnight at room temperature on a roller, with constant mixing. The beads were then washed twice in 10 mL PBS and resuspended in 1 mL filter-sterilized PBS/1% bovine serum albumin (BSA) (1 g BSA in 100 mL PBS). The antibody-coated beads were stored at 4°C. Flow cytometric analysis was used to verify that the beads were coated with antibody (results not shown).
Conjugate formation and immunofluorescence
Glass multiwell slides (Hendley-Essex, London, United Kingdom) were precoated with poly-L-lysine (Sigma-Aldrich). CD4+ T cells that had been incubated overnight were then washed in serum and glutamine-free RPMI and were resuspended to a final concentration of approximately 5 × 106 cells/mL in RPMI. MS9 cells were used at a concentration of approximately 3 × 106 cells/mL and Jβ2.7 cells at approximately 5 × 106/mL.
For the cyclic peptide inhibition studies where only one half of the cells were treated, each sample of CD4+ PBMCs was divided into 2 halves. One half was stained with CellTracker Blue CMAC, 7-amino-4-chloromethyl-coumarin (CMAC; Cambridge Bioscience, Cambridge, United Kingdom) by incubating 2.5 × 106 cells/mL in a 30-μM CMAC solution for 30 minutes at 37°C, 5% CO2. The cells were washed twice in RPMI/20% FCS, incubated at 37°C, 5% CO2 for a further 30 minutes and washed again before use in cell conjugation experiments. In each experiment, either the CMAC-stained cells or the unstained cells were treated with the cyclic peptides. The frequency of MTOC polarization to the cell-cell junction was quantified only in unstained cells in conjugates formed between a CMAC-stained cell and an unstained cell.
To form cell-cell conjugates, the cells were placed in 5-mL polystyrene round-bottom Falcon tubes (Becton Dickinson United Kingdom) for 5 minutes to allow any large clumps to settle out. Suspended cells were then plated onto the precoated glass multiwell slides and incubated at 37°C for 40 minutes or 1 hour. For experiments involving antibody-coated beads, the cells were further diluted to give a final concentration of 5 × 105 cells/mL and 2.5 × 106/mL of beads (1:5 ratio of cells to beads). Beads and cells were left to conjugate for 1 hour or 2 hours. Samples were fixed with 100% methanol (precooled to -20°C) for 5 minutes, then washed extensively in PBS, blocked in PBS/1% BSA, and processed for immunofluorescence. Primary antibodies were added in the presence of PBS/1% BSA for 40 minutes and washed in PBS/1% BSA.25 Secondary antibodies were added in the presence of PBS/1% BSA for 40 minutes, washed thoroughly in PBS and H2O, then mounted in PBS containing 90% glycerol and 2.5% DABCO (1,4-diazabicyclo[2,2,2]octane; Sigma-Aldrich).
Cyclic peptides
Cyclic peptides cIBR (cyclo-1, 12-Pen-PRGGSVLVTGC) and cLAB.L (cyclo-1,12-Pen-ITDGEATDSGC) were gifts from Dr Teruna J. Siahaan, University of Kansas. The cIBR peptide is derived from the sequence of ICAM-1 and inhibits homotypic T-cell adhesion in vitro by blocking the interaction between ICAM-1 and LFA-1.26 The introduction of a disulphide bond in the cyclic peptide stabilizes the secondary structure by imposing conformational rigidity on the peptide which results in stronger binding to ICAM-1 than that of linear peptides. The cLAB.L peptide is similarly derived from the I-domain of α subunit of LFA-1 and is also able to inhibit ICAM-1/LFA-1–dependent adherence of T cells.27
Control cyclic peptides RD-LBEC (GGLKKVNRLD), RD-IBL (IVKSPSVSTQ), and R2BAL (GRQEGYFLPA) were also a gift from Dr Teruna J. Siahaan. Each control peptide is derived from the reversed amino acid sequence of the respective native protein: RD-LBEC from the β2 subunit of LFA-1, R2BAL from the α subunit of LFA-1, and RD-IBL from ICAM-1.
CD4+ T cells from HTLV-1–infected patients were obtained as described above (under “Patients and cells”) and incubated overnight in Petri dishes. The cells were washed and resuspended at 5 × 105/mL in 5 mL-polystyrene round-bottom Falcon tubes. The cyclic peptides were then added at a final concentration of 200 μM. After 30 minutes excess peptide was washed out, and the cells were resuspended at 5 × 106/mL. The cultures were left for 5 minutes in the 5-mL polystyrene round-bottom Falcon tubes before incubating the cells on microscope slides for 1 to 2 hours at 37°C, to allow the cells to form conjugates.
