Abstract
Although improvement in outcomes has occurred in younger adults with acute myeloid leukemia (AML) during the past 4 decades, progress in older adults has been much less conspicuous, if at all. Approximately 50% to 75% of adults with AML achieve complete remission (CR) with cytarabine and an anthracycline such as daunorubicin or idarubicin or the anthracenedione mitoxantrone. However, only approximately 20% to 30% of the patients enjoy long-term disease survival. Various postremission strategies have been explored to eliminate minimal residual disease. The optimal dose, schedule, and number of cycles of postremission chemotherapy for most patients are not known. A variety of prognostic factors can predict outcome and include the karyotype of the leukemic cells and the presence of transmembrane transporter proteins, which extrude certain chemotherapy agents from the cell and confer multidrug resistance and mutations in or over expressions of specific genes such as WT1, CEBPA, BAX and the ratio of BCL2 to BAX, BAALC, EVI1, KIT, and FLT3. Most recently, insights into the molecular pathogenesis of AML have led to the development of more specific targeted agents and have ushered in an exciting new era of antileukemia therapy. Such agents include the immunoconjugate gemtuzumab ozogamicin, multidrug resistance inhibitors, farnesyl transferase inhibitors, histone deacetylase and proteosome inhibitors, antiangiogenesis agents, Fms-like tyrosine kinase 3 (FLT3) inhibitors, and apoptosis inhibitors.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of diseases characterized by uncontrolled proliferation of clonal neoplastic hematopoietic precursor cells and impaired production of normal hematopoiesis leading to neutropenia, anemia, and thrombocytopenia.1 If untreated, patients die of infection or bleeding usually in a matter of weeks. Some older adults may have a slower progressive clinical course. An estimated 10 600 new cases occurred in the United States in 2002 and 7400 patients died of the disease.2 The overall incidence is 3.4 cases per 100 000 population; 1.2 cases per 100 000 population at age 30 and more than 20 cases per 100 000 population at age 80 years.2 The median age is 20 years and has been increasing over the past decade.
Historically, the diagnosis and response to therapy were established from morphology and cytochemistry. Although morphology remains the initial diagnostic tool for any patient with acute leukemia, the past decade has witnessed increased reliance on cell-surface antigen expression by immunophenotyping, usually carried out by flow cytometry, as well as cytogenetic and molecular markers.
During the past 4 decades, many studies have investigated a wide variety of cytotoxic antileukemic agents. Most recently, insights into the molecular pathogenesis of AML have led to the development of the more specific targeted therapy. This review focuses on current and evolving drug therapy in the treatment of adults with AML.
Current treatment results
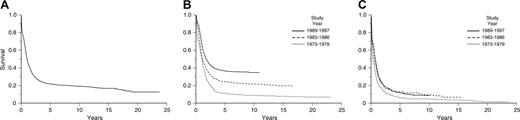
Approximately 50% to 75% of adults with AML achieve complete remission (CR) with the deoxycytidine analog cytarabine and an anthracycline antibiotic, such as daunorubicin or idarubicin, or the anthracenedione mitoxantrone, which inhibit the enzyme topoisomerase IIa. However, only 20% to 30% of patients enjoy long-term disease-free survival (DFS). The majority of patients die of their disease, primarily because of persistent or relapsed AML. In an Eastern Cooperative Oncology Group (ECOG) analysis of the outcome of approximately 3000 patients with previously untreated AML entered on 5 successive clinical trials with cytarabine and daunorubicin for induction and with increasingly more intensive postremission therapy, 62% achieved CR, but 76% relapsed or died.3,4 The 5-year overall survival (OS) rate among 2000 patients younger than 55 years has improved from 11% in the 1970s to 37% in the 1990s. (Figure 1A-C). In contrast, among 1000 patients age 55 years and older, progress over the past 3 decades has been very modest.5
Prognostic factors
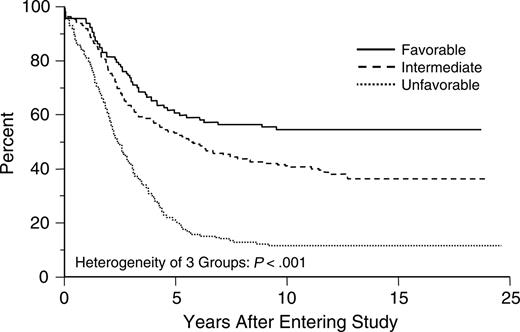
