Abstract
We monitored the number of intravascular platelet-leukocyte aggregates (PLAs) and thrombotic occlusions (TOs) by intravascular microscopy in the mesentery of rats receiving antiphospholipid (aPL) immunoglobulin G (IgG) purified from the sera of patients with antiphospholipid syndrome. aPL IgG had no procoagulant effect, but it caused rapid endothelial deposition of fibrinogen, followed by PLA and TO in rats receiving an intraperitoneal injection of bacterial lipopolysaccharide 3 hours before IgG infusion. Anti-β2-glycoprotein I-depleted aPL IgG failed to induce PLAs and TOs. C3 and C9 colocalized with aPL IgG on the mesenteric vessels. The number of PLAs and TOs was markedly reduced in C6-deficient rats and in animals treated with anti-C5 miniantibody, suggesting the contribution of the terminal complement (C) complex to the aPL antibody-mediated intravascular thrombosis. In conclusion, our data indicate that antibodies to β2-glycoprotein I trigger coagulation subsequent to a priming proinflammatory factor and that the terminal C complex is the main mediator of the coagulation process.
Introduction
Antiphospholipid syndrome (APS) is a clinical condition characterized by recurrent thrombosis at diverse anatomic sites and fetal losses that affects young people and causes significant morbidity with high social costs. The diagnosis is supported by the finding of arterial/venous occlusions and/or obstetric complications as well as by the detection of antiphospholipid (aPL) antibodies.1 It is now widely accepted that aPL antibodies react to a large extent with PL-binding plasma proteins rather than with negatively charged PLs. The β2-glycoprotein I (β2GPI) and prothrombin account for greater than 90% of the target antigens recognized by these antibodies.2
Clinical and experimental studies have provided evidence for the direct implication of aPL antibodies in the development of APS, and data obtained from in vitro models strongly suggest that anti-β2GPI antibodies play a major role.3,4 Passively infused aPL antibodies, although effective in causing fetal loss in pregnant mice,5,6 are apparently unable to induce thrombotic manifestations per se in the animals.7,8 The results of in vivo experiments have shown that the main effect of aPL antibodies is to increase the size of the clot triggered by mechanical or photochemical injury of the vessels, suggesting that they act by potentiating the procoagulant effect of an initiating factor.7,8 A 2-hit hypothesis has been proposed to justify the requirement of the vessel injury for aPL-dependent clotting enhancement and to explain the clinical observation of occasional and localized thrombi observed in patients with APS despite the persistent presence of aPL antibodies in the circulation.4 According to this hypothesis, aPL antibodies provide the first hit that increases the risk of thrombotic events and potentiates the procoagulant effect of a later thrombophilic condition that acts as a second hit. In line with this hypothesis, concomitant risk factors for thrombosis have been identified in up to 50% of patients with APS, including surgical operations and prolonged immobilization associated with venous thrombotic occlusions, dyslipidemia, and arterial hypertension observed in arterial thrombosis.9 Infections have also been found to precede the onset of APS, and their frequency can be as high as 24% in patients with the catastrophic variant of the syndrome.10,11 Microbial agents may act as a second hit that favor a procoagulant state per se eventually resulting in thrombus formation.4
In an attempt to elucidate the sequence of events triggered by aPL antibodies and leading to the formation of thrombi, we have used intravital microscopy to monitor the vascular changes that occur in rats following the intraarterial injection of aPL antibodies. This in vivo model has proved useful in evaluating the contribution of additional factors other than aPL antibodies to promote the localization of thrombi in restricted areas of the vascular tree. Data will be reported showing for the first time that human polyclonal immunoglobulin G (IgG) with anti-β2GPI activity triggers clotting in the microcirculation of rat mesentery in the presence of a priming proinflammatory stimulus and that the terminal complement (C) complex is required for aPL antibody-dependent thrombus formation.
Patients, materials, and methods
Patients and antibody assays
All patients had a history of arterial or venous thrombosis or both. In addition, they displayed medium-high titer of anti-β2GPI antibodies detected as described in a previous publication.12 The clinical features and the level of aPL, anti-β2GPI, and C-fixing antibodies are reported in Table 1. Anticardiolipin and anti-β2GPI antibodies were detected by enzyme-linked immunosorbent assay (ELISA), and values were expressed as IgG antiphospholipid units (GPL) or OD, respectively, as described.13 Values higher than 10 GPL or than the 95th percentile of 50 healthy subjects for the anti-β2GPI antibodies (OD, 0.16 for IgG) were considered positive. C-fixing antibodies were tested by an assay similar to that used to measure the anti-β2GPI antibodies except that purified IgG (150 μg/mL) was incubated with solid-phase-bound β2GPI (10 μg/mL) for 90 minutes at room temperature (RT) followed by purified C1q (Quidel, San Diego, CA) at the concentration of 20 μg/mL for 60 minutes at 37°C. The bound C1q was revealed by its reaction with goat anti-C1q IgG (Quidel) labeled with biotin, as previously described,14 followed by alkaline phosphatase-labeled streptavidin (Sigma-Aldrich, Milan, Italy). Details of the immunoenzymatic reaction are given in Pausa et al.15 The OD values observed in the control group did not exceed 0.25. An informed consent was obtained from patients and healthy control subjects according to the Declaration of Helsinki.