Analysis of polarization and conjugation
A Laborlux 12 Leitz fluorescent microscope with a BGR filter system and a water-based objective with a numerical aperture of 1.0 (Leitz, Wetzlar, Germany) was used to observe the bead and cell conjugates at room temperature. Only cell-cell conjugates with 2 cells were counted. Cell-bead conjugates were counted where the cell was not in contact with another cell and where there was only one point of contact between the cell and a bead. Conjugates were examined by visually dividing the cell into 5 radial sectors; the MTOC was deemed polarized when it was orientated toward the conjugated bead or cell within the sector in contact with the bead or cell. Thus, a frequency of MTOC polarization of 20% implies random orientation of the microtubules (ie, no significant effect of cell contact etc). In each experiment a minimum of 300 conjugates were counted. An Optronics Magnafire cooled CCD camera (Optronics, Goleta, CA) on the Leitz fluorescence microscope was used to take photographs of conjugates; images were displayed using Magnafire software (Optronics).
Statistical analysis
The frequency of MTOC polarization in HTLV-1–infected and uninfected cells was compared by using the odds ratio (OR). To test whether a given OR differed significantly from 1.0 and to compare 2 different odds ratios, we applied normal theory to the distribution of ln(OR), the natural logarithm of OR.28 To calculate the summary odds ratio for a given effect over a number of experiments, we used the inverse variance method of weighting individual values of ln(OR).28 To calculate the statistical significance of a given effect over different experiments, we used Fisher chi-square method of combining probabilities:
where pi is the significance level obtained from the ith experiment, and k is the number of experiments; -2Σln(pi) is distributed as a chi-square variate with 2k degrees of freedom.29
Results
Cross-linking of certain T-cell surface molecules leads to polarization of the MTOC
To test the ability of individual T-cell surface antigens to polarize the MTOC in uninfected CD4+ T cells, we incubated CD4+ T cells obtained from healthy HTLV-1 seronegative subjects with latex beads coated with antibodies to different T-cell surface molecules. T-cell surface molecules were chosen for study on the basis of their known importance in the formation of an IS or in T-cell signaling or costimulation. The frequency of MTOC polarization induced by the respective antibodies was compared with that induced by a matched isotype control antibody, to control for any nonspecific effects of immunoglobulin in contact with the T cell.
As previously described,6,8,9 beads coated with anti-CD3 antibodies caused efficient polarization of the MTOC toward the bead in CD4+ uninfected control T cells; the odds ratio of MTOC polarization induced by anti-CD3, compared with that induced by an isotype control antibody, was greater than 5 (Table 1). Beads coated with antibodies directed against CD2, LFA-1 (CD11a and CD18), CD28, and CD50 induced MTOC polarization with a frequency greater than 3 times that induced by their respective isotype control antibodies (Table 1); the polarization induced by anti-LFA-1 antibodies was greatest (ie, gave the highest odds ratio when compared with the isotype control). In contrast, relatively infrequent MTOC polarization (odds ratio, 1.0-2.0) was induced in uninfected CD4+ cells by beads coated with antibodies directed against CD54 (ICAM-1), CD58, and CD25 (Table 1); that is, the frequency of polarized cells was close to the random expectation of 20% (see “Patients, materials, and methods”).
Activation of T cells using antibodies cross-linking CD2 requires antibodies of 2 specificities: either 2 anti-CD2 antibodies, one of which is directed against a CD58-binding site and the other directed against an activation-related epitope known as CD2 R30,31 ; or one anti-CD2 and one anti-Ig antibody which cross-links the anti-CD2 antibody.32 For this reason, 2 different antibodies to CD2 were chosen, clones 4B2, CLB-T11/1 and 4B2, CLB-T11.2/1. However, the frequency of MTOC polarization in CD4+ control T cells toward beads coated with either of the 2 anti-CD2 antibodies alone was already high and there was no significant increase in polarization of the MTOC to beads coated with both antibodies (data not shown).