The outcome for adults with AML depends on a variety of factors, including age of the patient, intensity of postremission therapy, and biologic characteristics of the disease, the most important of which are the cytogenetics at presentation.4,6-8 The karyotype of the leukemic cells can distinguish 3 groups with either favorable, intermediate, or poor prognostic risk6-8 (Figure 2). Other factors include the presence of transmembrane transporter proteins, which extrude certain chemotherapy agents from the cell and confer multidrug resistance,9 and mutations in or overexpression of specific genes such as WT1,10,11 CEBPA,12 BAX and the ratio of BCL2 to BAX,13 BAALC,14 EVI1,15 KIT,16 and FLT3.17,18
Estimates of overall survival. (A) Kaplan-Meier product-limit estimate of overall survival for patients with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. Reprinted with permission.5 (B) Kaplan-Meier product-limit estimate of overall survival for younger patients (ages ≤ 55 years) with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. Reprinted with permission.5 (C) Kaplan-Meier product-limit estimate of overall survival for older patients (ages > 55 years) with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. From Appelbaum et al.5 Copyright American Society of Hematology, used with permission.
Estimates of overall survival. (A) Kaplan-Meier product-limit estimate of overall survival for patients with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. Reprinted with permission.5 (B) Kaplan-Meier product-limit estimate of overall survival for younger patients (ages ≤ 55 years) with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. Reprinted with permission.5 (C) Kaplan-Meier product-limit estimate of overall survival for older patients (ages > 55 years) with newly diagnosed acute myeloid leukemia treated on ECOG protocols between 1973 and 1997. From Appelbaum et al.5 Copyright American Society of Hematology, used with permission.
Induction chemotherapy
Development of a standard regimen
During the past 35 years, a series of studies has established an induction regimen that has become a standard of care for patients not participating on a clinical trial. A widely used combination for induction is the cell cycle–specific agent cytarabine 100 mg/m2 by continuous infusion for 7 days and the non–cell-cycle–specific anthracycline antibiotic daunorubicin 45 to 60 mg/m2/d intravenously for 3 days.19-21 To improve the CR rate, studies have tested alternative and higher doses of anthracyclines or the anthracenediones,22-33 higher doses of cytarabine,34-37 new agents combined with cytarabine and daunorubicin such as etoposide, the purine analog fludarabine or the camptothecin topotecan,38-40 or sequential standard therapy followed by high doses of cytarabine.41-44 Despite theoretic advantages,45 none of these approaches is definitively better than the standard regimen (Table 1).
Hematopoietic growth factors
Multiple studies have established the safety of hematopoietic growth factors when administered in induction and consolidation. Growth factors have a role in the supportive care of AML when given after induction therapy to reduce the period of neutropenia.46,47 Recently, there has been renewed interest in the use of growth factors as priming agents, to move leukemia cells into a phase of the cell cycle, which might render them more susceptible to cytotoxic chemotherapy. However, benefits of such a strategy have not been definitively established. One large study using granulocyte colony-stimulating factor (G-CSF) showed no effect on CR rate but an improvement in DFS.48 Another study, also using G-CSF, reported the opposite, an improvement in CR rate but no effect on DFS.47 A study with granulocyte-macrophage colony-stimulating factor (GM-CSF) showed no effect on either CR rate or DFS.29
Estimated distributions of overall survival by cytogenetic risk status. CI indicates confidence interval. From Slovak et al.8 Copyright American Society of Hematology, used with permission.
Estimated distributions of overall survival by cytogenetic risk status. CI indicates confidence interval. From Slovak et al.8 Copyright American Society of Hematology, used with permission.
Postremission therapy
Various strategies have been explored to eliminate minimal residual disease not apparent in the bone marrow of patients in CR which could contribute to relapse. Such strategies have included intensive consolidation therapy, high-dose chemotherapy, or chemoradiotherapy with either allogeneic or autologous hematopoietic stem-cell transplantation (HSCT), or low-dose maintenance therapy.