Purification of aPL and anti-β2GPI antibodies
The IgG was purified on HiTrap Protein G column (Pharmacia, Milan, Italy) from the sera of 6 patients with APS diagnosed according to the Sapporo criteria.16 Control IgG fractions were purified from the sera of 5 blood donors negative for aPL antibody. The IgG from 3 patients was depleted of anti-β2GPI antibodies by affinity chromatography through β2GPI-sepharose column as previously reported.17
Preparation of anti-C5 miniantibodies
Miniantibody containing TSA12/22 was generated by amplifying and cloning the single-chain fragment variable TS12/2218 in the PUT-sec19 vector containing the rat CH2-CH3 crystallizable fragment (Fc) region. The miniantibody (MB-12/22) was then subcloned as EcoRI-HindIII fragment in the pCDNA3 (Invitrogen, Milan, Italy) vector. Purified plasmid DNA was transfected with Lipofectamine 2000 (Invitrogen) in HEK293T cells grown in Dulbecco Modified Eagle Medium (Gibco, Milan, Italy) supplemented with 10% fetal calf serum previously depleted of IgG using HiTrap Protein G column (Pharmacia). MB-12/22 was purified from cell-conditioned medium loaded on Protein G column and eluted with 1 M NaCl in phosphate-buffered saline. Fractions containing the MB-12/22 were selected by ELISA and checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
A similar procedure was followed to prepare a miniantibody to gliadin from the original single-chain fragment variable (scFv) previously described by Marzari et al.20
Animals
Male rats (270-300 g) of 3 different strains, including Wistar, C6+/+ PVG, and C6-/- PVG rats, were used for the in vivo experiments. The Wistar rats were obtained from a local colony kept under controlled dietary conditions in the University Animal House. C6+/+ PVG male rats were purchased from Harlan Italy (Corezzano, Italy), and C6-/- PVG male rats were from a previously described rat colony21 established in our Animal House. All the experimental procedures were performed in compliance with the guidelines of European (86/609/EEC) and the Italian (D.L.116/92) laws and approved by the Italian Ministry of University and Research as well as by the Administration of the University Animal House.
Bleeding and coagulation times
The tail bleeding time and the coagulation time were evaluated in C6+/+ and C6-/- PVG rats according to De Clerck et al.22
Rat model of thrombosis
The in vivo experiments were performed on male Wistar rats (270-300 g) that received an intraperitoneally injection of bacterial lipopolysaccharide (LPS) from Escherichia coli O55:B5 (2.5 mg/kg body weight; Sigma-Aldrich) or sterile saline, as a control, and 3 hours later were anesthetized with sodium thiobarbital (Inactin; 80 mg/kg) purchased from BYK (Konstanz, Germany). The left carotid artery and the femoral vein were cannulated with polyethylene catheters (0.45 and 0.60 inner diameter, respectively) (Intramedic Clay-Adams, Sparks, MD) connected to a micro-infusion pump (Harward Apparatus, Holliston, MA). The tip of the catheter was pushed inside the carotid artery to the aortic arch to inject the IgG directly into the arterial circulation. The fluorescent vital dye Rhodamine 6G (Sigma), which stains leukocytes and platelets, was infused slowly into the femoral vein at the concentration of 0.025 mg/kg/min and at a rate of 0.25 mL/h 30 minutes prior to the intraarterial administration of IgG.
Details of the surgical procedure and analysis of mesenteric microvessels by intravital microscopy to monitor the thrombus formation were reported in previous publications.23,24 At least 3 microvascular areas containing arterioles, capillaries, and postcapillary venules were analyzed for the formation of fluorescent aggregates of leukocytes and platelets that partially or completely occluded the vessels. The results are expressed as a ratio between the number of thrombi and the total number of microvessels examined and also as percentage of occluded microvessels.
