Abstract
The human immunodeficiency virus (HIV) has been reported to target noninfected CD4 and CD8 cells for destruction. This effect is manifested in part through up-regulation of the death receptor Fas ligand (FasL) by HIV-1 negative factor (Nef), leading to bystander damage. However, the signal transduction and transcriptional regulation of this process remains elusive. Here, we provide evidence that p38 mitogen-activated protein kinase (MAPK) is required for this process. Loss-of-function experiments through dominant-negative p38 isoform, p38 siRNA, and chemical inhibitors of p38 activation suggest that p38 is necessary for Nef-induced activator protein-1 (AP-1) activation, as inhibition leads to an attenuation of AP-1-dependent transcription. Furthermore, mutagenesis of the FasL promoter reveals that its AP-1 enhancer element is required for Nef-mediated transcriptional activation. Therefore, a linear pathway for Nef-induced FasL expression that encompasses p38 and AP-1 has been elucidated. Furthermore, chemical inhibition of the p38 pathway attenuates HIV-1-mediated bystander killing of CD8 cells in vitro. (Blood. 2005;106:2059-2068)
Introduction
Human immunodeficiency virus (HIV) infection typically results in the eradication of the host's immune system through eventual depletion of its CD4 cells.1-3 Although initially controlled, the virus persists and invariably replicates to high titers. In this regard, disregulated apoptosis is considered a major pathogenesis event leading to severe CD4 lymphopenia during HIV-1 infection.3 HIV-induced apoptosis of host cells has been reported to both involve and not involve the Fas/Fas ligand (FasL) apoptotic pathway. FasL is not present on resting T cells, but activated T cells may undergo apoptosis through the CD95/CD95 ligand (CD95L) pathway.4-7
Specifically, negative factor (Nef) has been reported to induce apoptosis of bystander cells while protecting infected cells. Nef expression in T cells induces FasL expression on the infected cell.8,9 This proposed mechanism is also detected in lymph nodes of simian immunodeficiency virus (SIV)-infected monkeys and HIV-infected patients, further suggesting its importance.5 Concomitantly, Nef expression in T cells induces signals that protect the infected cell from the same Fas-mediated cell death via B-cell lymphoma 2 (Bcl2)-antagonist of cell death (Bad) phosphorylation and apoptosis signal-regulating kinase 1 (ASK1) inhibition.10,11 Ideally, this would allow the virally infected cell to persist in the face of the host immune response by becoming resistant to apoptosis while provoking localized destruction of neighboring cells and effector T cells attempting to mediate viral factory clearance.
Previously, stimulation by Nef of FasL has been directly linked to Nef's ability to bind to the CD3 ζ chain of the T-cell receptor (TCR).8 This interaction is mediated by the proline-rich domain of Nef, which potentiates its interaction with Nef-associated kinase (NAK/p62).9,10 However, the downstream signals that regulate this Nef-mediated FasL transcription remain undetermined. Here, we report that mitogen-activated kinase p38 is necessary for Nef-mediated FasL transcription. Further, disruption of p38 leads to the inhibition of activator protein 1 (AP-1)-dependent transcription, which we show is necessary for FasL up-regulation.
Materials and methods
Cell culture
Jurkat, 293T, and the monocyte line U937 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were passaged in RPMI-1640 or Dulbecco modified Eagle medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin. Cells were maintained at 37°C and 5% CO2. Leukopacks from individual donors were obtained from the CFAR clinical core facilities at the University of Pennsylvania (UPENN) School of Medicine to isolate T cells as well as monocytes. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density centrifugation and cultured as described previously.4,12 CD14 monocytes were purified from PBMCs by positive enrichment using autoMACS (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Monocytes were cultured in complete RPMI-1640 medium with 500 U/mL recombinant human interleukin-4 (rhIL-4) and 1000 U/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; both from R&D Systems, Minneapolis, MN) per 106 cells.4,12
Mitogen-activated protein kinase (MAPK) inhibitor and plasmids
The p38 inhibitors SB203580 and RWJ67657 have been previously described.13,14 The wild-type Nef (pNef) or pNef (PxxPxR) alanine substitution plasmids were amplified by single-round polymerase chain reaction (PCR) using Nef-specific primers or overlap extension PCR and subcloned into the pVax vector (Invitrogen, Frederick, MD). The wild-type p38 MAP kinase, dominant-negative p38 (KM mutant; K to M mutation at the adenosine triphosphate [ATP]-binding site), was constructed.15 Human FasL (hFasL) reporter expression vector hFasL-Luc (1.2-kb FasL promoter reporter construct) and hFasLmut (mutations in the AP-1 site were introduced into the 1.2-kb FasL promoter construct) were previously described.16
Four sets of p38 shRNA oligonucleotides (oligos) were designed for p38 sequence using siRNA target finder with appropriate controls from Ambion (Austin, TX) web resources. For each set, top- and bottom-strand oligos were synthesized separately and annealed together. The primers for siRNA corresponding to the coding region were made corresponding to the sequence as follows: clone p38-61: top, 5′-gatccACATGAGAAGCTAGGCGAGTTcaagagaCTCGCCTAGCTTCTCATGTTTTTTTGGAAA-3′ and bottom, 5′-AGCTTTTCCAAAAAA CTCCTGGTAGAGAAGAAAAtctcttgAATTTTCTTCTCTACCAGGAGg-3′; clone p38-352: top, 5′-gatccGCTCATGAAACATGAGAAGTTcaagagaCTTCTCATGTTTCATGAGCTTTTTTGGAAA-3′ and bottom, 5′-AGCTTTTCCAAAAAAGCTCAT GAAACATGAGAAGtctcttgAACTTCTCATGTTTCATGAGCg-3′; and clone p38-1016: top, 5′-gatccTGGAAGCGTGTTACTTACATTcaagagaTGTAAGTAACACGCTTCCATTTTTTGGAAA-3′ and bottom, 5′-AGCTTTTCCAAAAAATGGAAGCGTGTTACTTACAtctcttgAATGTAAGTAACACGCTTCCAg-3′. The selection of siRNA sequences followed the criteria provided by Ambion. The BamHI restriction site was included on each top strand at its 5′ end; similarly, on the bottom strand, the HindIII site was included at the 3′ end to facilitate cloning into the pSilencer 2.0-U6 neo vector (Ambion). In the top strand, the uppercase nucleotides correspond to the nucleotide open reading frame (ORF) of the mRNA sequence. Next to the mRNA sequence, a 7-base (caagaga) spacer sequences was included to facilitate to form a small hairpin loop. The second stretch of uppercase nucleotides is the reverse complement of the first. The 2 oligos were annealed and inserted into a pSilencer 2.0-U6 neo vector (Ambion) that had been linearized with BamHI and HindIII enzymes. Positive clones were identified and sequences were conformed by automated DNA sequencing. The pSilencer 2.0-U6 neo vector, which contains a 21-base scrambled sequence and has no significant homology to mouse, rat, or human gene sequences, was used as a negative control to transfect cells.17
Transfection and luciferase reporter assay
Jurkat or U937 (2 × 106) cells were washed twice with phosphate-buffered saline (PBS), resuspended in 500 μL Opti-MEM (Gibco) culture medium, and transfected with LipofectAMINE Plus (Gibco). The plasmid pNef (5 μg), with a mixture of 2 μg cytomegalovirus (CMV) vector expressing green fluorescence protein (GFP), was transfected with the FuGENE 6 Reagent (Roche Applied Science, Indianapolis, IN). Cells were washed twice with complete RPMI-1640 and incubated for 48 hours. Transfection efficiencies were assessed by fluorescence-activated cell sorter (FACS) analyses of GFP expression. For determination of AP-1 and FasL reporter assay, 2.5 μg reporter plasmid (pAP1-Luc or hFasL-Luc) alone or with pNef (5 μg), mutated pNef with or without inhibitors (1 μM), pNef or pNef plus p38Wt, or pNef plus p38 dominant-negative construct (5 μg) were added to the cells and mixed well as indicated. Electroporation was carried out at 250 V and 960 μF in a Bio-Rad Gene Pulse II (Bio-Rad, Hercules, CA).16 Cells were grown in complete RPMI 1640 medium for 48 hours at 37°C.