Intensive consolidation chemotherapy
Retrospective analyses of cooperative group studies and a prospective randomized trial show that increasing the intensity of postremission therapy is beneficial in younger but not older adults.3,4,49 Several studies have evaluated the role of intensive postremission consolidation with high-dose (3 gm/m2/dose) cytarabine (HiDAC). A prospective study by the Cancer and Leukemia Group B (CALGB) demonstrated that 4 courses of HiDAC are significantly better than 4 courses of intermediate- (400 mg/m2/dose) or standard-dose cytarabine (100 mg/m2/dose), confirming a dose-response effect in younger patients and a benefit in patients with good-risk cytogenetics.50 Cerebellar dysfunction, particularly in older adults and in those with hepatic or renal dysfunction, is an important toxicity.
General conclusions about postremission consolidation chemotherapy
Although postremission therapy is a sine qua non for curing AML, fundamental issues remain unresolved (Table 1). The optimal dose, schedule, and number of cycles of consolidation chemotherapy for most patients with AML who achieve CR have not been established. In younger patients, cycles of intensive consolidation chemotherapy, often with, but not limited to, high-doses of cytarabine, prolong DFS and OS.
Prospective studies of intensive postremission chemotherapy, allogeneic hematopoietic stem-cell transplantation, and autologous hematopoietic stem-cell transplantation
Hematopoietic stem-cell transplantation refers to the administration of very intensive chemotherapy with or without radiation and infusion of previously collected hematopoietic stem cells harvested from either the patient (autologous), or a human leukocyte antigen (HLA)–matched donor (allogeneic). Autologous HSCT is limited by the lack of the immunologic reaction referred to as graft-versus-leukemia (GVL) effect present in patients undergoing allogeneic HSCT, in which the donated allogeneic cells recognize the recipient's leukemic cells as foreign. Furthermore, there is a theoretic risk of infusion of occult residual leukemic cells.51 To decrease toxicities associated with such intensive doses of chemotherapy, lower doses have been explored, relying more on the immunologically mediated GVL effect to eradicate the disease.52 Although the benefits of this strategy of nonmyeloablative HSCT is currently under investigation,52,53 its ultimate role may be in older adults in first CR who are unable to tolerate standard conditioning regimens. Whether such a regimen can reduce the toxicity associated with matched unrelated donor HSCT is currently being explored.54 A potential limitation is the 3 to 9 months required for the immunologic GVL effect to develop in the presence of rapidly proliferating leukemia cells.
Allogeneic HSCT for patients with AML in first CR is associated with the lowest relapse rate and provides the best antileukemic potential, but it is associated consistently with a higher risk of treatment-related mortality than either autologous HSCT or consolidation chemotherapy.55-58 As a result, the benefit of a lower relapse rate is offset by a higher treatment-related mortality. The decision to recommend allogeneic transplantation in first CR must be made on a patient-by-patient basis, evaluating the risks and patient age, type of donor match, and disease prognosis. However, it is reasonable to consider such a strategy in patients with poor-risk cytogenetics and some younger patients with intermediate-risk cytogenetics who are otherwise suitable candidates.
Current HSCT techniques have changed significantly, and the transplant-related mortality with contemporary autologous HSCT is 0% to 3% compared with 14% to 18% in published studies, a fact that, historically, has obscured the potential benefit of the lower relapse rate. Furthermore, current understanding of the pathophysiology of AML mandates that therapeutic decisions be made, in part, on cytogenetic and molecular prognostic factors at diagnosis. This has led to subgroup analyses of small patient cohorts that limit confidence in the results.