Evaluation of fibrinogen deposition
Rat fibrinogen (3 mg/mL; Sigma) was incubated with fluorescein isothiocyanate (FITC; Sigma) dissolved in dimethyl sulfoxide (DMSO) at the concentration of 10 mg/mL for 1 hour at RT at a ratio of 70 μg FITC/mg proteins. The labeled proteins were dialyzed for 2 days against several changes of sterile saline at 4°C and filtered through a 0.2-μm pore filter (BD Biosciences, Milan, Italy). FITC-fibrinogen was infused intravenously over a 5-minute period at the concentration of 13 mg/mL to a final volume of 250 μL in sterile saline immediately before the videomicroscopy analysis at the time of the intraarterial infusion of aPL-positive IgG. Deposition of FITC-fibrinogen on the endothelium of the mesenteric microvessels was monitored at 5-minute intervals up to 1 hour after the infusion of aPL-positive IgG, and the kinetics of fibrinogen deposition was compared with that of the distribution of leukocytes and platelets labeled with rhodamine 6G. Control rats received LPS followed 3 hours later by FITC-fibrinogen infusion and aPL-negative IgG to evaluate the effect of LPS on fibrinogen deposition.
Immunofluorescence
The mesenteric tissue harvested from rats at the end of the in vivo experiment was analyzed for the deposition of IgG and C components. To reduce nonspecific protein binding, blood was washed from the mesenteric microvessels by infusing sterile saline through the cannulated carotid artery at a flow rate of 2 mL/min and drained through the jugular vein. The rats were then killed and, after exposing and washing the intestine, the mesenteric tissue was dissected and cut into small pieces, which were then stretched on polylysine-treated glass slides (BDH Laboratory Supplies, Poole, United Kingdom). The slides were dried at 37°C overnight and stored at -20°C until use. To detect IgG, the mesenteric tissue was fixed in acetone, permeabilized with a solution of 0.1% Triton X-100 for 15 minutes at RT, and finally incubated with 1/50 FITC-labeled goat anti-human IgG (Sigma) for 1 hour at RT. The tissue was double-stained for C3 or C9 using 5 μg/mL biotin-labeled IgG against rat C3 or C9 for 1 hour at RT followed by 1/100 R-phycoerythrin (RPE)-conjugated streptavidin (Dako, Glostrup, Denmark). In other experiments deposition of C components was also evaluated using the same biotin-labeled primary antibodies against C3 and C9 followed by FITC-conjugated streptavidin (Dako). The cells were viewed under the fluorescence microscope (Zeiss 250-CF Jenalumar; Zeiss, Jena, Germany).
Statistical analysis
The results were expressed as mean ± SD. Data were compared by analysis of variance (ANOVA) using post hoc analysis for paired multiple comparisons with Fisher corrected t test. Probabilities of 0.05 or less were considered statistically significant.
Results
aPL antibodies induce thrombosis in LPS-primed rats
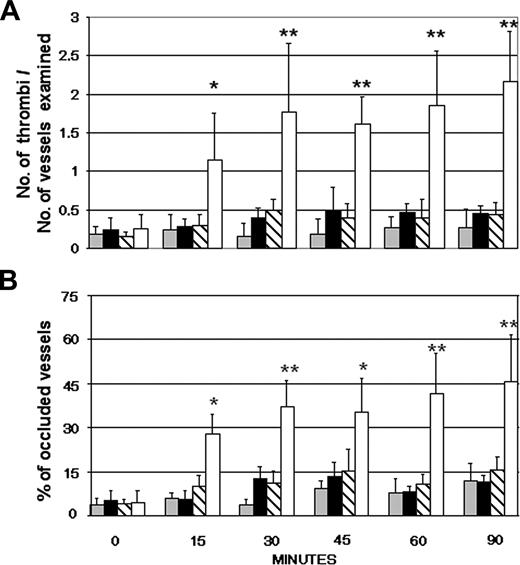
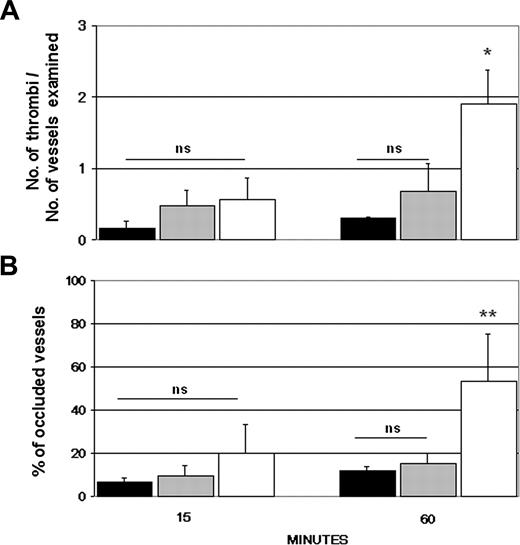
Initial experiments were performed to establish a rat model of thrombosis induced by the ischemia of the superior mesentery artery for 40 minutes followed by reperfusion. Tranexamic acid, an antifibrinolytic agent, was slowly infused intravenously at the concentration of 200 mg/kg prior to ligation of the artery to enhance the coagulation process. Partial or complete thrombotic occlusions of the microvessels occurred within 20 to 60 minutes from the start of reperfusion (data not shown). Using this as a reference model of thrombosis, we monitored the intravascular changes that occurred in the microvessels of rat mesentery following the intraarterial infusion of IgG purified from aPL-positive sera. Rhodamin 6G was injected 30 minutes prior to IgG to label circulating leukocytes and platelets involved in thrombus formation. None of the IgG samples had an overt