Abstract
A somatic mutation in the JH2 autoinhibitory domain of the Janus kinase 2 (JAK2) tyrosine kinase was recently described in polycythemia vera, essential thrombocythemia, and myelofibrosis with myeloid metaplasia. The prevalence of this mutation in either “atypical” myeloproliferative disorders (MPDs) or the myelodysplastic syndromes (MDSs) is unknown. Bone marrow–derived genomic DNA from 245 patients—119 with chronic myelomonocytic leukemia (CMML), 101 with MDS, 11 with hypereosinophilic syndrome (HES), 8 with systemic mastocytosis (SM), and 6 with chronic neutrophilic leukemia (CNL)—was screened for the JAK2 V617F mutation. A mutant allele was detected in 11 patients: 3 with CMML (3%), 5 with MDS (5%), 2 with SM, and 1 with CNL. Interestingly, one of the patients with SM and the patient with CNL with JAK2 V617F had a history of lymphoma, and this patient with SM also had associated myelofibrosis and CMML. The current observation strengthens the specific association between JAK2 V617F and classic MPD, but also suggests an infrequent occurrence in other myeloid disorders.
Introduction
Recently, a potentially major breakthrough for understanding the pathobiology of myeloproliferative disorders (MPDs) was reported: several groups independently detected a somatic point mutation in a highly conserved residue of the pseudokinase domain of the JAK2 tyrosine kinase (V617F) in most patients with polycythemia vera (PV), as well as one-third to one-half of patients with either essential thrombocythemia (ET) or myelofibrosis with myeloid metaplasia (MMM).1-4 In the present study, we screened a large number of patients with either atypical MPD (aMPD) or myelodysplastic syndrome (MDS) to determine whether this activating JAK2 mutation is associated only with classic BCR/ABL-negative MPD,5 or if, instead, it also occurs in other chronic myeloid disorders (CMDs). In addition, we studied possible phenotypic alterations accompanying the occurrence of JAK2 V617F in these disorders.
Other than chronic myeloid leukemia (CML), which is characterized by the presence of the Philadelphia chromosome or its molecular equivalent (ie, the BCR/ABL fusion oncogene, encoding a constitutively active tyrosine kinase), precise molecular definition has proven elusive for the CMD.6 In part, this is due to the mixed clonality of these disorders and the diversity of clinical presentations. Rare cases of CMML exhibit gene rearrangements involving the beta chain of the platelet-derived growth factor receptor (PDGFR) that result in cellular transformation,7 some patients with hypereosinophilic syndrome (HES) have rearrangements of the alpha chain of the PDGFR,8 and somatic activating mutations of c-KIT (eg, the recurrent D816V missense mutation) underlie a proportion of cases of SM,9 but for the majority of CMDs, a specific molecular mechanism is not yet known. The striking clinical success seen in CML therapy with pharmacologic BCR/ABL kinase inhibition by rationally designed agents including imatinib mesylate,10 AMN-107,11 and BMS-35482512 highlights the pressing need for defining comparable targets in the other CMDs.
The recurrent JAK2 mutation was identified independently by a candidate gene approach1,3,4 and by high-throughput DNA sequencing of the functional domains of 85 tyrosine kinases in PV, MMM, and ET blood samples.2 In the latter analysis, JAK2 V617F was detected at the genomic DNA level in blood from 74% of 164 patients with PV, 32% of 115 patients with ET, and 35% of 46 patients with MMM who were recruited through the internet.2 Subsequent ongoing analysis of more than 250 well-characterized MPD blood and marrow samples from the Mayo Clinic CMD Cell Bank has demonstrated an even greater proportion of patients with PV, MMM, and ET with the mutation (approximately 85%, 50%, and 40%, respectively, of patients diagnosed using currently applicable screening criteria; T.L.L., D.P.S., A.T., unpublished data, March 2005). JAK2 V617F results in constitutive activation of the tyrosine kinase, phosphorylation of STAT5, and factor-independent growth of hematopoietic cells, and was not observed in blood from 209 healthy individuals, nor in buccal cells from 110 patients with PV who had the mutation in hematopoietic cells, strongly suggesting pathobiologic relevance.2 This hyperactive tyrosine kinase is an attractive target for pharmacologic inhibition, so it is important to understand how applicable such approaches might be to other myeloid disorders besides the 3 classic BCR/ABL-negative MPDs. In this study, we demonstrate that the JAK2 V617F mutation is uncommon in aCMD and MDS, despite the myeloproliferative features frequently observed in the former group; when present, it may be associated with marrow fibrosis or a history of lymphoma.