Maintenance therapy
Treatment for acute promyelocytic leukemia
Acute promyelocytic leukemia (APL) is treated differently from all other subtypes of AML and has become the most curable in adults. This subtype is associated with the t(15;17) translocation which results in a fusion transcript, the promyelocytic leukemia–retinoic acid receptor α (PML-RARα), derived from the juxtaposition of the PML gene on chromosome 15 and the retinoic acid receptor α gene on chromosome 17.61 The vitamin A derivative, all-trans retinoic acid (ATRA), has the remarkable ability to induce differentiation of leukemic promyelocytes in patients with APL, resulting in high CR rates.62-67 Therefore, APL is the first subtype of AML treated with an agent targeted to a specific genetic mutation. The benefits of ATRA in APL were identified as part of Chinese herbal medicine treatments rather than as a conscious effort to target the molecular abnormality in APL. Two prospective randomized trials demonstrate that ATRA with chemotherapy significantly improves DFS and OS, and approximately 70% of patients are cured of APL.65-67 The concepts that cytarabine may not be required in the treatment of a subtype of AML and that maintenance appears advantageous represent departures from conventional management.65-69
Treatment of older adults
The treatment of older adults (ages ≥ 55-60 years) with AML is very disappointing with modest, if any, improvement in OS over the past 4 decades.5,70 Older patients do not tolerate intensive chemotherapy as well as younger patients, particularly if they have preexisting comorbidities.71 In addition, older adults frequently have leukemia cells with poor-prognosis karyotypes9 and frequently express the multidrug resistance marker P-glycoprotein, rendering their cells more resistant to chemotherapy.9 It is possible that multidrug resistance marker expression identifies an immature stem-cell–like leukemia. Historic studies using attenuated doses of chemotherapy or delaying intensive therapy until disease progression led to poor results.72,73 Contemporary studies in older patients have established that approximately half can achieve CR.29,31 The major problem is that older adults have a high rate of relapse, and OS rates are less than 10%.5 No clearly effective postremission therapy has been established. Considering that AML occurs much more frequently in older patients,2 this represents the most important challenge in drug therapy for AML. The discouraging overall outcome in older adults has spurred major efforts in the development of new targeted drug therapies.
Treatment of patients with relapsed or refractory AML
The treatment of adults with relapsed or refractory AML is also unsatisfactory. The most important factor predicting response to reinduction chemotherapy appears to be the duration of first CR.74 High-dose cytarabine has been an effective reinduction regimen.75 However, the addition of other agents to HiDAC does not improve outcome.75 Allogeneic HSCT may provide the highest likelihood of cure, presumably related to the generation of an immunologically mediated graft-versus leukemia reaction not present with salvage chemotherapy, preferably after induction of a second CR.76-79 However, there is a lack of prospective data to definitively demonstrate a superior outcome with such a strategy. If a suitable histocompatible sibling donor is not available, transplantation from an alternative donor such as a matched unrelated donor80 or a haploidentical stem-cell donor81,82 can be considered. However, given the risks of reactivation of cytomegalovirus (CMV) and other opportunistic infections, this strategy is still investigational. A small number of patients in second CR also may be cured with autologous HSCT.83 Standard chemotherapy alone is rarely curative in patients who have relapsed. Most of these patients, as well as older adults who have not relapsed but who have a poor prognosis, are candidates for novel investigational approaches (Table 2). Much of the search for new agents focuses on biologically targeted strategies that may be effective without the severe myeloablative toxicity that, historically, has been the hallmark of drug therapy for AML.
Insights into novel therapy based on genetic mechanisms of disease
There is more known about the genetic basis of AML than perhaps any other human cancer. More than 100 different mutations and/or gene rearrangements have been identified in AML. Because AML is a sporadic disease, only rarely occurring as a heritable trait, many of the initial insights were derived from cloning of acquired recurring chromosomal translocations from bone marrow of affected individuals. More recently, a spectrum of subcytogenetic point mutations and gene rearrangements have been identified through characterization of candidate loci, and it is likely that many more will be identified.84,85
Mutations that activate signal transduction cascades in AML
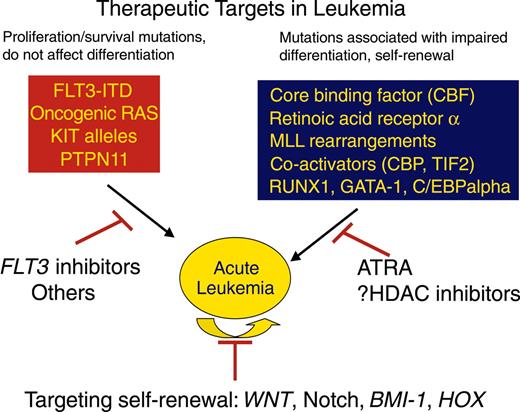
