In a recent Blood article, Mims and colleagues1 reported the phenotype of a patient with anemia and iron overload who was homozygous for a novel mutation in the iron transporter SLC11A2 (DMT1). SLC11A2 (solute carrier family 11, member 2) is the only known transporter involved in cellular iron uptake in mammals. We recently showed that Slc11a2 was critical for both intestinal iron absorption and erythroid iron assimilation in mice.2 Slc11a2-/- mice died within the first few days of life with severe systemic iron deficiency and consequent anemia. Animals lacking Slc11a2 only in the intestine had a similar but less severe phenotype that could be rescued by parenteral iron administration. Lethally irradiated wild-type animals transplanted with Slc11a2-/- hematopoietic stem cells were anemic (hemoglobin [Hgb] 8.53 ± 0.20 g/dL, mean corpuscular volume [MCV] 26.33 ± 0.59; n = 6) compared with animals that had received wild-type cells (Hgb 13.20 ± 0.13, MCV 50.32 ± 0.63; n = 5) 8 to 12 weeks after transplantation (P < .001). However, liver iron stores were increased, with liver nonheme iron averaging 109.6 μg/g wet weight (n = 2) in animals that received transplants versus 63.0 μg/g wet weight in controls (n = 2) 12 weeks after transplantation and later.2 This phenotype, observed in mice lacking Slc11a2 only in hematopoietic cells, was strikingly similar to that in the human patient.
The human mutation reported by Mims et al has 2 molecular consequences. First, it favors the production of a splice isoform missing exon 12, which encodes the eighth predicted transmembrane segment of SLC11A2. Second, transcripts retaining exon 12 encode an E399D substitution. To attempt to understand why the phenotype resulting from the human mutation differed from that seen in mice lacking Slc11a2, we analyzed the functions of the 2 mutant gene products (Figure 1).
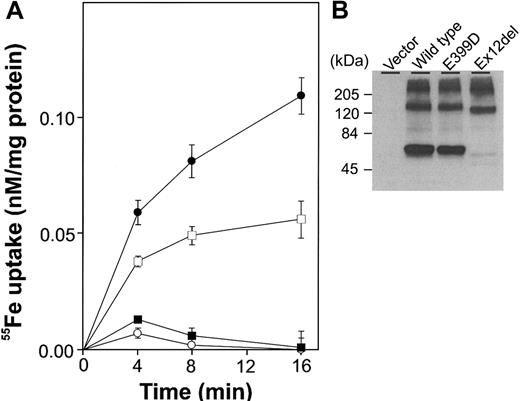
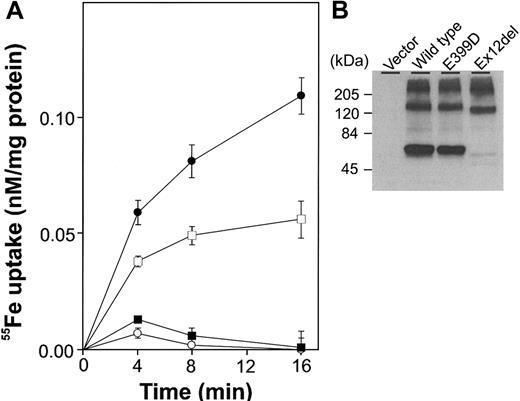
SLC11A2 that lacked exon 12 was poorly expressed in transiently transfected HEK293T cells and had little, if any, iron transport activity. In contrast, the E399D mutant protein showed expression that was comparable to the wild-type protein and had substantial iron transport activity. We infer that tissues expressing only the exon 12-deleted form would lack SLC11A2 activity and that tissues expressing the E399D form would have SLC11A2 activity in proportion to the amount of protein present.
Function of mutant forms of SLC11A2. (A) HEK293T cells were transiently transfected with wild-type SLC11A2 (•), exon-12-deleted SLC11A2 (▪), E399D SLC11A2 (□) or vector alone (○). 55Fe uptake experiments were carried out twice, in duplicate or triplicate, as described previously.3 A representative experiment is shown. (B) Immunoblot analysis was performed with an anti-FLAG M2 monoclonal antibody (Sigma, Saint Louis, MO) recognizing an epitope tag.
Function of mutant forms of SLC11A2. (A) HEK293T cells were transiently transfected with wild-type SLC11A2 (•), exon-12-deleted SLC11A2 (▪), E399D SLC11A2 (□) or vector alone (○). 55Fe uptake experiments were carried out twice, in duplicate or triplicate, as described previously.3 A representative experiment is shown. (B) Immunoblot analysis was performed with an anti-FLAG M2 monoclonal antibody (Sigma, Saint Louis, MO) recognizing an epitope tag.
While it is possible that humans have a distinct, unknown iron uptake activity that is absent in mice, as proposed by Mims et al, we favor a different hypothesis. If the patient expressed substantial amounts of functional E399D SLC11A2 in the intestine, her increased liver iron stores could be explained by the combined effects of enhanced intestinal absorption in response to anemia and redistribution of iron from the erythron to the liver.
Human DMRT1 mutation and mechanism for increased absorption
We recently reported on a patient with microcytic hypochromic anemia who is homozygous for a mutation in DMT1 that exaggerates the alternative splicing normally present in hematopoietic cells but not in normal duodenal enterocytes.1 Unlike animal models of DMT1 mutation, the iron-deficient mk mouse and Belgrade rat, our patient was not only anemic but also iron overloaded. In their letter, Gunshin and coworkers formally prove that, in HEK293T cells, the alternatively spliced DMT1 isoform present as the major species in the hematopoietic cells of our patient does not code for a functional iron transporter and that the full length DMT1 isoform with the missense mutation codes for a functional transporter. We predicted in our paper that this would indeed be the case.
Gunshin and colleagues propose that the observed difference in phenotype between our patient (who is iron overloaded) and the mk mouse and Belgrade rat (which are iron deficient) is explained in the patient by the combined effects of enhanced intestinal absorption in response to anemia and redistribution of iron from the erythron to the liver. We view the explanation for our patient's increased liver iron content differently, and propose that absorption of heme iron may account for some of the difference. In our study we found no difference in total DMT1 mRNA between the patient's duodenum and normal duodenum; however, the patient's duodenum (unlike normal duodenum) had primarily alternatively spliced DMT1 mRNA with a small amount of full-length DMT1 transcript. Although the Western blots of duodenum were not completely quantitative, there did not appear to be a significant difference in DMT1 between the patient and healthy controls. Thus, one would need to predict that up-regulation of ferroportin (due to the low level of hepcidin demonstrated in our patient) combined with 5% to 10% of normal iron uptake by enterocytes (based on ∼10% full length DMT1 mRNA translated into protein with 50% activity as predicted by Gunshin et al) would be enough to result in iron overload in a menstruating female. This uncertainty both emphasizes our incomplete knowledge of how much DMT1 protein is required for “normal” iron absorption in the intestine compared with the level required for “normal” iron utilization by erythroid precursors and demonstrates the need for further work in the area.
Correspondence: Josef Prchal, Hematology 802E, 1 Baylor Plaza, Baylor College of Medicine, Houston, TX 77030 OR Dept of Pathophysiology, Charles University School of Medicine, Prague, Czech Republic; e-mail: jprchal@bcm.tmc.edu.