procoagulant effect over 90 minutes of observation, giving results essentially similar to those obtained with the IgG from aPL-negative sera (Figure 1). To ascertain whether a proinflammatory stimulus was required for the aPL-dependent procoagulant effect, the rats received LPS intraperitoneally 3 hours prior to infusion of aPL-positive IgG. The dose of LPS (2.5 mg/kg body weight) was selected because it caused only a mild adhesion of leukocytes and platelets to the endothelium in the absence of cell aggregates. In rats that received aPL-positive IgG in addition to LPS, stable cell microaggregates of platelets and leukocytes started to be seen 10 to 15 minutes after IgG infusion. The cell aggregates became progressively larger by 25 to 30 minutes, and their number almost doubled after 60 minutes (Figure 1). The thrombi caused partial or complete occlusions of the mesenteric microvessels, particularly of the distal small arteries, resulting in a reduced blood flow and, in some cases, in a complete stop of circulating blood. No thrombi were observed in LPS-treated rats that received an infusion of aPL-negative IgG (Figure 2).
Effects of infusion of human aPL-positive and aPL-negative IgG in rats with or without pretreatment with LPS. Human IgG (10 mg/1 mL sterile saline) purified from 6 aPL antibody-positive sera was infused into the carotid artery of Wistar rats 3 hours after the intraperitoneally injection of either LPS (□) or sterile saline (▧). Another group of rats was treated with 5 aPL-negative IgG (10 mg/1 mL sterile saline) with (▪) or without (▦) pretreatment with LPS. The procoagulant effect of the various treatments was evaluated by counting the number of microvessels with partial or total occlusions (A) and the number of occluded vessels, as shown by the complete and persistent stop of the blood flow (B). These parameters were evaluated on 2 rats for each IgG sample to a total number of 12 and 10 rats for the aPL-positive and aPL-negative IgG, respectively. The results are expressed as mean ± SD. **P < .01, *P < .05 versus control rats receiving aPL-negative IgG.
Effects of infusion of human aPL-positive and aPL-negative IgG in rats with or without pretreatment with LPS. Human IgG (10 mg/1 mL sterile saline) purified from 6 aPL antibody-positive sera was infused into the carotid artery of Wistar rats 3 hours after the intraperitoneally injection of either LPS (□) or sterile saline (▧). Another group of rats was treated with 5 aPL-negative IgG (10 mg/1 mL sterile saline) with (▪) or without (▦) pretreatment with LPS. The procoagulant effect of the various treatments was evaluated by counting the number of microvessels with partial or total occlusions (A) and the number of occluded vessels, as shown by the complete and persistent stop of the blood flow (B). These parameters were evaluated on 2 rats for each IgG sample to a total number of 12 and 10 rats for the aPL-positive and aPL-negative IgG, respectively. The results are expressed as mean ± SD. **P < .01, *P < .05 versus control rats receiving aPL-negative IgG.
To examine whether the coagulation process was involved in the formation of cell aggregates, FITC-fibrinogen was infused together with aPL-positive or aPL-negative IgG. Deposition of labeled fibrinogen on the endothelium was already observed 5 to 10 minutes after aPL-positive IgG infusion and increased thereafter up to 30 minutes (Figure 2) prior to the formation of visible cell aggregates. This effect was not seen in rats receiving aPL-negative IgG. Infusion of both FITC-fibrinogen and rhodamine, which labels circulating leukocytes and platelets, with aPL-positive IgG resulted in an early deposition of fibrinogen and in the adhesion of a small number of platelets to endothelial cells eventually developing into large intravascular thrombi (data not shown).