From a therapeutic perspective, these mutations can be subdivided into several subgroups. For example, a majority of patients with AML have mutations that result in constitutive activation of signal transduction pathways that confer proliferative and/or survival advantage to hematopoietic progenitors (Table 3). These include mutations that constitutively activate receptor tyrosine kinases, such as FLT3 or C-KIT receptor tyrosine kinase (C-KIT), oncogenic mutations in RAS, or activating mutations in protein tyrosine phosphatases such as PTPN11 (SHP2). These appear to segregate as a complementation group, in that only very rarely are any 2 of these observed in the same patients with AML. Thus, as discussed under “New agents directed at molecular and other specific targets,” small molecule inhibitors of these pathways such as FLT3 or C-KIT inhibitors, or inhibitors of RAS through farnesyl transferase inhibition, are attractive as potential therapeutic agents. These mutations collectively account for approximately 50% of patients with AML, but it is plausible that the high-throughput genomic approaches to gene discovery will ultimately identify activating mutations in signal transduction pathways in all patients with AML. In addition, the high frequency of mutations in signal transduction pathways suggests that small molecule inhibitors of common downstream effectors shared by all mutations may also be effective (eg, MEK inhibitors to impair oncogenic RAS signals, or mTOR with rapamycin as a target for PI3K/AKT activation86 ). Such agents could be used alone or in combination with inhibitors of upstream effectors such as FLT3.
Mutations in genes that result in dysregulated gene transcription
A second broad complementation group of mutations and gene rearrangements is defined by loss-of-function mutation in transcription factors and transcriptional coactivators that are important for normal hematopoietic development. These mutations also rarely if ever are present in the same patient with leukemia, appear to confer the property of impaired hematopoietic differentiation to leukemic blasts, and may contribute to self-renewal potential, a hallmark of all human cancers. Novel therapeutic approaches have focused on compounds that might override the block in differentiation, as exemplified by the paradigm of ATRA therapy for APL. The mechanistic basis for therapeutic efficacy of ATRA in APL is complex, but the phenotypic consequence of ATRA therapy is induction of terminal differentiation and apoptosis of leukemic blasts. A key component of ATRA activity is release of the nuclear corepressor complex, containing histone deacetylase (HDAC), that is aberrantly recruited to promoters by the PML-RARα fusion.87 Aberrant recruitment of nuclear corepressor complexes by leukemogenic fusion proteins appears to be a central theme in AML. For example, RUNX1-ETO and CBFβ-MYH11 associated with t(8;21) and inv(16), respectively, aberrantly recruit the nuclear corepressor complex to RUNX1 target genes.88-90 Thus, development of small molecule inhibitors of HDAC may be of therapeutic value in induction of differentiation and might be used in combination with agents that target signal transduction pathways. Other loss-of-function mutations that impair hematopoietic differentiation and are potential targets for therapy are listed in Table 3 and may involve the hematopoietic transcription factors RUNX1, GATA-1, CEBPA, and PU.1, as well as mutations that affect components of the transcriptional apparatus itself, such as MLL fusions and fusions involving transcriptional coactivators. In addition, there is evidence for overexpression of certain transcription factors such as EVI1 in AML as a consequence of the t(3;21) translocation,91,92 or through as yet unidentified trans- or cis-acting mutations. Overexpression of EVI1 also results in a myelodysplastic phenotype in murine models of disease, suggesting that it may be a viable target for therapeutic intervention.93
Finally, there are several mutations that occur in genes that encode proteins involved in nuclear cytoplasmic shuttling. These include direct involvement of components of the nuclear pore, such as the NUP98 gene that is fused through chromosomal translocation to a spectrum of partners, including homeobox-containing (HOX) family members,94 and the NUP214 gene that is fused to DEK oncogene (DEK) in patients with t(6;9) AML. Although the fusion partners must play an important role in disease pathogenesis, it also seems likely that the frequent selection for fusions containing components of the nuclear pore in AML must also connote a functional relevance for the nucleoprorins themselves. Most recently, it has been reported that nucleophosmin (NPMI), a nucleolar protein that is involved in nucleocytoplasmic shuttling, is mutated and mislocalized to the cytoplasm of AML cells in approximately 35% of patients.95 It is not known what the functional consequence of this observation is, but it again suggests that dysregulation of the normal nuclear to cytoplasmic trafficking machinery may be important in the pathogenesis of AML.