Three different antibodies to CD11a (LFA-1) were used because the LFA-1/ICAM-1 interaction plays an important role in the formation of the immunologic synapse, and because each of the antibodies used has a different action upon binding to LFA-1. The anti-CD11a antibody clone 38 blocks binding of CD11a to ICAM-1. However, this antibody is also a partial agonist; that is, its binding leads to signal transduction in the T cell through LFA-1. The antibody G25.2 cross-links CD11a but does not block or stimulate—that is, has no detected effect on—the function of LFA-1. Mab24 binds only to the active form of LFA-1.22 Each of the 3 anti-CD11a antibodies caused a similar frequency of polarization of the MTOC.
Although all 3 anti–ICAM-1 antibodies used are described as blocking antibodies, more than one antibody was used because ICAM-1 is up-regulated in HTLV-1–infected cells33 and is thought to be important in the initiation of formation of the virologic synapse. However, none of the antibodies used was effective in inducing polarization of the MTOC in the CD4+ T cells from uninfected control subjects. That is, each anti–ICAM-1 antibody induced less-frequent MTOC polarization than that induced by beads coated with antibodies to other cell surface molecules (Table 1). The anti–ICAM-1 antibody 84H10 blocks ligation of ICAM-1 to LFA-1 and reduces syncytium formation in HIV-1–infected cultures of a T-cell leukemic cell line, CEM.34 The anti–ICAM-1 antibodies HA5835 and 15.236 are also known to block ligation with LFA-1. Antibodies to ICAM-1 are not known to cause T-cell activation.
A significant increase in polarization of the MTOC is observed in HTLV-1–infected CD4+ T cells
The results reported in Table 1 showed that monoclonal antibodies against several different T-cell surface molecules each caused MTOC polarization in normal uninfected CD4+ T cells. Igakura et al2 reported a strong association between MTOC polarization and HTLV-1 infection in T cells that make contact with another cell. We, therefore, wanted to test whether engagement of single specific T-cell surface molecules causes a higher frequency of MTOC polarization selectively in HTLV-1–infected T cells. To do this we examined conjugates formed between antibody-coated beads and PBMCs from HTLV-1–infected individuals.
We compared the frequency of polarization of the MTOC toward antibody-coated beads in infected and uninfected cells within the CD4+ PBMC population from HTLV-1–infected individuals. The results of these experiments are summarized in Table 2. There was a strong association between polarization of the MTOC and polarization of Gag p19, regardless of the antibody used. Figure 1 shows an example of polarization induced by a bead coated with anti-CD11a. HTLV-1 infection did not significantly alter the frequency of MTOC polarization induced by antibodies to CD2, CD18, CD50, or CD58 and isotype control antibodies (data not shown). However, cross-linking of each of 3 surface molecules caused a significantly higher frequency of MTOC polarization in HTLV-1–infected cells than in uninfected cells (Table 2): CD54 (ICAM-1), CD25 (IL-2Rα), and CD11a (a constituent chain of LFA-1). This synergistic effect of HTLV-1 infection and antibody cross-linking was both strongest (ie, gave the highest odds ratio) and most statistically significant in the case of cross-linking of ICAM-1. Interestingly, the MTOC polarization induced by cross-linking of either CD3 (T-cell receptor complex) or CD28 (T-cell costimulatory molecule) was in each case significantly and reproducibly reduced in frequency by HTLV-1 infection of the cell (Table 2).
Effect of HTLV-1 infection on microtubule polarization in an HTLV-1–infected T-cell line
In addition to testing the effects of antibody-coated beads on CD4+ T cells from HTLV-1–infected individuals and uninfected control subjects, we examined the effect of such beads on MTOC polarization in 2 human CD4+ T-cell lines: Jurkat cells and MS9 cells, which are continuously infected with HTLV-1.16 The results are shown in Table 3. As in the experiments carried out on freshly isolated CD4+ T cells (data not shown), antibodies directed against CD50, CD58, and clone mAb24 against the CD11a chain of LFA-1 induced a similar frequency of MTOC polarization in the HTLV-1–infected MS9 cells as in the uninfected Jurkat cells. The strongest polarization in MS9 cells versus Jurkat cells was again induced by antibodies against CD25 and ICAM-1. However, the frequency of MTOC polarization induced by beads coated with anti-CD3 was greater than 30-fold lower (OR = 0.02) in the MS9 cells than in Jurkat cells. Similarly, the frequency of polarization induced by anti-CD28 was 6-fold lower (OR = 0.17) in the MS9 cells. These observations confirmed the inhibitory effect of HTLV-1 infection on cytoskeletal polarization induced by anti-CD3 or anti-CD28 that was observed in freshly isolated infected CD4+ T cells (Table 2). MTOC polarization in MS9 cells was also significantly less frequent than in Jurkat cells when mixed with beads coated in antibodies to CD18 or CD28. The anti-CD11a (clones 38 and G25.2)–coated beads induced significantly more frequent MTOC polarization in the HTLV-1–infected MS9 cells than in Jurkat cells; this effect was partly due to the weak polarization that these antibodies induced in the Jurkat cells. The frequency of polarization in freshly isolated uninfected CD4+ T cells with these same anti-CD11a–coated beads was also significantly higher than that seen in Jurkat cells. In summary, these observations confirmed the findings made in freshly isolated CD4+ PBMCs (Table 2); that is, HTLV-1 increased the frequency of MTOC polarization induced by cross-linking of ICAM-1, CD25, or CD11a but inhibited the polarization induced by antibodies to CD3 or CD28.