Thrombosis is induced by antibodies to β2GPI
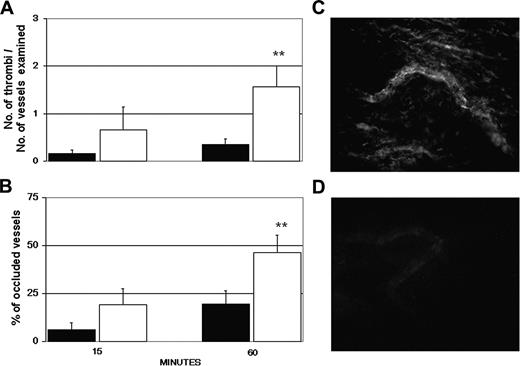
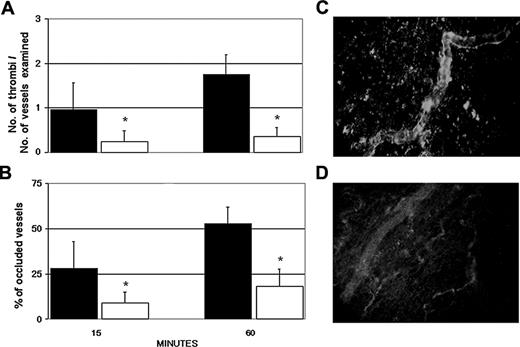
The target specificity of the aPL-positive IgG was evaluated by examining the ability of the IgG, purified from 3 patients (A, C, and D), in causing vessel occlusions before and after depletion of antibodies to β2GPI. Removal of these antibodies by affinity chromatography resulted in a significant decrease in the number of thrombi from 1.61 to 0.32 and in the number of occluded vessels from 46% to 20% at 60 minutes after injection. The values obtained with the anti-β2GPI-depleted IgG were essentially similar to those seen in rats receiving aPL-negative IgG (Figure 3).
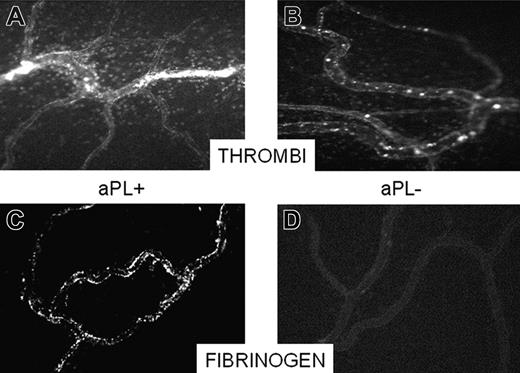
Occlusion of a mesenteric microvessel by thrombi and fibrinogen deposition on the endothelium. Video-photomicrographs of mesenteric microvessels of rats receiving an intraperitoneally injection of LPS and 3 hours later an infusion of either aPL-positive IgG or aPL-negative IgG. The micrographs were taken 30 minutes after the IgG infusion. Thrombi occluding 2 vessels can be seen in the aPL-positive IgG-treated rats (A) and absence of thrombi in the microvessels of aPL-negative IgG-treated rats (B). To evaluate deposition of fibrinogen, the rats were treated as indicated in “Patients, materials, and methods,” except that a solution of FITC-labeled fibrinogen (3.25 mg/250 μL sterile saline) was infused at the same time as the IgG. The micrographs were taken 10 minutes after the infusion of FITC-fibrinogen. Note the deposition of FITC-fibrinogen on the endothelium of aPL-positive IgG-treated rats (C) as opposed to the absence of staining of the microvessel in rats receiving aPL-negative IgG (D). Original magnification, × 100. Details of figure acquisition are given in Dobrina et al.23
Occlusion of a mesenteric microvessel by thrombi and fibrinogen deposition on the endothelium. Video-photomicrographs of mesenteric microvessels of rats receiving an intraperitoneally injection of LPS and 3 hours later an infusion of either aPL-positive IgG or aPL-negative IgG. The micrographs were taken 30 minutes after the IgG infusion. Thrombi occluding 2 vessels can be seen in the aPL-positive IgG-treated rats (A) and absence of thrombi in the microvessels of aPL-negative IgG-treated rats (B). To evaluate deposition of fibrinogen, the rats were treated as indicated in “Patients, materials, and methods,” except that a solution of FITC-labeled fibrinogen (3.25 mg/250 μL sterile saline) was infused at the same time as the IgG. The micrographs were taken 10 minutes after the infusion of FITC-fibrinogen. Note the deposition of FITC-fibrinogen on the endothelium of aPL-positive IgG-treated rats (C) as opposed to the absence of staining of the microvessel in rats receiving aPL-negative IgG (D). Original magnification, × 100. Details of figure acquisition are given in Dobrina et al.23
At the end of the experiment, the animals received an intraarterial infusion of sterile saline (40 mL) to remove circulating blood, and the ileal mesentery was gently detached from the gut and analyzed for the presence of IgG and C components by direct immunofluorescence analysis. As shown in Figure 3, IgG was deposited with a patchy distribution on the endothelium of the vessels in rats treated with aPL-positive IgG, but it was undetectable on the vessel of rats receiving anti-β2GPI-depleted IgG. Deposits of IgG were also seen on the ileal microvessels of rats treated with aPL-positive IgG in the absence of LPS, but they were generally fainter and more evenly distributed than those of aPL-positive IgG in the LPS-treated rats (data not shown). The ability of aPL IgG to activate C was evaluated by analyzing the ileal mesentery of rats treated with