Attention has turned to the self-renewal potential of leukemic cells as a therapeutic target. A small population of blasts has been identified in human AML that has the properties of leukemic stem cells96 and is thought to be responsible for continuous growth and propagation of leukemia blasts and for relapse of disease in patients after remission induction with intensive chemotherapy. Several genes and pathways have been identified that are important for this critical property of self-renewal, including the WNT/β-catenin pathway, Notch, Bmi-1, and HOX family members.97-101 Although it is not yet certain how these pathways might be effectively targeted therapeutically, or whether it will be possible to target these pathways without excessive toxicities, these are ultimately the most important target cells to ablate (Figure 3).
New agents directed at molecular and other specific targets
Among the most exciting developments has been the remarkable ability to classify subtypes of AML by characterizing the expression profiles of all known genes in the human genome using microarray technology.102-105 The expression profile of clusters of genes will be useful not only to classify patients but also to identify specific molecular targets for therapeutic intervention. Currently, cytogenetics at presentation provides the most important prognostic information in AML. However, 70% of patients are in the intermediate-risk category and, of these, about 70% have a normal karyotype. Therefore, the most common karyotype in AML, normal, is a large, heterogeneous and poorly defined population. Expression profiling has already refined the diagnostic classification of leukemias and holds promise for individualized approaches to therapy.
Some targets to which therapy has already been directed include cell-surface antigens such as CD33, a glycoprotein expressed on most AML blast cells and not on normal hematopoietic stem cells; P-glycoprotein, a mediator of multidrug resistance which acts as an efflux pump to extrude chemotherapy from the cell; enzymes involved in signal transduction such as farnesyl transferase; vascular endothelial growth factor and other angiogenic proteins; enzymes that contribute to the enhanced proliferative and survival capacity of leukemia blasts, such as FLT3 or RAS; enzymes that contribute to impaired hematopoietic differentiation, such as HDAC inhibitors, and the antiapoptotic gene BCL-2. While these new strategies have not yet translated into the kind of clinically meaningful advances as has ATRA in APL, they represent a first wave of targeted therapies in AML and provide a platform for development of more efficacious targeted therapies. In addition, it is likely that none of these will be curative as single agents in treatment of this complex disease, but rather they will need to be used in combination with conventional therapy (as is ATRA) or with other molecularly targeted agents.
Two broad classes of mutations are associated with acute leukemia. One class of mutations, exemplified by activating mutations in tyrosine kinases such as BCR/ABL, FLT3, TEL/PDGFBR, or oncogenic RAS mutations result in enhanced proliferative and survival advantage for cells. These mutations can be targeted by small molecule inhibitors of the respective tyrosine kinases or potentially by farnesyl transferase inhibitors. A second class of mutations is loss-of-function mutations in hematopoietic transcription factors, as exemplified by the acute myeloid leukemia 1 (AML1)/ETO or PML/RARα gene rearrangements, or point mutations in AML1 or C/EBPα. Treatment that targets this class of mutations can include agents that specifically induce differentiation and apoptosis of leukemic cells, as demonstrated by the use of ATRA in PML/RARα-positive acute promyelocytic leukemia and potentially by HDAC inhibitors. Finally, although not known to be mutant in leukemia, genes and pathways that are responsible for the self-renewal potential of leukemia stem cells, such as WNT, Notch, BMI-1, or HOX family members may also be candidates for molecularly targeted therapy.
Two broad classes of mutations are associated with acute leukemia. One class of mutations, exemplified by activating mutations in tyrosine kinases such as BCR/ABL, FLT3, TEL/PDGFBR, or oncogenic RAS mutations result in enhanced proliferative and survival advantage for cells. These mutations can be targeted by small molecule inhibitors of the respective tyrosine kinases or potentially by farnesyl transferase inhibitors. A second class of mutations is loss-of-function mutations in hematopoietic transcription factors, as exemplified by the acute myeloid leukemia 1 (AML1)/ETO or PML/RARα gene rearrangements, or point mutations in AML1 or C/EBPα. Treatment that targets this class of mutations can include agents that specifically induce differentiation and apoptosis of leukemic cells, as demonstrated by the use of ATRA in PML/RARα-positive acute promyelocytic leukemia and potentially by HDAC inhibitors. Finally, although not known to be mutant in leukemia, genes and pathways that are responsible for the self-renewal potential of leukemia stem cells, such as WNT, Notch, BMI-1, or HOX family members may also be candidates for molecularly targeted therapy.