Total amounts of DNA and equal molar ratios of promoters were kept constant in all setups by using empty vectors.16 Because of differences in transfection efficiencies, an expression plasmid pCMV β-galactosidase (β-gal) was cotransfected as a transfection efficiency control, and luciferase activities were normalized based on β-gal activity with the β-gal reporter gene assay.16 Cells were harvested, washed 3 times with PBS, and lysed in 100 μL reporter lysis buffer (RLB) according to the manufacturer's instructions (Roche Applied Science). Cell debris was removed by centrifugation, and the supernatant was used in the luciferase assay using LUMAT-LB9501 (Berthold, Bad Wildbad, Germany).16
HIV-1 pseudotype viruses and infection
The HIV-1 proviral infectious constructs pNL4-3/HSA, pNL4-3/HSA/ΔEnv, or pNL4-3/HSA/ΔEnv/ΔNef and primary clade specific isolates (subtype A-/94UG103, subtype-B/92US723, subtype-C/96USNG31, and subtype-D/92UG001) were obtained through the AIDS Research and Reference Reagent (RRR)-Program, National Institute of Allergy and Infectious Diseases (NIAID), NIH.18 Constructs containing ΔNef were generated by recombinant PCR mutagenesis.19 HIV-1 viral particles were generated by transfection with pNL4-3/HSA alone or cotransfection of pNL4-3/HSA/ΔEnv or pNL4-3/HSA/ΔEnv/ΔNef with vesicular stomatitis virus G (VSV-G) envelope18 by FuGENE 6 transfection in 293T cells. Infection was carried out by incubating the cells with HIV-1 virus at a concentration of 100 tissue-culture infective dose (TCID50)/106 cells per milliliter. These retroviruses encode as a specific cell-surface marker, the murine CD24 antigen (heat-stable antigen [HSA]), which allows for identification of infected target cells by FACS analysis.12,18 Culture supernatant was collected at 6-, 12-, and 24-hour intervals and assayed for virus production by measuring p24 antigen by enzyme-linked immunosorbent assay (ELISA) kit (Beckman Coulter, Fullerton, CA). Data are presented as mean plus/minus SEM.
Flow cytometry
Cell suspensions (106) were washed in PBS (pH 7.2) containing 0.2% bovine serum albumin and 0.1% NaN3. Cells were stained with 1 to 2 μg of the following monoclonal antibodies (mAb): CD3 fluorescein isothiocyanate (FITC) mouse immunoglobulin G2a (IgG2a), CD4 phycoerythrin (PE) mouse IgG1, CD8 FITC mouse IgG1, CD14 FITC and PE mouse IgG2a (PharMingen, San Diego, CA), and FasL (NOK-1) mouse IgG1 (eBioscience, San Diego, CA). For intracellular p24gag, staining was performed by fixing 106 cells in 1% paraformaldehyde containing 20 μg/mL lysolecithin (Sigma, St Louis, MO) for 5 minutes at 4°C. Samples were then washed, permeabilized in 2 mL ice-cold methanol while vortexing, placed on ice for 15 minutes, washed again, and resuspended in 1 mL PBS/0.1% nonidet P-40 (NP-40; Sigma) for 5 minutes at 4°C. Cells were washed and incubated with 1 μg PE-conjugated KC57-RD1 (Coulter) for 15 minutes at 4°C. Cells were washed with PBS and resuspended in 200 μL PBS and analyzed directly on a Coulter EPICS Flow Cytometer (Coulter) using FlowJo software (TreeStar, San Carlos, CA). All samples were compared with their isotype-matched controls. In the case of dual flow cytometry, individual samples treated with each isotype alone were used to determine the background levels of autofluorescence.12
Apoptosis, caspase, and FasL ELISA assays
FACS analysis was performed to identify cells undergoing apoptosis.4 Apoptosis was evaluated by using an annexin-V assay kit (PharMingen) and analyzed directly on a Coulter EPICS Flow Cytometry using FlowJo software. Caspase-3 activity was determined using Caspase-3/CPP32 colorimetric protease assay kit according to the manufacturer's instructions (MBL, Watertown, MA). FasL in the supernatant or lysates was quantified by sandwich ELISA with commercial ELISA (R&D Systems) according to the manufacturer's instructions.
Protein extraction and Western blotting
Cells were washed with ice-cold PBS and lysed with protein lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane, pH 7.4], 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethylene glycol tetraacetic acid], 1% triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3PO4, 1 μM/mL leupeptin, and 1 mM phenyl methyl-sulfonyl fluoride). After brief sonication, the lysates were clarified by centrifugation at 12 100g/10 minutes and protein content was measured by the Bradford method (Bio-Rad). Protein (50 μg per lane) was loaded into acrylamide gels, separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted to polyvinylidenefluoride (PVDF) transfer membrane. Primary antibodies to p38 and phospho-p38 (Thr180/Tyr182; Cell Signaling, Beverly, MA), hFasL (USBiological, Swampscott, MA), and HIV-1 p24gag (NIH-AIDS reagent; NIH, Rockville, MD) were used. Immunoblot was carried out using the specific primary antibody and detected with secondary horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG using Amersham (Arlington Heights, IL) enhanced chemiluminescence (ECL) system and was visualized by autoradiography.12
CD8 T-cell apoptosis assay
Monocytes were purified as described in cell culture techniques and purified CD8 T cells were isolated from PBMCs by negative immunoselection using Dynal magnetic beads (Dynal, Lake Success, NY)4,12 for depletion of CD4 T cells. Monocytes were infected with HIV-1 NL4-3 wild-type (Wt)- or NL4-3 ΔNef-deleted virus at a concentration of 100 TCID50/106 cells per milliliter/well. Different experimental groups were set up as follows: group I, as a mock (untreated); group II, incubated with latex beads only; group III, incubated with latex beads and treated with 20 μg/mL neutralizing anti-FasL mAb (NOK-2; PharMingen); and group IV, incubated with latex beads and treated with 1 μM p38 inhibitor (RWJ67657). On day 7 after infection, 0.5 × 106 polystyrene latex beads (IDC Spheres; IDC, Portland, OR) was added to groups II, III, and IV for 3 hours at 37°C. Anti-FasL mAb was added for 90 minutes, before 0.5 × 106 autologous purified CD8 T cells were added as described.4 After 14 hours of incubation of monocytes plus T cells, cells were harvested and stained with mAbs including FITC-conjugated anti-CD8 and further stained with annexin-V-PE. The analysis was performed on gated low forward scatter and CD8 T cells and further analyzed for the CD8/annexin-V-positive cells (CD8 population).
Results
HIV infection stimulates p38 phosphorylation
Activation of p38 kinase following HIV infection in some target cells has been previously demonstrated.14,20 Activation was not a result of virus binding to the cell surface but apparently occurs after virus internalization. We sought to gain insight into the molecular interactions responsible for this activation; therefore, we examined the effect of HIV-1 infection on p38 phosphorylation, a major indicator of p38 kinase activity. HIV-1-infected Jurkat T cells or PBMCs were harvested 24 hours after infection for protein analysis of p38 activation. Western blot analysis was carried out using antibodies specific for either the Thr180 and Thr182 phosphorylated forms of p38 or controls (Figure 1A). We observed that p38 was rapidly phosphorylated following HIV infection in either the cell line or in primary PBMCs, confirming the activation of p38 signaling during HIV infection.
p38 MAPK activation is required for HIV-1-related apoptosis. (A) p38 MAPK is activated by HIV-1 infection. Immunoblot analysis of protein extracted from PBMCs or Jurkat T cells infected with mock or NL4-3 virus. Total cellular protein extract (50 μg) was extracted 24 hours after infection and analyzed by 12% SDS-PAGE. p38 and phospho-p38 MAPK activation was detected by using specific antibodies that recognize phospho-p38 MAPK (top) only when phosphorylated or the p38 MAPK (middle). HIV infection strongly activates phosphorylation in both cell types. The same lysates were blotted with anti-p24gag(bottom) to monitor viral infection. (B-C) HIV-1-induced apoptosis is blocked by p38 MAPK inhibitor. Human PBMCs were infected with (B) NL4-3 Wt virus or (C) different HIV-clade specific primary viral isolates. Cells were uninfected (mock) or infected with HIV-1 virus with or without addition of 1 μM p38 inhibitor-I or -II (SB203580 or RWJ67657, respectively). Cells were collected 2 days after infection and stained with annexin-V-FITC; p24gag-PE and HIV-induced apoptosis analysis was performed. The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 (B) or 2 (C) independent experiments. Both p38 inhibitors block HIV-driven apoptosis. (D) p38 MAP kinase inhibitors block HIV-induced caspase-3 activity. Cell lysates were prepared from the groups described in panel B. Total protein from each cell lysate (100 μg) was used for the colorimetric caspase assay as described in “Materials and methods.” Each column represents the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. All these experiments gave similar results.