Polarization of the MTOC and Gag p19 toward an antibody-coated latex bead. The MTOC was polarized toward beads coated with anti-CD11a antibody in HTLV-1–infected and uninfected CD4+ T cells after 1 hour of incubation. Tubulin α is green, stained using Fluorescein (FITC), original magnification × 400. If the MTOC was polarized, then invariably Gag p19 (red, stained with Alexa Fluor 568) was also polarized toward an antibody-coated bead. Similar polarization of both the MTOC and Gag p19 was induced by latex beads coated with either anti-CD2, CD3, CD18 (LFA-1), CD25 (IL-2Rα), CD28, CD50 (ICAM-3), CD54 (ICAM-1), or CD58 (LFA-3).
Polarization of the MTOC and Gag p19 toward an antibody-coated latex bead. The MTOC was polarized toward beads coated with anti-CD11a antibody in HTLV-1–infected and uninfected CD4+ T cells after 1 hour of incubation. Tubulin α is green, stained using Fluorescein (FITC), original magnification × 400. If the MTOC was polarized, then invariably Gag p19 (red, stained with Alexa Fluor 568) was also polarized toward an antibody-coated bead. Similar polarization of both the MTOC and Gag p19 was induced by latex beads coated with either anti-CD2, CD3, CD18 (LFA-1), CD25 (IL-2Rα), CD28, CD50 (ICAM-3), CD54 (ICAM-1), or CD58 (LFA-3).
Cyclic peptides derived from ICAM-1 and LFA-1 abolished the preferential polarization of MTOC to the cell-cell junction in freshly isolated CD4+ T cells
Coculture of antibody-coated beads and CD4+ T cells showed (Table 2) that cross-linking of ICAM-1 leads to frequent polarization of the MTOC. The antibody-coated beads were used to identify which cell surface molecules were involved in the polarization event. However, antibody cross-linking may differ in important respects (eg, in affinity and kinetics) from engagement of ICAM-1 by its physiologic ligand LFA-1 on the opposing cell surface. To test whether the ICAM-1/LFA-1 interaction is indeed important in the MTOC polarization associated with formation of the virologic synapse between an HTLV-1–infected cell and another cell, we used cyclic peptides that are known to block this interaction.26,27 The LFA-1–derived cyclic peptide (cLAB.L) and the ICAM-1–derived peptide (cIBR) were added to freshly isolated CD4+ T cells from HTLV-1–infected HAM/TSP patients to test the effect on polarization of the MTOC in CD4+:CD4+ T-cell conjugates.
As shown previously,2 the odds ratio of MTOC polarization in a Gag p19+ cell, compared with a Gag p19- cell in conjugates formed between CD4+ PBMCs of an HTLV-1–infected subject, typically lies between 3.5 and 4.5. The results of experiments with the cyclic blocking peptides showed (Table 4) that treating the cells with either cLAB.L or cIBR reduced the odds ratio of MTOC polarization to the cell-cell junction in HTLV-1–infected T cells. Treatment of the CD4+ T cells simultaneously with both blocking peptides completely abolished the HTLV-1–associated MTOC polarization (Table 4): the OR of 1.25 was not significantly different from 1.0. To test the specificity of this inhibition, we used 3 different control cyclic peptides (see “Patients, materials, and methods”). When the control peptides RD-LBEC, RD-IBL, or R2BAL were added, in each case the odds ratio of MTOC polarization (2.85, 2.54, and 3.70, respectively) did not differ significantly from that seen in untreated cells (2.83), whereas the blocking peptides cIBR and cLABL, as before, significantly reduced the odds of polarization (ORs, 1.31 and 1.14, respectively). Simultaneous addition of all 3 control peptides similarly had no significant effect on the frequency of MTOC polarization (OR = 2.87).