Gemtuzumab ozogamicin
The first of these new targeted agents to be approved by the US Food and Drug Administration is gemtuzumab ozogamicin. This agent is an immunoconjugate of an anti-CD33 antibody chemically linked to a potent cytotoxic agent, calicheamicin.106 Complete remission by conventional criteria is achieved in approximately 15% of patients with AML in first relapse.107,108 Occasionally, patients have developed a veno-occlusive diseaselike syndrome, and caution is indicated for those patients proceeding to transplantation. Two recent studies suggest that gemtuzumab ozogamicin may be associated with a higher CR rate when administered with intensive chemotherapy, and major cooperative groups are conducting phase-3 studies that incorporate gemtuzumab ozogamicin as part of standard chemotherapy in patients newly diagnosed with AML.109,110 If randomized clinical trials confirm these findings, the standard of care for induction in AML will change for the first time in 30 years. Attempts to offer gemtuzumab ozogamicin to older adults as initial induction therapy have not been encouraging.111,112 Data suggest that the effects of gemtuzumab ozogamicin may be potentiated by the use of a P-glycoprotein inhibitor.113
Multidrug resistance inhibitors
Although several agents inhibit P-glycoprotein, with the exception of a Southwest Oncology Group (SWOG) trial testing cyclosporine given with cytarabine and infusional daunorubicin,114 randomized trials of MDR modulators such as PSC-833 have not shown benefit.115,116 It is also possible that expression of multidrug resistance simply identifies an immature stem-cell–like leukemia. More potent modulators such as zosuquidar are currently being studied.117
Farnesyl transferase inhibitors
Mutations and dysregulation of Ras have been associated with the development of myeloid leukemias.118 Farnesyl transferase inhibitors (FTIs) interfere with RAS signaling by precluding farnesylation of RAS and transfer to the plasma membrane.119 These agents have activity in refractory AML120 and are modestly active in patients newly diagnosed with AML.121 Responses do not correlate with mutational status of Ras, which suggests that activation of native RAS by upstream effectors (such as mutant FLT3) might also be of therapeutic value. However, in some cases response does not even correlate with the degree of FT inhibition, suggesting that there may be other important targets. Preliminary data reporting a CR rate of 21% in 92 patients newly diagnosed has led to a current major intergroup trial evaluation of the FTI tipifarnib (Zarnestra) in older adults who are unable to tolerate conventional chemotherapy.
Histone deacetylase and proteosome inhibitors
Aberrant recruitment of the nuclear corepressor complex by leukemogenic fusion proteins is a recurring theme in AML and may result both in modification of chromatin structure at critical hematopoietic promoters, as well as aberrant acetylation of proteins that regulate cell-cycle progression and other functions.122 HDAC inhibitors induce differentiation of malignant cells and are under active investigation in AML.123,124
The proteosome inhibitor bortezomib, a newly approved drug for multiple myeloma, has demonstrated preclinical synergistic activity with the histone deacetylase inhibitors, as well as potential single-agent activity in leukemia.125,126 Clinical efficacy in AML is intriguing, although as yet, unproven.