p38 MAPK activation is required for HIV-1-related apoptosis. (A) p38 MAPK is activated by HIV-1 infection. Immunoblot analysis of protein extracted from PBMCs or Jurkat T cells infected with mock or NL4-3 virus. Total cellular protein extract (50 μg) was extracted 24 hours after infection and analyzed by 12% SDS-PAGE. p38 and phospho-p38 MAPK activation was detected by using specific antibodies that recognize phospho-p38 MAPK (top) only when phosphorylated or the p38 MAPK (middle). HIV infection strongly activates phosphorylation in both cell types. The same lysates were blotted with anti-p24gag(bottom) to monitor viral infection. (B-C) HIV-1-induced apoptosis is blocked by p38 MAPK inhibitor. Human PBMCs were infected with (B) NL4-3 Wt virus or (C) different HIV-clade specific primary viral isolates. Cells were uninfected (mock) or infected with HIV-1 virus with or without addition of 1 μM p38 inhibitor-I or -II (SB203580 or RWJ67657, respectively). Cells were collected 2 days after infection and stained with annexin-V-FITC; p24gag-PE and HIV-induced apoptosis analysis was performed. The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 (B) or 2 (C) independent experiments. Both p38 inhibitors block HIV-driven apoptosis. (D) p38 MAP kinase inhibitors block HIV-induced caspase-3 activity. Cell lysates were prepared from the groups described in panel B. Total protein from each cell lysate (100 μg) was used for the colorimetric caspase assay as described in “Materials and methods.” Each column represents the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. All these experiments gave similar results.
HIV-1-induced apoptosis is blocked by p38 MAPK inhibitors
Studies support that active host cell signaling is involved in the induction of apoptosis.21 A host cell-signaling pathway that may have relevance for bystander apoptosis is the p38 MAP kinase pathway. Genetic studies conducted in mice suggest that MAP kinase kinase 3 (MKK3), an upstream kinase of p38, is required for activation-induced cell death of T cells.22 Therefore, we examined the role of the p38 pathway in HIV-1-induced apoptosis. Human PBMCs were infected with NL4-3 virus at a concentration of 100 TCID50/106 cells per milliliter and tested for apoptosis under various conditions, including in the presence or absence of known specific p38 inhibitors designated as inhibitor-I or inhibitor-II (SB203580 or RWJ67657, respectively) at 1 μM as described.14 Cells were collected 2 days after infection from each treatment group and analyzed for apoptosis induction in HIV-infected cells. HIV-1 infection promoted significant apoptosis in target cells (Figure 1B). Of importance, both p38 inhibitors could block HIV-driven apoptosis. Both inhibitors inhibited apoptosis driven by virus more than 70% at 1-μM concentration. However, p38 inhibitor-II exhibited a greater antiapoptotic potency. To confirm this observation on primary viral isolates, we analyzed the induction of apoptosis by 4 different subtype-divergent HIV viral isolates (Figure 1C). All 4 primary subtype-divergent viruses induced strong and clearly detectable levels of target cell apoptosis in this infection system. Again, both inhibitors were effective at inhibiting apoptosis. We wanted to ensure that the observed apoptosis was also activating caspases. Accordingly, we chose the caspase-3 assay as a downstream marker for all caspase-induced apoptosis,21 and caspase-3 activation was attenuated by pharmacologic inhibition of p38 with 70% potency with a 1-μM concentration of inhibitor-II (Figure 1D). These results support an essential role for the p38 MAPK pathway in apoptosis induction by diverse isolates of HIV-1 in PBMCs.
HIV-1-mediated FasL up-regulation requires p38 signaling
Previous studies indicate that HIV-infected cells can up-regulate FasL expression and selectively induce the apoptosis of FasL-susceptible T cells from HIV-positive individuals.4 Recent reports have identified that up-regulation of FasL on HIV-infected cells can be induced by Nef.23 However, the signaling pathways that are required for this activity remain unclear. Based on the inhibition of apoptosis by p38 inhibitors observed and previous studies implicating Fas/FasL signaling in HIV-induced apoptosis, we decided to examine the relevance of the p38 pathway to HIV-induced FasL expression. We first sought to confirm the induction of FasL by HIV infection. Strong induction of FasL was observed in HIV infection in PBMCs (Figure 2A). Further FasL expression induction was also confirmed using FasL-specific antibody by immunoblot analysis using the infected samples (Figure 2B). This result confirms a relationship between the FasL induction and HIV infection. Next, PBMCs or Jurkat cells were infected with mock or NL4-3 virus in the presence or absence of p38 inhibitor-II (RWJ67657), and FasL expression was analyzed by FACS using FasL-specific antibody. We observed strong inhibition of FasL expression induced by the pharmacologic blockade of p38 signaling (Figure 2C-D). This inhibition appears independent of the cell type used in the experiment. Further, the up-regulation of FasL by individual HIV genes suggests that Nef but not Tat, Vpr, Vpu, or Env is sufficient to activate high-level FasL expression in Jurkat and the monocytic U937 cells (data not shown). This effect was dosage dependent with saturating effects observed at 1 μM.
p38 MAPK activation is required for HIV-induced FasL expression. (A) HIV-1-mediated FasL induction in HIV-1-infected cells. Quantification of FasL expression in human PBMCs infected with HIV-1 NL4-3 virus. Cell lysates or supernatants were prepared at the indicated times after infections. As a control, mock cells were stimulated with phorbol myristate acetate (PMA, 10 ng/mL) plus ionomycin (500 ng/mL) to induce FasL expression as described in “Materials and methods.” Results are expressed in concentration to demonstrate that the assay detects both forms of FasL. Data represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (B) Immunoblot analysis of FasL expression. Total cellular proteins were prepared from the groups described in panel A. Protein lysates were blotted with anti-FasL-specific antibody or anti-p24gag (bottom) to confirm the FasL expression and monitor viral infection status. (C-D) FasL induction is blocked by p38 inhibitors. PBMCs (C) or Jurkat T cells (D) were infected with HIV-1 NL4-3 virus in the presence or absence of 1 μM p38 inhibitor RWJ67657. At 48 hours after infection, FasL expression was measured by flow cytometry, and analysis was performed on a gated low forward scatter and CD24(HSA)-positive cells using FasL-specific antibody. It is noteworthy that the p38 inhibitor strongly blocks FasL induction in both cell types tested. Data shown are representative of 1 of 3 independent experiments. Histograms show the indicated surface marker, and filled histograms represent the isotype-matched control antibodies.
p38 MAPK activation is required for HIV-induced FasL expression. (A) HIV-1-mediated FasL induction in HIV-1-infected cells. Quantification of FasL expression in human PBMCs infected with HIV-1 NL4-3 virus. Cell lysates or supernatants were prepared at the indicated times after infections. As a control, mock cells were stimulated with phorbol myristate acetate (PMA, 10 ng/mL) plus ionomycin (500 ng/mL) to induce FasL expression as described in “Materials and methods.” Results are expressed in concentration to demonstrate that the assay detects both forms of FasL. Data represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (B) Immunoblot analysis of FasL expression. Total cellular proteins were prepared from the groups described in panel A. Protein lysates were blotted with anti-FasL-specific antibody or anti-p24gag (bottom) to confirm the FasL expression and monitor viral infection status. (C-D) FasL induction is blocked by p38 inhibitors. PBMCs (C) or Jurkat T cells (D) were infected with HIV-1 NL4-3 virus in the presence or absence of 1 μM p38 inhibitor RWJ67657. At 48 hours after infection, FasL expression was measured by flow cytometry, and analysis was performed on a gated low forward scatter and CD24(HSA)-positive cells using FasL-specific antibody. It is noteworthy that the p38 inhibitor strongly blocks FasL induction in both cell types tested. Data shown are representative of 1 of 3 independent experiments. Histograms show the indicated surface marker, and filled histograms represent the isotype-matched control antibodies.