In a second set of experiments with the cyclic peptides, only half the cells were pretreated with the cyclic peptides, then washed before being allowed to form conjugates with nonpeptide-treated cells. Washing neither removes the bound peptide nor abolishes its effect on cell-cell adhesion, because the peptide-ligand complex is rapidly internalized by the cell.37 One half was stained with a fluorescent dye, CMAC, to distinguish the cells that had been treated with peptide. By examining conjugates formed between stained and unstained cells, we quantified the effect of blocking ICAM-1 or LFA-1 on either an infected cell or an uninfected cell.
The results of experiments with the cyclic blocking peptides showed (Table 5) that treating the cells with cIBR reduced the odds ratio of MTOC polarization to the cell-cell junction in HTLV-1–infected T cells by approximately 2-fold. However, treatment of the CD4+ T cells with cLAB.L, the blocking peptide that mimics LFA-1 and therefore blocks engagement of ICAM-1, completely abolished the HTLV-1–associated MTOC polarization (Table 4): the OR of 1.14 was not significantly different from 1.0.
Preferential polarization of the MTOC in Gag p19+ cells does not occur when conjugation is with a cell lacking LFA-1 expression
A Jurkat cell line selected for the loss of LFA-1 expression (Jβ2.7) was used as a further test of the effect of ICAM-1 cross-linking in an HTLV-1–infected cell on the triggering of polarization of the MTOC. The Jβ2.7 cell line had been transfected either with a vector encoding wild-type LFA-1 (Jβ2.7/wtLFA-1), or a mutant form of LFA-1 that is constitutively expressed in the active (high affinity) form (Jβ2.7/ΔLFA-1), or the empty vector (Jβ2.7/mock).17 The 3 respective cell lines were used to form conjugates with the HTLV-1–infected cell line MS9. The odds ratio of polarization of the MTOC in Gag p19+ (MS9) cells versus Gag p19-(Jβ2.7) cells was lower than that induced in ex vivo CD4+ PBMCs (summary OR of 1.83; Table 6) by the wild-type LFA-1–expressing cell line. The cell line that constitutively expressed the active form of LFA-1 induced a higher frequency of MTOC polarization (summary OR = 2.50; Table 6). However, when there was no LFA-1 expression (Jβ2.7/mock cells), the summary OR was below 1.00, indicating that, without cross-linking of ICAM-1 or other LFA-1 ligands, the polarization of the MTOC was inhibited by HTLV-1 infection. The data in Table 7 confirm that the frequency of polarization was reduced in the infected (MS9) cells in contact with cells that lacked LFA-1 expression.
Discussion
Polarization of the MTOC to the cell-cell junction is associated with formation of both the immunologic synapse3 and the virologic synapse.2 The role of MTOC polarization in the IS appears to be to direct the focal delivery of secreted proteins (lymphokines or lytic granules) to the appropriate antigen-presenting cell (APC), but not to nearby cells.38 In the IS, polarization is triggered by engagement of the TCR5 with the major histocompatibility complex (MHC)/peptide complex on the surface of the antigen-presenting cell. But in the HTLV-1–associated VS, polarization is strongly associated with HTLV-1 infection of the T cell; that is, the MTOC is polarized inside an infected T cell, not toward an infected cell. This observation implies that TCR-mediated recognition of HTLV-1 antigens plays no essential role in VS formation; rather, the VS must be triggered by a combination of 2 signals: HTLV-1 infection and cell contact. The present study was, therefore, designed to test the hypothesis that a ligand-receptor interaction between 2 CD4+ T cells is synergistic with HTLV-1 antigen expression in causing polarization of the infected T cell's MTOC toward the cell-cell junction. To test this hypothesis, we examined the effect of cross-linking individual T-cell surface molecules using monoclonal antibodies coated onto latex beads. Cross-linking by antibody can mimic physiologic stimuli: eg, anti-CD3 antibody delivers an activating signal through the T-cell receptor that mimics physiologic engagement with MHC/peptide complexes.39
The results of the antibody cross-linking experiments showed that cross-linking of several different T-cell surface molecules cause MTOC polarization. However, cross-linking of either of 2 molecules, ICAM-1 (CD54) or CD25, caused significantly more frequent polarization in an HTLV-1–infected cell than an uninfected cell, indicating a synergistic interaction between HTLV-1 infection and cross-linking of the respective surface molecule in triggering the cytoskeletal rearrangement. Similar results were obtained both in PBMCs from several individuals infected with HTLV-1 and in a CD4+ T-cell line (MS9) continuously infected with HTLV-1.