Antiangiogenesis agents
The potential role for antiangiogenesis therapy in AML is suggested by evidence that bone marrow biopsies for patients with AML demonstrate increased microvessel density that is associated with a poor prognosis.127 Furthermore, vascular endothelial growth factor (VEGF) plays a role in stimulating growth and proliferation of leukemic cells, and an increased endogenous level of VEGF is also associated with a poor prognosis.128 Receptor tyrosine kinase inhibitors of VEGF are another strategy under active study.129 Preliminary data suggest that SU5416, a small molecule inhibitor of phosphorylation of VEGF receptors 1 and 2, C-KIT, the stem cell factor (SCF) receptor and FLT3, has activity in AML.130 Bevacizumab, an anti-VEGF antibody, has been demonstrated to be safe in AML and is currently undergoing phase-2 studies.131
FLT3 inhibitors
Mutations that constitutively activate the FLT3 receptor tyrosine kinase occur in approximately 30% of AML and confer a poor prognosis.17 These observations have fueled the development of FLT3-selective–targeted tyrosine kinase inhibitors with in vitro cytotoxicity to leukemia cells.132-134 There are 4 FLT3 inhibitors that are currently in clinical trials, including PKC-412 (Novartis, Basel, Switzerland), CEP-701 (Cephalon, Frazer, PA), MLN518 (Millennium, San Francisco, CA), and SU11248 (SuGen, New York, NY).133 Although data are still preliminary, several generalizations have emerged. The compounds, which like imatinib mesylate are selective but not completely specific, are well tolerated at doses that achieve inhibition of the target FLT3. Each of the inhibitors have activity in patients with relapsed AML with activating mutations, although responses have been quite modest and characterized by transient reduction in peripheral blood blasts, less frequent reduction in bone marrow blasts, and rare hematologic responses. In some cases, patients that lack ITD or activation loop mutations have responded to FLT3 inhibitors, although mutations that activate FLT3 outside of these regions have been identified and may explain response in this subset. These data are perhaps not surprising, in that it would not be expected that a kinase inhibitor as a single agent in this setting would be any more effective than imatinib mesylate in patients experiencing chronic myeloid leukemia (CML) blast crisis. Current clinical trials are focusing on use of FLT3 inhibitors in combination with chemotherapy.
Activating alleles of KIT occur in approximately 5% of patients with AML, but in contrast with activating juxtamembrane deletions observed in gastrointestinal stromal-cell tumors that are imatinib mesylate sensitive, the most common KIT alleles in AML include KIT D816V or KIT D816Y that are resistant to imatinib mesylate. It has recently been reported that PKC-412, however, is a potent inhibitor of these mutant KIT proteins16 and may have clinical therapeutic value for these patients with AML, as well as in systemic mast-cell disease associated with KIT D816V or KIT D816V.
Apoptosis inhibitors
Overexpression of the apoptosis inhibitor protein bcl-2 can render tumor cells resistant to induction of apoptosis by drug therapy. A high level of expression of BCL-2 in AML is associated with a poor prognosis.135 Down-regulation of BCL-2 by antisense oligonucleotides in vitro sensitizes leukemic cells to chemotherapy in AML cell lines.136 A phase-1 trial of bcl-2 antisense oligonucleotide (GNS, oblimersen sodium) showed a response in 8 of 20 patients with relapsed or refractory AML.137 In a subsequent phase-1 trial in untreated older adults, to determine feasibility, the bcl-2 antisense oligonucleotide was administered with chemotherapy.138 Ten (45%) of 22 patients achieved CR without unexpected toxicities. A randomized phase-3 study by the CALGB is now evaluating the role of the BCL-2 antisense oligonucleotide both in induction and in consolidation.
Summary and future directions
Consistent incremental progress has occurred in younger adults with AML because increasingly intensive chemotherapy can be administered to patients whose cells are relatively sensitive. Older adults usually cannot tolerate such intensive chemotherapy, and their leukemic cells are inherently more resistant. Many new antileukemic agents with novel mechanisms of action and direction are available to explore.
Several of the current new agents being investigated are likely to have a role in the future therapy for AML. However, diverse drug-resistance mechanisms are a prominent feature of AML, and none alone appear likely to alter the standard of care and have an effect comparable to that of ATRA in APL. Combinations of several agents, targeting more than one gene mutation or antigenic determinant, may hold greater promise. The future also rests with new techniques such as cDNA microarray.102-105,139 and genome-wide approaches to gene discovery, in combination with high-throughput screens for modulators of validated targets. These technologies will likely provide important insights into the molecular pathogenesis of AML and identify critical genes that may be targeted.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2005-01-0178.
We thank Dr Charles Schiffer for his review of the manuscript and Ms Kristen Burton for her assistance with manuscript preparation.