Study design
Sample collection and processing
The current study was approved by the institutional review board of the Mayo Clinic, and State of Minnesota and Federal Health Insurance Portability and Accountability Act (HIPAA) guidelines regarding access to medical records were followed. Genomic DNA was obtained from 2 sources: marrow samples donated by patients to the Mayo Clinic CMD Cell Bank (MDS and aCMD except for some CMML), and methanol-preserved cultured waste cells from marrow obtained for cytogenetic studies (some CMML samples). In addition, 20 MDS DNA samples (British patients with acquired thalassemia13 ) and DNA from 50 healthy donors were a gift from Professor Doug Higgs (Weatherall Institute of Molecular Medicine, Oxford, United Kingdom). Other than these latter samples, all cases were reviewed by Mayo Clinic hematopathologists and diagnoses assigned according to standard World Health Organization (WHO) criteria.14 Patient characteristics are summarized in Table 1. Granulocytes were isolated from marrow samples by density centrifugation with Ficoll-Hypaque (Sigma Chemical, Saint Louis, MO). Genomic DNA was extracted using High Pure PCR Template Preparation Kit (Roche Diagnostics, Penzberg, Germany).
JAK2 mutation analysis
Genomic DNA was amplified by polymerase chain reaction (PCR), and successful amplification was confirmed by electrophoresis on an ethidium bromide–impregnated 1% agarose gel. Each 50-μL PCR reaction contained approximately 25 ng DNA template, 5 μL 10× Roche buffer (final concentration of MgCl2: 1.5 mM), 1.5 U Taq polymerase (Roche), 0.8 mM deoxynucleoside triphosphates (dNTPs; Roche), and 20 pM each of sense and antisense primers (5′-TGCTGAAAGTAGGAGAAAGTGCAT-3′ and 5′-TCCTACAGTGTTTTCAGTTTCAA-3′, respectively). PCR cycling parameters were: one cycle of 94°C for 2 minutes; 35 cycles of 94°C for 30 seconds, 52°C for 40 seconds, and 72°C for 40 seconds; followed by one cycle of 72°C for 2 minutes.
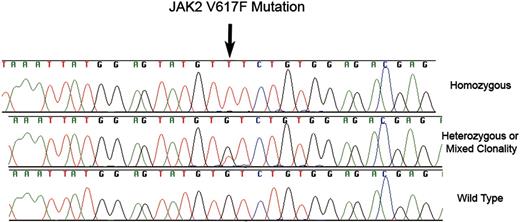
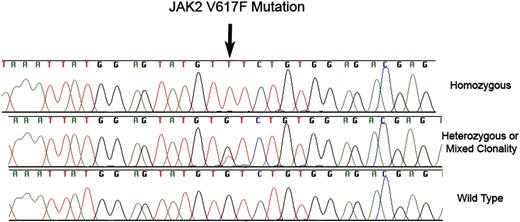
JAK2 V617F mutations. Fluorescent dye chemistry sequencing chromatograms showing homozoygous (top) and heterozygous/mixed clonality (middle) guanine to thymine substitution (arrow) in exon 12 of JAK2 in granulocyte genomic DNA from 2 patients with MDS. The predicted protein consequence is substitution of phenyalanine for valine at position 617 of NP_004 963. The bottom chromatogram is the wild-type sequence.
JAK2 V617F mutations. Fluorescent dye chemistry sequencing chromatograms showing homozoygous (top) and heterozygous/mixed clonality (middle) guanine to thymine substitution (arrow) in exon 12 of JAK2 in granulocyte genomic DNA from 2 patients with MDS. The predicted protein consequence is substitution of phenyalanine for valine at position 617 of NP_004 963. The bottom chromatogram is the wild-type sequence.
PCR products were cleaned with the QIAquick PCR purification Kit (Qiagen, Valencia, CA). Fluorescent dye chemistry sequencing was performed using the same primers used for amplification, on an ABI PRISM 3700 DNA Analyzer (Applied Biosystems, Foster City, CA). Sequencher 4.2 (Gene Codes, Ann Arbor, MI) and GenBank Accession NM_004 972 (JAK2 mRNA) and the corresponding region from the NC_000 009 chromosome 9 contig were used for sequence analysis.