LPS and aPL-positive IgG for deposition of C3 and C9. Figure 4 shows that both these C components were found to colocalize with IgG on the endothelium of the mesenteric microvessels, suggesting that C was activated by the antibodies bound to β2GPI. Similar results were obtained with all the other aPL-positive IgG, but we failed to detect C deposition in rats receiving anti-β2GPI-depleted aPL-positive IgG (data not shown). The ability of all the aPL-positive IgG to activate human C was confirmed in in vitro assays, as shown by the results presented in the Table 1.
Antibodies to β2GPI are responsible for thrombus formation in aPL-positive IgG-treated rats. The animals received LPS and 3 hours later aPL-positive IgG (□) or anti-β2GPI-depleted aPL-positive IgG (▪) from 3 patients with APS (A, C, and D). The number of thrombi (A) and vessel occlusions (B) were evaluated at various time intervals on 4 rats per group (1 sample, D, was repeated twice), and the results are expressed as mean ± SD. **P < .01 versus anti-β2GPI-depleted aPL-positive IgG. Immunofluorescence analysis of mesenteric tissue showing deposits of IgG in rats treated with aPL-positive IgG (C) or absence of IgG in rats receiving anti-β2GPI-depleted aPL-positive IgG (D). Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
Antibodies to β2GPI are responsible for thrombus formation in aPL-positive IgG-treated rats. The animals received LPS and 3 hours later aPL-positive IgG (□) or anti-β2GPI-depleted aPL-positive IgG (▪) from 3 patients with APS (A, C, and D). The number of thrombi (A) and vessel occlusions (B) were evaluated at various time intervals on 4 rats per group (1 sample, D, was repeated twice), and the results are expressed as mean ± SD. **P < .01 versus anti-β2GPI-depleted aPL-positive IgG. Immunofluorescence analysis of mesenteric tissue showing deposits of IgG in rats treated with aPL-positive IgG (C) or absence of IgG in rats receiving anti-β2GPI-depleted aPL-positive IgG (D). Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
Localization of IgG and C components on mesenteric tissue of rats treated with LPS followed 3 hours later by aPL-positive IgG. The mesenteric tissue was double-stained for IgG (A,C) using FITC-labeled goat anti-human IgG and for C3 (B) or C9 (D) using biotin-labeled specific antibodies to rat C components (5 μg/mL) followed by RPE-conjugated streptavidin. Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
Localization of IgG and C components on mesenteric tissue of rats treated with LPS followed 3 hours later by aPL-positive IgG. The mesenteric tissue was double-stained for IgG (A,C) using FITC-labeled goat anti-human IgG and for C3 (B) or C9 (D) using biotin-labeled specific antibodies to rat C components (5 μg/mL) followed by RPE-conjugated streptavidin. Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
The membrane attack complex promotes formation of aPL antibody-induced thrombi
To ascertain whether the C components deposited on the endothelium contributed to the formation of thrombi, aPL-positive IgG were infused into C6-/- and C6+/+ PVG rats pretreated with LPS. The numbers of thrombi and occluded microvessels observed in the C6+/+ rats at 60 minutes were essentially similar to those seen in the Wistar rats (Figure 5). By contrast, aPL-positive IgG infused into C6-/- rats had only a marginal effect, which was slightly higher than that obtained with aPL-negative IgG, although not statistically different (Figure 5). Control IgG had a similar effect on C6+/+ and C6-/- rats. The 2 groups of rats did not differ for the bleeding and the coagulation times, suggesting that the markedly reduced procoagulant effect of aPL-positive IgG in C6-/- rats cannot be attributed to a defect of coagulation in these rats (data not shown).