p38 MAPK is necessary for FasL expression by HIV-1 Nef
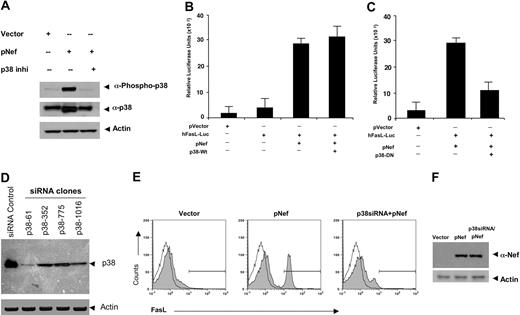
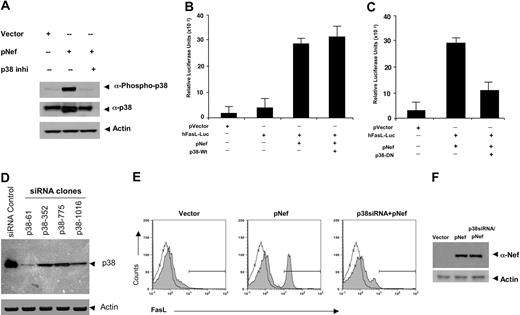
To investigate the requirement of p38 activity in Nef-induced FasL expression, we first sought to demonstrate that Nef transfection is sufficient to activate p38 and that this activation can be reversed by a p38 inhibitor. Nef induced a strong increase in phosphorylation of p38 above basal levels (Figure 3A). In contrast, there was no detectable increase in the phosphorylation of p38 in Nef-treated cells in the presence of p38 inhibitor. These data indicate that Nef can induce phosphorylation of the p38 MAP kinase and also that Nef is an activator of this pathway. Next, we studied the p38 requirement for FasL transcription in a promoter-specific fashion. Jurkat cells were transfected with pNef in the presence of cotransfected wild-type p38 (Figure 3B) or dominant-negative p38 constructs (Figure 3C), and activation of a FasL promoter-driven luciferase construct was assayed. The dominant-negative p38 contains a K-to-M mutation at the ATP-binding site, and this molecule has been previously reported to block p38 activity in vitro by protein interference.15 Nef strongly induced FasL promoter activation in the presence of native p38 (Figure 3B). In contrast, induction of FasL transcription was reduced in cells cotransfected with this dominant-negative mutant p38 and the Nef construct (Figure 3C). To test directly if p38 is required to activate Nef-mediated FasL expression, we sought to develop specific siRNAs that target p38. We targeted the p38-α isoform, which is the dominant isoform expressed in immune cells. These siRNAs were studied for their ability to suppress the expression of p38. Four different sets of p38 siRNA oligos were examined. Jurkat cells that were transfected with siRNA vector clone p38-61 demonstrated a dramatic reduction of p38 expression, while the other clones, p38-352, p38-775, and p38-1016, showed only moderate decreases in p38 expression (Figure 3D). Next, Jurkat cells were cotransfected with pNef and siRNA (clone p38-61) construct, and activation of FasL expression was assayed in equal numbers of cells by flow cytometry. Nef-mediated FasL expression was significantly reduced by clone p38-61 (Figure 3E), and the effect of p38 siRNA appears independent of the expression level of Nef (Figure 3F). Therefore, these loss-of-function experiments (chemical inhibitor, dominant negative, and siRNA) suggest that p38 MAPK is necessary for FasL induction by HIV-1 Nef.
Nef-induced FasL induction requires p38 MAPK activation. (A) Nef induced phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells transfected with 5 μg vector control or pNef in the presence or absence of p38 inhibitor-II. Samples were prepared as described in “Materials and methods” and resolved on 12% SDS-PAGE gels. Gels were transferred to PVDF membranes and immunoblotted with p38MAPK- and phospho-p38MAPK-specific antibodies as indicated. Nef induced phosphorylation of p38, and this activation can be blocked by the p38 inhibitors (p38 inhi). (B-C) Inhibition of Nef-induced FasL promoter activity by a dominant-negative p38. Jurkat cells were transfected with plasmid containing pVector, the hFasL-Luc promoter, the pNef plus hFasL-Luc promoter, or the pNef plus hFasL-Luc promoter together with wild-type p38 (B) or p38-DN (C) plasmid constructs as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments and were reproducible. Nef strongly induced the FasL promoter activation (B). In contrast, induction of FasL transcriptions was reduced in cells cotransfected with dominant-negative mutant p38 (C). (D) Construction and expression of p38siRNAs. Four different target siRNA expression vectors were constructed as described in “Materials and methods.” Jurkat T cells were transiently transfected with 5 μg siRNA control vector or p38siRNA expression constructs as indicated clones. Cell lysates were extracted 48 hours after transfection, and immunoblotting was performed by using an anti-p38 antibody and an actin antibody as a control for equal loading. p38 expression was suppressed significantly in cells transfected with clone p38-61 and moderately in cells transfected with the other clones compared with control vector transfected. (E) FasL expression is significantly reduced by p38siRNA. Jurkat T cells were electroporated with 5 μg plasmid-expressing vector, pNef, or p38siRNA (clone p38-61) and pNef plasmids as indicated. At 48 hours after transfection, the surface levels of FasL expression were determined by flow cytometry using a FasL-specific antibody. Filled histograms show the FasL expression, and open histograms represent isotype-matched control antibodies. p38 siRNA inhibited Nef-induced FasL induction. Similar results were obtained in 3 independent experiments. Transfection efficiency was monitored by cotransfection of a pCMV plasmid encoding GFP, which also served as a marker for gating on transfected cells. (F) p38 siRNA does not affect Nef expression. Total protein extracts were prepared from the groups transfected with vector, pNef, or p38siRNA plus pNef. Of each protein sample, 50 μg was separated on 12% SDS-PAGE and analyzed by Western blot with a polyclonal antibody against Nef antibody. Immunoblotting was also performed using an antiactin antibody as an internal control.
Nef-induced FasL induction requires p38 MAPK activation. (A) Nef induced phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells transfected with 5 μg vector control or pNef in the presence or absence of p38 inhibitor-II. Samples were prepared as described in “Materials and methods” and resolved on 12% SDS-PAGE gels. Gels were transferred to PVDF membranes and immunoblotted with p38MAPK- and phospho-p38MAPK-specific antibodies as indicated. Nef induced phosphorylation of p38, and this activation can be blocked by the p38 inhibitors (p38 inhi). (B-C) Inhibition of Nef-induced FasL promoter activity by a dominant-negative p38. Jurkat cells were transfected with plasmid containing pVector, the hFasL-Luc promoter, the pNef plus hFasL-Luc promoter, or the pNef plus hFasL-Luc promoter together with wild-type p38 (B) or p38-DN (C) plasmid constructs as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments and were reproducible. Nef strongly induced the FasL promoter activation (B). In contrast, induction of FasL transcriptions was reduced in cells cotransfected with dominant-negative mutant p38 (C). (D) Construction and expression of p38siRNAs. Four different target siRNA expression vectors were constructed as described in “Materials and methods.” Jurkat T cells were transiently transfected with 5 μg siRNA control vector or p38siRNA expression constructs as indicated clones. Cell lysates were extracted 48 hours after transfection, and immunoblotting was performed by using an anti-p38 antibody and an actin antibody as a control for equal loading. p38 expression was suppressed significantly in cells transfected with clone p38-61 and moderately in cells transfected with the other clones compared with control vector transfected. (E) FasL expression is significantly reduced by p38siRNA. Jurkat T cells were electroporated with 5 μg plasmid-expressing vector, pNef, or p38siRNA (clone p38-61) and pNef plasmids as indicated. At 48 hours after transfection, the surface levels of FasL expression were determined by flow cytometry using a FasL-specific antibody. Filled histograms show the FasL expression, and open histograms represent isotype-matched control antibodies. p38 siRNA inhibited Nef-induced FasL induction. Similar results were obtained in 3 independent experiments. Transfection efficiency was monitored by cotransfection of a pCMV plasmid encoding GFP, which also served as a marker for gating on transfected cells. (F) p38 siRNA does not affect Nef expression. Total protein extracts were prepared from the groups transfected with vector, pNef, or p38siRNA plus pNef. Of each protein sample, 50 μg was separated on 12% SDS-PAGE and analyzed by Western blot with a polyclonal antibody against Nef antibody. Immunoblotting was also performed using an antiactin antibody as an internal control.