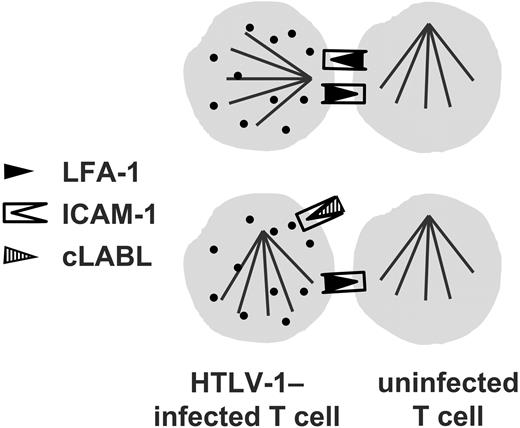
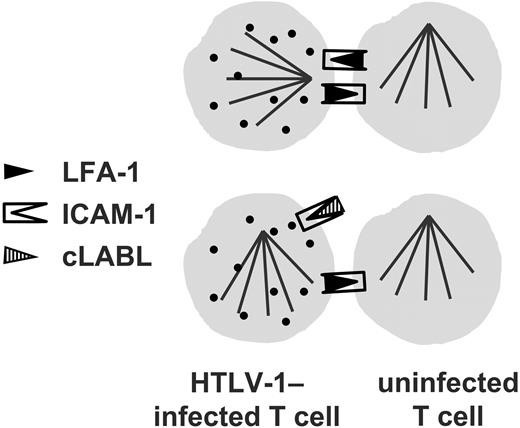
Cross-linking of any molecule that leads to a degree of polarization of MTOC and Gag has the potential to contribute to cell-cell spread of HTLV-1. However, the characteristics of the stimulus delivered by antibody cross-linking—that is, the strength and kinetics of the signal—may differ substantially from the physiologic stimulus. Therefore, any conclusions drawn from antibody-induced cross-linking experiments require corroborative evidence from experiments that involve a physiologic ligand-receptor interaction. To test the importance of the interaction between ICAM-1 and LFA-1 in the cytoskeletal polarization associated with the HTLV-1–induced VS in a more physiologic system than antibody-coated beads, we blocked this interaction in spontaneous conjugates formed between unstimulated CD4+ T cells from HTLV-1–infected individuals. We used cyclic peptides (cIBR and cLABL) derived from either ICAM-1 or LFA-1. Each peptide alone reduced the odds of MTOC polarization in HTLV-1–infected cells; when both peptides were used together with the CD4+ cells, there was no significant difference in the frequency of polarization in infected cells compared with uninfected cells. None of 3 control cyclic peptides significantly altered the frequency of MTOC polarization.
Since T cells express both ICAM-1 and LFA-1, we then wanted to test whether the direction of the interaction was important; that is, whether engagement of ICAM-1 or LFA-1 on the HTLV-1–infected cell was more effective in triggering the observed cytoskeletal polarization. Therefore, in the second set of experiments, only one of the cells within a conjugate was blocked with the cyclic peptides. The MTOC polarization that is strongly associated with the HTLV-1–infected T cell in the VS (Igakura et al2 ; Tables 4, 5) was reduced in frequency by blocking LFA-1 with the ICAM-1–derived blocking peptide, but it was completely abolished by blocking ICAM-1 on an infected cell with the LFA-1–derived blocking peptide (OR not significantly > 1.0). These observations strongly imply that the ICAM-1/LFA-1 interaction indeed plays a necessary part in triggering the polarization of the cytoskeleton that is observed in the HTLV-1–associated VS. As a further independent test of the importance of cross-linking of ICAM-1 in an infected cell on polarization of the MTOC, the LFA-1–knock-out Jurkat cell lines were used. The results of these experiments showed that, in the absence of LFA-1 on a neighboring cell, the MTOC in an uninfected cell was polarized toward the neighboring cell with a higher frequency than in an infected cell. Thus, HTLV-1 infection inhibited cell-contact–induced MTOC polarization in the cell in the absence of ICAM-1 cross-linking. These results corroborate the conclusion that engagement of ICAM-1 on the surface of the HTLV-1–infected cell is particularly effective in triggering microtubule reorientation.