APL-positive IgG fail to induce thrombus formation and vessel occlusions in C6-deficient rats. Six C6+/+ PVG rats (□) and an equal number of C6-/- PVG (▦) rats received LPS and 3 hours later aPL-positive IgG from 3 patients with APS (A, C, and D). An additional group of 6 C6+/+ PVG rats (▪) received LPS followed by aPL-negative IgG. All rats were examined for the number of thrombi (A) and occluded vessels (B). Thrombi and vessel occlusions were significantly reduced in C6-/- rats. These parameters were evaluated on 2 rats for each IgG sample. The results are expressed as mean ± SD. *P < .05, **P < .01 versus C6-/- PVG receiving aPL-positive IgG; ns indicates not significant. Error bars indicate standard deviation (SD).
APL-positive IgG fail to induce thrombus formation and vessel occlusions in C6-deficient rats. Six C6+/+ PVG rats (□) and an equal number of C6-/- PVG (▦) rats received LPS and 3 hours later aPL-positive IgG from 3 patients with APS (A, C, and D). An additional group of 6 C6+/+ PVG rats (▪) received LPS followed by aPL-negative IgG. All rats were examined for the number of thrombi (A) and occluded vessels (B). Thrombi and vessel occlusions were significantly reduced in C6-/- rats. These parameters were evaluated on 2 rats for each IgG sample. The results are expressed as mean ± SD. *P < .05, **P < .01 versus C6-/- PVG receiving aPL-positive IgG; ns indicates not significant. Error bars indicate standard deviation (SD).
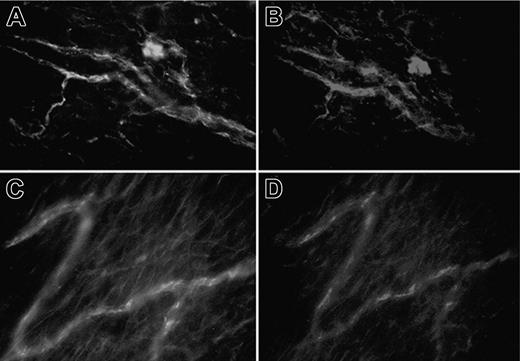
Because the results obtained with the C6-/- rats were highly suggestive for a critical role played by the terminal C components in thrombus formation, we examined the effects of the anti-C5 miniantibody in preventing the procoagulant activity of aPL-positive IgG in LPS-treated Wistar rats. This is a neutralizing antibody that inhibits the activation of C5 from human and other animal species, including rat C5, and blocks the release of C5a and the assembly of C5b-9.18 Intravenous administration of anti-C5 antibodies markedly reduced the vascular occlusions induced by aPL-positive IgG in the Wistar rats that have received LPS. Immunofluorescence analysis of the mesenteric tissue obtained from rats treated with the anti-C5 miniantibody showed deposition of C3, whereas C9 was undetectable, suggesting that the antibody was effective in neutralizing circulating C5 (Figure 6).
Discussion
Although the association between aPL antibodies and vascular thrombosis is now well established,1 the development of thrombi in restricted areas of the vascular tree, despite the fact that these antibodies circulate in the whole bloodstream, suggests that additional factors may contribute to promote the local formation of thrombi. Our inability to trigger the coagulation process in rats with passively infused aPL-positive IgG, as analyzed by intravital microscopy, supports this concept in agreement with previous observations by Pierangeli et al25 who failed to detect thrombi at autopsy in mice receiving human aPL antibodies. The requirement for additional factors to induce aPL antibody-mediated thrombus is clearly different from the direct effect of aPL antibodies in causing fetal loss in mice.5,6
Because we were searching for cofactors required for aPL antibody-mediated thrombus formation, we deliberately avoided inducing a thrombogenic injury by a mechanical or photochemical damage of the vessel wall.8,25 These experimental conditions were used previously to evaluate the procoagulant activity of aPL-positive IgG based on their ability to enhance the thrombus formation initiated by the vessel injury. Our approach, on the contrary, was to mimic more closely the in vivo situation using a cofactor commonly occurring in patients with APS and unable to trigger clotting per se. LPS was selected for our experimental model because infections are reported to precede the vascular events in a significant proportion of both catastrophic and simple/classic APS variants.9-11 Furthermore, LPS injected intraperitoneally failed to cause partial or complete thrombotic occlusions of the microvessels even after 90 minutes of observation, thus meeting the other requirement for a cofactor acting as a second hit in APS. LPS is only an example of a number of potential cofactors, and similar effects are likely to be obtained with other proinflammatory mediators activating endothelial cells, platelets, and leukocytes that have been implicated in the development of the coagulation process in patients with APS.7,8,26 The endothelial cells seem to play a critical role in the initiation of aPL antibody-mediated thrombosis in our experimental model. This conclusion is supported by the finding that aPL antibodies injected into LPS-treated rats targeted to the endothelium of the mesenteric microvessels, as shown by the fluorescence