HIV-1 Nef-deleted virus fails to induce FasL on human PBMCs
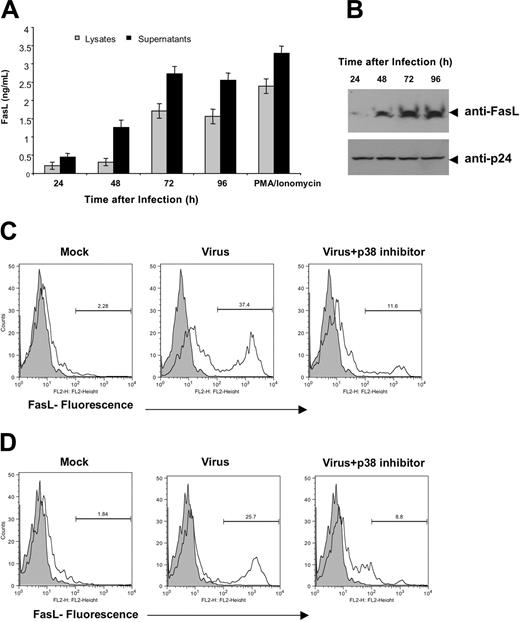
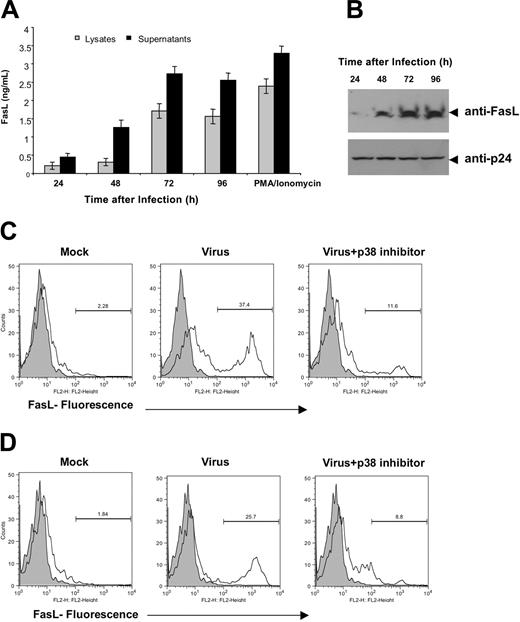
Next, we wanted to ascertain if p38 is also necessary for FasL induction in HIV-1 infection. Accordingly, we constructed a virus that was devoid of Nef expression to ascertain if there was an effect on FasL in the absence of Nef. Nef-deleted virus was constructed by introducing a frameshift mutation in the Nef ORF, thus eliminating Nef from this virus, and confirmed via proviral transfection into 293T cells via ELISA and Western blotting (Figure 4A-B). To analyze the effect of viral delivery of the nef gene on FasL expression, we used a pseudoviral infection assay for infecting PBMCs with the NL4-3 Wt or NL4-3 Δ Nef-deleted virus in the presence or absence of p38 inhibitor at 1 μM. Flow cytometry analysis of intracytoplasmic p24gag and FasL levels was determined 4 days after infection, allowing us to follow both markers in infected cells (Figure 4C). A direct correlation was observed between the presence of the Nef gene and the ability of the virus to induce FasL expression. The percentage of positive cells is depicted in the upper-right quadrant for each group. Approximately half of the infected (p24gag positive) cells were FasL positive on day 4. The FasL-positive cells constitute a unique separate population of cells that was observed only in the Nef-containing virus infection group. Of interest, viral Nef-mediated FasL expression was suppressed by treatment of the culture with 1 μM p38 inhibitor. These results in conjunction with Figure 3 suggest that Nef is critical for HIV-1-mediated FasL up-regulation. Furthermore, Nef-deleted viruses exhibit strong attenuation of p38 activation (Figure 4D). A modest activation of p38 was also observed in Nef-deleted viral infections, which could be due to influence from other HIV antigens, including the envelope antigen.24,25 These results extend the association between Nef, FasL induction, and the p38 pathway within an infection setting.
Nef is required for HIV-1-mediated FasL induction and efficient p38 activation. (A) Expression and analysis of the proviral expression constructs. A frameshift mutation (5′ Nef) was introduced in the nef gene in the proviral constructs as described in “Materials and methods.” Cell lysates derived from 293T cells transfected with pNef, pNL4-3 Wt, or pNL4-3 ΔNef HIV-1 proviral constructs were separated by 12% SDS-PAGE and then transferred to nitrocellulose membranes. Samples were probed with an HIV-1 Nef-specific antiserum. Nef was not expressed in the pNL4-3 ΔNef constructs. WB indicates Western blot. (B) HIV-1 infection was monitored by measuring p24 levels in culture supernatants derived from PBMCs infected with NL4-3 Wt or NL4-3 ΔNef virus. Data were obtained 96 hours after infection. These experiments were repeated 3 times and similar results were obtained. Error bars represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (C) Induction of FasL expression by NL4-3 Wt or NL4-3 ΔNef viruses in human PBMCs. Cells (1 × 106) were infected with NL4-3 Wt or NL4-3 ΔNef virions and then treated with or without 1 μM p38 inhibitor RWJ67657 as indicated. Two days after infection, an equal number of cells (analysis was performed on a gated low forward scatter and CD24[HAS]-positive cells) were assayed for intracellular p24gag and surface FasL expression by flow cytometry as described in “Materials and methods.” The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 independent experiments and observations of similar suppression of FasL were obtained. (D) Nef-deleted viruses failed to induce phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells infected with mock, NL4-3 Wt, or NL4-3 ΔNef virus. Samples were prepared 24 hours after infection as described in “Materials and methods” and immunoblotted with p38MAPK and phospho-p38MAPK-specific antibodies as indicated.
Nef is required for HIV-1-mediated FasL induction and efficient p38 activation. (A) Expression and analysis of the proviral expression constructs. A frameshift mutation (5′ Nef) was introduced in the nef gene in the proviral constructs as described in “Materials and methods.” Cell lysates derived from 293T cells transfected with pNef, pNL4-3 Wt, or pNL4-3 ΔNef HIV-1 proviral constructs were separated by 12% SDS-PAGE and then transferred to nitrocellulose membranes. Samples were probed with an HIV-1 Nef-specific antiserum. Nef was not expressed in the pNL4-3 ΔNef constructs. WB indicates Western blot. (B) HIV-1 infection was monitored by measuring p24 levels in culture supernatants derived from PBMCs infected with NL4-3 Wt or NL4-3 ΔNef virus. Data were obtained 96 hours after infection. These experiments were repeated 3 times and similar results were obtained. Error bars represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (C) Induction of FasL expression by NL4-3 Wt or NL4-3 ΔNef viruses in human PBMCs. Cells (1 × 106) were infected with NL4-3 Wt or NL4-3 ΔNef virions and then treated with or without 1 μM p38 inhibitor RWJ67657 as indicated. Two days after infection, an equal number of cells (analysis was performed on a gated low forward scatter and CD24[HAS]-positive cells) were assayed for intracellular p24gag and surface FasL expression by flow cytometry as described in “Materials and methods.” The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 independent experiments and observations of similar suppression of FasL were obtained. (D) Nef-deleted viruses failed to induce phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells infected with mock, NL4-3 Wt, or NL4-3 ΔNef virus. Samples were prepared 24 hours after infection as described in “Materials and methods” and immunoblotted with p38MAPK and phospho-p38MAPK-specific antibodies as indicated.
p38-mediated AP-1 activation is required for Nef-induced FasL transcription
AP-1 is an important transcription factor in immune activation and is induced by many stimuli, including growth factors, cytokines, T-cell activators, neurotransmitters, and ultraviolet (UV) irradiation.16,26,27 Eukaryotic cells respond to external stresses and inflammatory factors through the activation of MAPKs, leading to altered transcriptional activity. Specifically, c-Jun N-terminal kinases (JNKs) and p38 MAPK have been implicated in these responses. In most cases, p38 is activated by MKKs 3 and 6 through their phosphorylation.28,29 Of interest, MAPK-activated transcription has been implicated in driving the transcriptional activation of FasL via AP-1.16,30 Accordingly, we hypothesized that Nef may exploit this host pathway for inducing FasL during HIV infection.