Results and discussion
The 50 normal samples were all wild type for exon 12 of JAK2. Only 3 JAK2 V617F mutations were detected among 119 CMML samples; all 3 sequence chromatograms displayed peaks from both wild-type and mutant genomic DNA at the locus of interest (Figure 1). Since JAK2 is encoded by an autosomal gene, when 2 chromatogram peaks are present in a particular DNA sample, this finding may represent a heterozygous mutation, or DNA derived from an admixture of clonal and residual normal hematopoietic elements.15
Some patients with CMML have primarily myelodysplastic features (eg, ineffective hematopoiesis and cytologic dysplasia) and others have more myeloproliferative features (eg, leukocytosis and splenomegaly).16-19 Appropriate classification of these patients has been controversial for many years. Attempts to separate CMML into dysplastic and proliferative subtypes using a peripheral white blood count (WBC) of 13 × 109/L as a cutoff have been widely challenged, because WBC alone is not a sensitive or specific marker for proliferative/dysplastic features.20,21 The 1982 French-American-British MDS classification incorporated CMML,22 but the most recent World Health Organization classification of myeloid neoplasia places CMML in a distinct myeloproliferative-myelodysplastic overlap category.14 The fact that CMML is defined principally by an arbitrary peripheral blood monocytosis threshold limits clinicians' ability to offer a prognosis on the basis of disease subtype alone. A specific molecular lesion might aid nosology and prognostication, but this study demonstrates that JAK2 V617F is not useful for these purposes. Among the 3 patients with CMML in this study with mutations, one had primarily proliferative features, one was primarily dysplastic, and the third was mixed; 2 of the 3 had mild to moderate marrow reticulin fibrosis, compared with 22 of 119 (18%) with 1+ and 2+ fibrosis in the series as a whole.
JAK2 V617F was detected in 5 of 101 MDS samples: 1 was homozygous (ie, no wild-type DNA detected; Figure 1) and 4 had heterozygous/mixed clonality mutations. One of these patients had insufficient metaphases for cytogenetic analysis whereas the other 4 had normal karyotypes. There were no distinguishing clinical or pathologic features among these patients: moderate marrow fibrosis was present in 1 individual, but proliferative features including leukocytosis and hepatosplenomegaly were not present. Marrow fibrosis was not present in other cases. With respect to MDS subtype, 2 had refractory anemia and ringed sideroblasts (RARS), 1 had refractory cytopenia with multilineage dysplasia (RCMD), 1 had refractory anemia (RA), and 1 had refractory anemia with excess of blasts 1 (RAEB-1).
The JAK2 mutation was not detected in 11 patients with HES, and was found in only 2 of 8 SM samples. In addition to mastocytosis, one of these 2 patients with SM had monocytosis and moderate reticulin fibrosis in the marrow, consistent with underlying CMML. The patient also had a history of B-cell lymphoma. The 2 patients with JAK2 V617F both lacked the D816V c-KIT mutation; 2 other patients in the series who had the c-KIT mutation did not have the JAK2 mutation.
True CNL is extremely rare23 and only 6 DNA samples were available for analysis; one of these had a homozygous JAK2 V617F mutation. Both peripheral blood mononuclear cell and granulocyte samples were also available from this patient, and mutant JAK2 was exclusively present in both samples, suggesting either complete replacement of the marrow by a clone bearing the mutation or a germ line mutation (buccal cells or fibroblasts were not available for comparison). Interestingly, this patient, as was the case with the single mutation–positive patient with SM, also had a history of B-cell lymphoma. Regardless, the patient continued to do well on oral hydroxyurea therapy, more than 2 years from initial diagnosis of CNL. Future analyses might include more CNL samples to better assess the true JAK2 mutation rate, as well as DNA from other rare CMD subtypes such as chronic basophilic leukemia and juvenile myelomonocytic leukemia. It will also be of interest to separate out various hematopoietic lineages including lymphocytes from patients with CMD with JAK2 V617F mutations, to assess the clonality and lineage specificity of this finding in a more rigorous fashion.
In conclusion, while the JAK2 V617F mutation is extraordinarily common in classic BCR/ABL-negative MPDs, it appears to be rare in both MDS and the aCMD subtypes analyzed here, with the possible exception of CNL. The aberrant proliferative signal in these other cases remains mysterious. Future investigations might focus on other molecules in the same signaling pathway as JAK2,24 or on other parallel signal transduction cascades. Meanwhile, it may also be of interest to search for activating JAK2 mutations in acute leukemia samples (especially erythroleukemia, given the high mutation prevalence in PV) and in other neoplasms. Given the history of lymphoma in a subset of patients with the JAK2 mutation in this series, the potential for involvement of the JAK–signal transducers and activators of transcription (STAT) signaling pathway or its negative regulators (eg, suppressor of cytokine signaling-1, Src homology 2–containing protein tyrosine phosphatase-1 [SHP-1], SHP-2) in lymphoproliferative disorders should also be investigated.
Prepublished online as Blood First Edition Paper, April 28, 2005; DOI 10.1182/blood-2005-03-1183.
Supported by National Institutes of Health grant K12 CA 90 628 (D.P.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.