The ICAM-1/LFA-1 interaction is known to play a significant role in the formation of the immunologic synapse3,11 and in reducing the threshold for T-cell activation.40 LFA-1 is expressed chiefly on T cells,41 whereas ICAM-1 is expressed on both T cells and many other nucleated cells.42 Signal transduction from bound LFA-1 on the T cell has been well described.43 But signaling into the T cell from ICAM-1, which has a very short cytoplasmic tail (28 amino acids), is less well understood.44,45 The activated form of LFA-1 binds with high affinity to ICAM-1, which itself is up-regulated by HTLV-1 infection.14 It remains possible that additional molecules on the HTLV-1–infected cell act to recruit or activate LFA-1 on the recipient cell. Intriguingly, there is evidence that the ICAM-1/LFA-1 interaction is also important in the spread of HIV-1 virions46,47 and in HIV-1–induced syncytium formation.48 However, the effects of blocking this interaction on the HIV-1 virologic synapse49 have not yet been reported.
Blocking ICAM-1 on the HTLV-1–infected cell abolishes the preferential microtubule polarization induced by cell contact in an infected cell.
Blocking ICAM-1 on the HTLV-1–infected cell abolishes the preferential microtubule polarization induced by cell contact in an infected cell.
The effects on the cytoskeleton of cross-linking CD25 on the T-cell surface have not previously been reported. Since the ligand of CD25 (IL-2) is soluble and is not presented to the T cell bound to the surface of another cell, like the related cytokine IL-15,50 the significance of the effect of CD25 cross-linking on cytoskeletal polarization in HTLV-1–infected T cells was at first unclear. However, it has been shown51 that CD25 and ICAM-1 are physically associated in the plasma membrane; eg, they can be coimmunoprecipitated directly from solubilized cell membranes. Two inferences may be drawn: first, this observation corroborates the conclusion that ICAM-1 cross-linking is synergistic with HTLV-1 infection in inducing cytoskeletal polarization in the T cell. Second, it is likely that the effects of cross-linking either CD25 or ICAM-1 are mediated by a common pathway.
The transcriptional transactivator protein of HTLV-1, Tax, up-regulates expression of both ICAM-114 and CD25 (Figure 2).13,15 Furthermore, Yamamoto et al33 obtained evidence that cross-linking of ICAM-1 on the cell surface up-regulates HTLV1 gene expression. As previously suggested,2 this may constitute a positive feedback loop that increases the efficiency of cell-cell spread of HTLV-1.
In contrast to cross-linking with antibodies to ICAM-1 and CD25, cytoskeletal polarization induced by antibodies to either CD3 or CD28 was significantly inhibited by HTLV-1 infection; again, this effect was seen both in PBMCs and in the infected T-cell line, MS9. The effect of this inhibition may be to reduce the frequency of reinfection of a T cell that is already infected with HTLV-1.
We conclude that the interaction between LFA-1 and ICAM-1 plays an important part in the polarization of the T cell's cytoskeleton that is associated with the HTLV-1–induced virologic synapse. Engagement of either LFA-1 or ICAM-1 on an infected T cell caused strong polarization of the MTOC toward the cross-linked molecules. In particular, ICAM-1 engagement appeared to be synergistic with HTLV-1 infection in causing a higher frequency of cytoskeletal polarization. Since the physiologic ligand of ICAM-1 is LFA-1, which is expressed mainly on T cells, this synergistic interaction may contribute to the T-cell tropism of HTLV-1 observed in vivo.
Prepublished online as Blood First Edition Paper, April 14, 2005; DOI 10.1182/blood-2004-07-2850.
Supported by the Wellcome Trust, United Kingdom, and the Leukaemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Teruna Siahaan from the University of Kansas for providing the cyclic peptides, Dr David Derse for the MS9 cells, and Dr Caterina Hioe for the Jβ2.7 cells. We thank the staff and patients of St Mary's Hospital for donations of blood. We also thank Dr Keith Gould, Dr Angelina Mosley, and Dr Sara Marshall for helpful comments on the manuscript.