As discussed in the section entitled “p38 MAPK is necessary for FasL expression by HIV-1 Nef,” Nef-induced p38 activation is necessary for the up-regulation of FasL induction in both an infection and a transfection setting. However, the requirement of the AP-1 binding enhancer element for Nef-induced FasL transcriptional activation was unclear, particularly since multiple elements have been proposed to drive FasL induction, including nuclear factor of activated T cell (NF-AT), nuclear factor κB (NF-κB), AP-1, and c-Myc.16,28-30
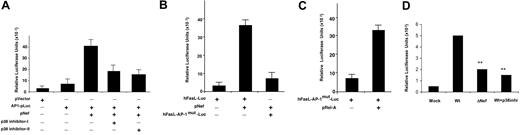
Accordingly, transfection of Jurkat cells with an AP-1 promoter reporter plasmid in conjunction with Nef induced strong AP-1 promoter activation in T cells and was sensitive to p38 inhibitors at 1-μM concentration. This suggests that Nef requires p38 to activate the transcription induced by the AP-1 enhancer elements (Figure 5A). To test whether these observations were related, we mutated the AP-1 enhancer element from the hFasL promoter plasmid by site-directed mutagenesis.16 Cotransfection with Nef failed to activate the AP-1mut promoter, indicating that the activation of AP-1 transcriptional factors by Nef is required for FasL transcription (Figure 5B). However, transfection of Jurkat cells with the hFasL AP-1mut expression vector in conjunction with pRelA (a subunit of NF-κB) did induce FasL transcription (Figure 5C), indicating that mutation of AP-1 sites does not affect the ability of other transcriptional factors to induce FasL transcription. Transfection of the AP-1 reporter plasmid into Jurkat cells and subsequent infection with NL4-3 Wt or NL4-3 ΔNef virus suggest that Nef is necessary for optimal AP-1-dependent transcription induced by HIV-1 (Figure 5D). Collectively, these data show that Nef-induced FasL expression requires p38, which drives the FasL promoter through AP-1 induction.
The PxxP domain of Nef is required for FasL transcription and p38 activation
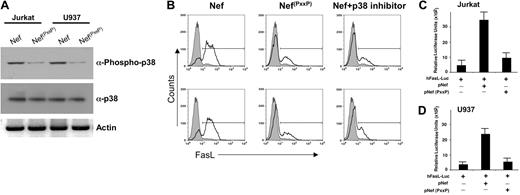
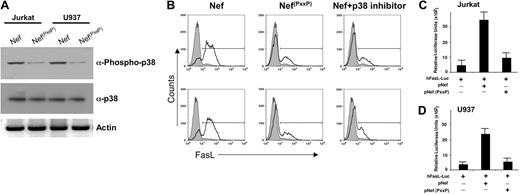
Previous experiments suggest that Nef possesses various protein-binding domains responsible for its activity.31 Of particular interest is the PxxP motif in Nef, which is a binding site for Src homology 3 (SH3)-mediated protein-protein interactions. We next examined if the PxxP region of Nef is required for phosphorylation of p38 in T-cell or in monocyte cell lines by mutating prolines to alanines, which retain Nef structure but block PxxP-associated activity.10,31-34 The PxxP mutation did not stimulate phosphorylation of p21-related kinase family (Pak) in T cells or hematopoietic cell kinase (Hck) phosphorylation in monocytic cell lines (data not shown). Next, Jurkat T cells and monocytic U937 cell lines were transfected with expression vectors for pNef or pNef(PxxP). The Nef containing the PxxP deletion did not stimulate phosphorylation of p38 in either T cells or monocytic cells (Figure 6A). These data suggest that Nef activation of p38 requires the PxxP domain of Nef.
We next examined FasL induction in T cells or monocytes transfected with pNef in the presence or absence of p38 inhibitor or PxxP-mutated Nef. While Nef strongly induced FasL on both T cells as well as monocytes (Figure 6B), mutation of the PxxP domain severely diminished FasL induction, indicating that the PxxP domain of Nef plays an essential role in Nef-driven FasL expression. These results were verified by FasL promoter reporter assays (Figure 6C-D). Collectively, these results illustrate that Nef activates FasL through its PxxP domain in monocytes and T cells. Furthermore, this activation appears to be upstream of Pak activation in T cells and of Hck activation in monocytes (data not shown). This activation in either cell phenotype eventually converges at p38, resulting in its activation, which is required for Nef-mediated AP-1 induction of FasL expression.
AP-1 is required for Nef-induced FasL promoter activation through p38. (A) Jurkat T cells were transfected with plasmid containing either the pVector or the AP1-Luc promoter plasmid, and the pNef plus AP1-Luc promoter plasmid or the pNef plus AP1-Luc promoter plasmid and treated with p38 inhibitors (1 μM) as indicated in “Materials and methods.” (B) Jurkat T cells were transfected with the hFasL-Luc promoter plasmid, hFasL-Luc promoter plasmid plus pNef, or pNef plus hFasLmut-Luc (mutation in the AP-1 binding site) promoter plasmid as indicated. (C) As a control, Jurkat cells were transfected with hFasLmut-Luc or hFasLmut-Luc plus an expression construct for pRelA (p65) as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal, and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments. (D) Nef-deleted virus failed to induce AP-1 transcription. Jurkat cells were transiently transfected with pAP1-Luc vector as indicated. At 48 hours after transfection, cells were infected with NL4-3 Wt with or without p38 inhibitor or NL4-3 ΔNef viruses as indicated. Luciferase activity in whole-cell lysates was monitored after 24 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal. These data are representative of 2 independent experiments. The asterisks indicate significant differences between cells infected with Wt virus (P < .02).
AP-1 is required for Nef-induced FasL promoter activation through p38. (A) Jurkat T cells were transfected with plasmid containing either the pVector or the AP1-Luc promoter plasmid, and the pNef plus AP1-Luc promoter plasmid or the pNef plus AP1-Luc promoter plasmid and treated with p38 inhibitors (1 μM) as indicated in “Materials and methods.” (B) Jurkat T cells were transfected with the hFasL-Luc promoter plasmid, hFasL-Luc promoter plasmid plus pNef, or pNef plus hFasLmut-Luc (mutation in the AP-1 binding site) promoter plasmid as indicated. (C) As a control, Jurkat cells were transfected with hFasLmut-Luc or hFasLmut-Luc plus an expression construct for pRelA (p65) as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal, and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments. (D) Nef-deleted virus failed to induce AP-1 transcription. Jurkat cells were transiently transfected with pAP1-Luc vector as indicated. At 48 hours after transfection, cells were infected with NL4-3 Wt with or without p38 inhibitor or NL4-3 ΔNef viruses as indicated. Luciferase activity in whole-cell lysates was monitored after 24 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal. These data are representative of 2 independent experiments. The asterisks indicate significant differences between cells infected with Wt virus (P < .02).
Inhibition of p38 is sufficient to prevent HIV-1-induced bystander killing of CD8 T cells
FasL is up-regulated in HIV-infected macrophages and T cells. Such FasL-positive cells have been hypothesized to be able to induce Fas-mediated apoptosis of CD8 effector T cells.3-5 Accumulating evidence indicates that antigen-presenting cells such as macrophages play a key role in the elimination of activated effector T cells.3,4,35 Accordingly, we next investigated the role of p38 activation of HIV-infected macrophages to induce bystander apoptosis of CD8 T cells.
PxxP domain of Nef is required for the FasL induction. (A) Loss of the PxxP domain of Nef significantly diminishes the phosphorylation of p38. Western blot analysis of extracts derived from Jurkat or U937 cells transfected with either the expression vector for wild-type Nef or the amino acid-mutated Nef. Cell extracts were prepared 48 hours after transfection as described in “Materials and methods” and subjected to 12% SDS-PAGE followed by PVDF membrane transfer and analyzed by Western blotting using specific p38 and phospho-p38 antibodies as indicated. Note, the amino acid-mutated Nef (pNef(PxxP)) failed to induce phosphorylation of p38. (B) Comparison of FasL induction by wild-type Nef versus amino acid-mutated Nef. Jurkat T cells or U937 cells were electroporated with 5 μg wild-type Nef, amino acid-mutated Nef, or wild-type Nef plus p38 inhibitor (1 μM). At 48 hours after transfection, the surface levels of FasL were determined by flow cytometry by staining with a FasL-specific antibody. Transfection efficiency was monitored by cotransfection of a pCMV plasmid encoding GFP. Thick line histograms show the indicated surface markers, and filled histograms represent the isotype-matched control antibodies. Similar results were obtained in 3 independent experiments. (C-D) The PxxP domain of Nef (amino-acid mutated) is essential for induction of FasL promoter activity in T cell or monocytic cells. Jurkat T cells or U937 cells were transfected with 5 μg hFasL-Luc promoter plasmid, wild-type Nef plus hFasL-Luc promoter plasmid, or amino acid-mutated Nef plus hFasL-Luc promoter plasmid as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments.
PxxP domain of Nef is required for the FasL induction. (A) Loss of the PxxP domain of Nef significantly diminishes the phosphorylation of p38. Western blot analysis of extracts derived from Jurkat or U937 cells transfected with either the expression vector for wild-type Nef or the amino acid-mutated Nef. Cell extracts were prepared 48 hours after transfection as described in “Materials and methods” and subjected to 12% SDS-PAGE followed by PVDF membrane transfer and analyzed by Western blotting using specific p38 and phospho-p38 antibodies as indicated. Note, the amino acid-mutated Nef (pNef(PxxP)) failed to induce phosphorylation of p38. (B) Comparison of FasL induction by wild-type Nef versus amino acid-mutated Nef. Jurkat T cells or U937 cells were electroporated with 5 μg wild-type Nef, amino acid-mutated Nef, or wild-type Nef plus p38 inhibitor (1 μM). At 48 hours after transfection, the surface levels of FasL were determined by flow cytometry by staining with a FasL-specific antibody. Transfection efficiency was monitored by cotransfection of a pCMV plasmid encoding GFP. Thick line histograms show the indicated surface markers, and filled histograms represent the isotype-matched control antibodies. Similar results were obtained in 3 independent experiments. (C-D) The PxxP domain of Nef (amino-acid mutated) is essential for induction of FasL promoter activity in T cell or monocytic cells. Jurkat T cells or U937 cells were transfected with 5 μg hFasL-Luc promoter plasmid, wild-type Nef plus hFasL-Luc promoter plasmid, or amino acid-mutated Nef plus hFasL-Luc promoter plasmid as indicated. Luciferase activity in whole-cell lysates was assayed after 12 to 18 hours and is shown as the mean value ± SEM. Transfection efficiency was monitored by cotransfection of a plasmid encoding β-gal and results were normalized to β-gal levels. Similar results were obtained in 3 independent experiments.
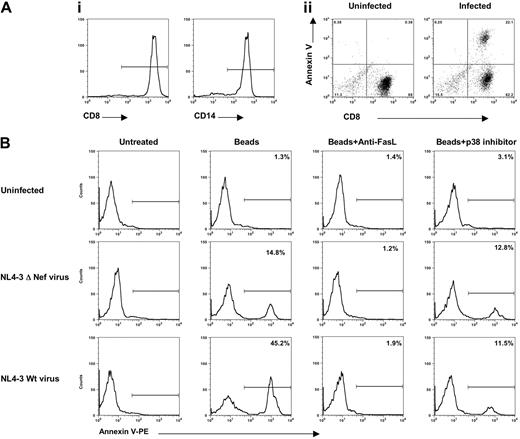
CD14 and CD8 cell populations were isolated from HIV-1-negative PBMCs (Figure 7Ai) by cell-negative immune selection. We then examined CD8 T-cell apoptosis after 12 hours of incubation with CD14 macrophages that were infected with the NL4-3 pseudovirus, which encoded VSV-G envelope, which had been activated through treatment with latex beads to stimulate phagocytosis. Annexin-V staining on the gated CD8 cells shows that 22.1% of the cells were induced to undergo apoptosis (Figure 7Aii). Uninfected macrophages, even when activated by polystyrene beads, induced a negligible level of apoptosis in the CD8 T-cell population under any of the conditions tested (Figure 7B, top panels). Also, the ΔNef virus did not induce bystander killing under these conditions (Figure 7B, middle panels). In contrast, HIV-infected macrophages stimulated with latex beads drove apoptosis in the CD8 T cells (Figure 7B, bottom panels). The addition of a neutralizing anti-FasL mAb to the cell culture completely blocked the induction of apoptosis, suggesting its required role. Of interest, p38 blockade was almost as effective as anti-FasL antibody at preventing bystander apoptosis, but there was clearly a residual level of apoptosis that was resistant to the inhibitor. This is consistent with prior published reports suggesting that other HIV antigens through p38-independent mechanisms can play a role in bystander apoptosis. These findings clearly illustrate that HIV-infected macrophages can induce Fas/FasL-mediated apoptosis of CD8 T cells during HIV infection, and inhibition of p38 is sufficient to prevent much of the resulting bystander apoptosis.
HIV-specific CD8+T-cell apoptosis is blocked by a p38 inhibitor. (Ai) CD14 macrophages and CD8 T cells were isolated from HIV-1-negative PBMC donors as described in “Materials and methods.” (Aii) Macrophages were infected with HIV-1 wild-type pseudovirus (NL4-3 virus that complemented with VSV-G envelope) (100 TCID50/1 × 106 cells per milliliter) and incubated with latex beads before being mixed with autologous CD8 T cells. Fourteen hours after CD8 T cells were added to infected macrophages, cells were harvested and stained with CD8-FITC/annexin-V-PE. Note that the CD8 T cells were induced to undergo apoptosis. (B) Autologous macrophages were uninfected or infected with pseudovirus HIV-1 NL4-3 ΔNef virus or infected with NL4-3 Wt virus (viruses that complemented with VSV-G envelope) at concentration of 100 TCID50/1 × 106 cells/mL, were stimulated with latex beads, and were incubated with purified mock CD8+ T cells in the presence or absence of neutralizing anti-FasL antibody or with 1 μM p38 inhibitor overnight. Cells were then harvested and stained for CD8 plus annexin-V. Apoptosis analysis was performed by gating the low forward and CD8 scatter of cells. The percentage values represent the gated CD8 annexin V-positive cells. A representative experiment of 3 performed is shown.
HIV-specific CD8+T-cell apoptosis is blocked by a p38 inhibitor. (Ai) CD14 macrophages and CD8 T cells were isolated from HIV-1-negative PBMC donors as described in “Materials and methods.” (Aii) Macrophages were infected with HIV-1 wild-type pseudovirus (NL4-3 virus that complemented with VSV-G envelope) (100 TCID50/1 × 106 cells per milliliter) and incubated with latex beads before being mixed with autologous CD8 T cells. Fourteen hours after CD8 T cells were added to infected macrophages, cells were harvested and stained with CD8-FITC/annexin-V-PE. Note that the CD8 T cells were induced to undergo apoptosis. (B) Autologous macrophages were uninfected or infected with pseudovirus HIV-1 NL4-3 ΔNef virus or infected with NL4-3 Wt virus (viruses that complemented with VSV-G envelope) at concentration of 100 TCID50/1 × 106 cells/mL, were stimulated with latex beads, and were incubated with purified mock CD8+ T cells in the presence or absence of neutralizing anti-FasL antibody or with 1 μM p38 inhibitor overnight. Cells were then harvested and stained for CD8 plus annexin-V. Apoptosis analysis was performed by gating the low forward and CD8 scatter of cells. The percentage values represent the gated CD8 annexin V-positive cells. A representative experiment of 3 performed is shown.
Discussion
Hallmarks of HIV-1 infection include the destruction of T cells and the suppression of cellular immune responses in vivo.3-6,35,36 A mechanism that has been previously reported is through the bystander killing of CD8 effector T cells, the cell population directly responsible for immune clearance and controlling viral load.3,5,35,36 Indirect cell killing has been proposed to involve the up-regulation of FasL-inducing apoptosis of effector cytotoxic T lymphocytes (CTLs) as they approach viral-harboring CD4 T cells and macrophages. Furthermore, lamina propria of SIV-infected rhesus macaques has been shown to harbor massive apoptosis of viral-specific memory T cells through the activation of the Fas-FasL pathway.37 Hence this strategy results in a significant advantage for HIV in evasion of immune recognition and viral clearance.
A recent report identified that Nef binds to the CD3ξ chain of the TCR complex, and this interaction is important for the induction of FasL expression.38 The effect required the PxxP domain, which consequently stimulated the transcriptional activation of FasL.38 Additional evidence suggests that Nef coprecipitates with the Nef-associated kinase (NAK), a member of the Pak39 in T cells. NAK is activated via the small guanosine triphosphatases (GTPases) CDC42 and Rac1 through Vav, and moreover, Pak1 and Pak2 are implicated in this activation.32-34 Although it is believed that activation of FasL transcription functions through the TCR-CD3+ complex mediated by the CD3+ξ chain,32,38,40 the downstream signals and their transcriptional regulation required for this activation have not been determined.
The results presented here indicate that p38 MAPK activation is necessary for T-cell and macrophage activation and eventual FasL transcription in various viral subtypes. Previous work suggests that multiple factors may be sufficient to induce FasL expression.16,26,27 Accordingly, we suggest that the AP-1 enhancer is required for Nef to induce FasL transcription. Further, p38 is also required for AP-1 activation, suggesting a linear pathway that involves p38 activation and its subsequent AP-1 activation. Recently, Biggs et al41 identified that AP-1 can be induced by Nef via extracellular signal-related kinase 1/2 (ERK1/2) MAPK signaling events. Our results suggest that p38-like ERK1/2 is required for AP-1 activation, and that this leads to a transcriptional up-regulation of FasL. Our evidence suggests that p38 MAPK is important for FasL-mediated killing and specifically targets AP-1 transcriptional factors for this effect. Thus our data extend these recent observations and link the AP-1 pathway with p38 activation and bystander killing.
Previous studies indicate that HIV infection of macrophages can induce FasL expression, which drives bystander killing of CD4+ T cells.4,11 Additionally, macrophages also drive FasL up-regulation to induce bystander killing of HIV-specific CD8 T cells.35 The destruction of the CD8 T cells by the FasL pathway is likely a significant damper for the cell-mediated immune response, which ultimately could limit immune clearance.4,6,7 We have also observed blockade of apoptosis using primary viral isolates.
Our results suggest that Nef is critical for efficient p38 activation and AP-1-driven transcription induced by HIV-1 infection. However, residual p38 phosphorylation and AP-1-mediated transcription can be observed in Nef-deleted viruses (Figure 5D), suggesting that other factors such as Env or other HIV accessory genes may also be capable of activating p38 and AP-1-driven transcription.24,25 On the other hand, Env is neither required nor sufficient to up-regulate FasL (data not shown), suggesting that either the inputs from Env are not sufficiently robust to drive FasL transcription or Nef is sufficient to activate another undetermined pathway that is concomitantly required with p38 to drive FasL transcription (data not shown), and overexpression fails to augment Nef-induced FasL transcription (Figure 3B).
In conclusion, our work identifies a linear signaling pathway that is necessary for HIV-1 Nef-induced bystander killing. We show that Nef activates and requires p38 MAPK activation and its AP-1 enhancer target within the FasL promoter to stimulate its transcription. Through loss-of-function experiments, we were able to delineate which factors are activated versus required for regulating FasL transcription. We suggest that this finding has important applications for the development of novel HIV therapeutics, as anti-p38 compounds could target a vital pathogenic function of HIV-1.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2005-03-0932.
Supported by a Johnson & Johnson Pharmaceutical Research and Development (PRD) grant to D.B.W. and K.M. Supported also by the National Institutes of Health (NIH) AIDS Research and Reference Reagents program and the Center for AIDS Research (CFAR), University of Pennsylvania.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Dr Craig B. Thompson, University of Pennsylvania, and Dr Mario Stevenson, University of Massachusetts Medical Center, for their continuous support, suggestions, and provision of reagents.


![Figure 4. Nef is required for HIV-1-mediated FasL induction and efficient p38 activation. (A) Expression and analysis of the proviral expression constructs. A frameshift mutation (5′ Nef) was introduced in the nef gene in the proviral constructs as described in “Materials and methods.” Cell lysates derived from 293T cells transfected with pNef, pNL4-3 Wt, or pNL4-3 ΔNef HIV-1 proviral constructs were separated by 12% SDS-PAGE and then transferred to nitrocellulose membranes. Samples were probed with an HIV-1 Nef-specific antiserum. Nef was not expressed in the pNL4-3 ΔNef constructs. WB indicates Western blot. (B) HIV-1 infection was monitored by measuring p24 levels in culture supernatants derived from PBMCs infected with NL4-3 Wt or NL4-3 ΔNef virus. Data were obtained 96 hours after infection. These experiments were repeated 3 times and similar results were obtained. Error bars represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (C) Induction of FasL expression by NL4-3 Wt or NL4-3 ΔNef viruses in human PBMCs. Cells (1 × 106) were infected with NL4-3 Wt or NL4-3 ΔNef virions and then treated with or without 1 μM p38 inhibitor RWJ67657 as indicated. Two days after infection, an equal number of cells (analysis was performed on a gated low forward scatter and CD24[HAS]-positive cells) were assayed for intracellular p24gag and surface FasL expression by flow cytometry as described in “Materials and methods.” The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 independent experiments and observations of similar suppression of FasL were obtained. (D) Nef-deleted viruses failed to induce phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells infected with mock, NL4-3 Wt, or NL4-3 ΔNef virus. Samples were prepared 24 hours after infection as described in “Materials and methods” and immunoblotted with p38MAPK and phospho-p38MAPK-specific antibodies as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-03-0932/6/m_zh80180584270004.jpeg?Expires=1766345433&Signature=EiAa9N5yynIbNGFIzXIGevYico~EVt3RRmQ4LkHzLlJ3nC1P-17v6fer3iIIPISqqcYhuMBkcK2ywK-p8jih4LrOvB1gsIwaj-YZBodHszBVIhwz8nnMmk1DPKco86WP6rD3rpR3sRMzibLZBqZPArAW9TLWwq5SgP6x~ZO4Vrn-HRQOo2UdAdbMSeFlL6VNmT1fKL6lTkzcr2TEYvFJLMjQ0OUvuTDPFp51sZB-ODz9H6lwl90Q5DelD6HTf7xBm6HQCDAai7SFxBJmYnFlu5szhJcoH4yX7TjS54Gkgtbw6sAuD-1LGdB2zqOz~OepT61AMVKtIZGmLqlUL-Fszw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 4. Nef is required for HIV-1-mediated FasL induction and efficient p38 activation. (A) Expression and analysis of the proviral expression constructs. A frameshift mutation (5′ Nef) was introduced in the nef gene in the proviral constructs as described in “Materials and methods.” Cell lysates derived from 293T cells transfected with pNef, pNL4-3 Wt, or pNL4-3 ΔNef HIV-1 proviral constructs were separated by 12% SDS-PAGE and then transferred to nitrocellulose membranes. Samples were probed with an HIV-1 Nef-specific antiserum. Nef was not expressed in the pNL4-3 ΔNef constructs. WB indicates Western blot. (B) HIV-1 infection was monitored by measuring p24 levels in culture supernatants derived from PBMCs infected with NL4-3 Wt or NL4-3 ΔNef virus. Data were obtained 96 hours after infection. These experiments were repeated 3 times and similar results were obtained. Error bars represent the mean ± standard deviation from triplicate samples derived from 1 of 3 independent experiments. (C) Induction of FasL expression by NL4-3 Wt or NL4-3 ΔNef viruses in human PBMCs. Cells (1 × 106) were infected with NL4-3 Wt or NL4-3 ΔNef virions and then treated with or without 1 μM p38 inhibitor RWJ67657 as indicated. Two days after infection, an equal number of cells (analysis was performed on a gated low forward scatter and CD24[HAS]-positive cells) were assayed for intracellular p24gag and surface FasL expression by flow cytometry as described in “Materials and methods.” The values indicated frequencies of positive cells of each quadrangle. Data were representative of 3 independent experiments and observations of similar suppression of FasL were obtained. (D) Nef-deleted viruses failed to induce phosphorylation of p38. Western blot analysis of protein extracted from Jurkat cells infected with mock, NL4-3 Wt, or NL4-3 ΔNef virus. Samples were prepared 24 hours after infection as described in “Materials and methods” and immunoblotted with p38MAPK and phospho-p38MAPK-specific antibodies as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-03-0932/6/m_zh80180584270004.jpeg?Expires=1766345434&Signature=WquYWiX5ftn-VWYgOd3-clVx7zDA5RitVNeiMdrtwWsR4Km63e2h5BqxjhQWTApQWU~oMTm4xptJ4g2pd4oNPBsyqmhUGonCiXj~JocTMu8ZfAtvGQe-s7btHEq2bEUbkg3lqvGMy0LX3ga8fCOqF~0rQK6oVBdWxJKiOBnRdtqWO3rz1W1a7RABcAkBqSL6IdgqJKyVdQLh4ulue9ed9O~7jV-6vLehWWnoFsKAF0F4bZw4a6ewInhhWFTv9SJ1xsvaU3Z-7ANLEPLtIFUzvo1Ld2PsASNbPDCk1wO4e4iLSzsvNqxvXff-674jAkT1UsfCL-2O61b8-9BHxz368A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)