staining of the inner layer of the vessel wall. The binding appears to be selective for the anti-β2GPI antibodies because deposits of IgG were not observed in rats receiving aPL-positive IgG depleted of anti-β2GPI antibodies. Weak deposits of IgG not associated with the formation of thrombi were seen in rats treated with aPL-positive IgG in the absence of LPS. The reason for the increased antibody deposition observed after injection of LPS and aPL antibodies is not apparent. It is possible that LPS up-regulates the expression of the ligand for β2GPI on endothelial cells or, more likely, synergizes with the antibodies in clustering the ligand capable of stimulating the endothelial cells, as suggested by the patchy distribution of IgG deposits.27
Effect of an anti-C5 miniantibody on thrombus formation and vessel occlusion induced by aPL-positive IgG in LPS-treated rats. The animals received 1 mg of either the anti-C5 miniantibody (□) or an unrelated miniantibody (▪) intravenously 15 minutes before the infusion of aPL-positive IgG (10 mg/1 mL sterile saline). The IgG from 3 patients with APS (A, C, and D) and from 3 healthy control subjects were used. In parallel experiments the miniantibody used at this concentration was found to completely neutralize the serum C activity for 20 minutes and to maintain this activity to 50% of the starting value up to 5 hours after infusion (data not shown). Thrombi (A) and vessel occlusions (B) were significantly reduced in the rats treated with the miniantibody to C5. These parameters were evaluated on 2 rats from each IgG sample. The results are expressed as mean ± SD. *P < .05 versus control rats receiving the unrelated miniantibody. Immunofluorescence analysis of the mesenteric tissue from LPS-treated rats that received aPL-positive IgG and anti-C5 miniantibody showing the deposits of C3 (C) and absence of C9 (D). Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
Effect of an anti-C5 miniantibody on thrombus formation and vessel occlusion induced by aPL-positive IgG in LPS-treated rats. The animals received 1 mg of either the anti-C5 miniantibody (□) or an unrelated miniantibody (▪) intravenously 15 minutes before the infusion of aPL-positive IgG (10 mg/1 mL sterile saline). The IgG from 3 patients with APS (A, C, and D) and from 3 healthy control subjects were used. In parallel experiments the miniantibody used at this concentration was found to completely neutralize the serum C activity for 20 minutes and to maintain this activity to 50% of the starting value up to 5 hours after infusion (data not shown). Thrombi (A) and vessel occlusions (B) were significantly reduced in the rats treated with the miniantibody to C5. These parameters were evaluated on 2 rats from each IgG sample. The results are expressed as mean ± SD. *P < .05 versus control rats receiving the unrelated miniantibody. Immunofluorescence analysis of the mesenteric tissue from LPS-treated rats that received aPL-positive IgG and anti-C5 miniantibody showing the deposits of C3 (C) and absence of C9 (D). Original magnification, × 250. Details of image acquisition are given in Dobrina et al.23
An additional and more compelling evidence for the involvement of endothelial cells in thrombus formation is the observation that the deposition of fibrinogen on the mesenteric endothelium after the infusion of aPL-positive IgG slightly precedes the formation of cell aggregates. This finding is compatible with the procoagulant effect induced on endothelial cells by aPL antibodies through the increased expression of tissue factor.28,29
Several in vitro and in vivo data have shown that aPL antibodies stimulate endothelial cells to express adhesion molecules, to release cytokines, and to manifest proadhesive properties for leukocytes.30 The expression of adhesion molecules on endothelial cells was found by Pierangeli et al31 to correlate with the enhanced thrombosis and leukocyte adhesion stimulated by these antibodies in in vivo models. More recently, Jankowski et al8 published data supporting the contribution of platelets to the development of thrombi. They used a photochemically induced model of arterial thrombosis in hamsters to evaluate the procoagulant effect of a murine monoclonal IgG with lupus anticoagulant activity reacting with hamster β2GPI. Their data showed that the monoclonal aPL antibodies bound preferentially to platelets and, to a much lesser extent, to endothelial cells. Leukocyte and platelet adhesion to the endothelium of mesenteric microvessels does not appear to be a primary event in the aPL antibody-dependent thrombus formation in our rat model, as suggested by our finding of an early deposition of fibrinogen on the endothelium prior to the formation of intravascular cell aggregates. Some cell adhesion was observed after injection of LPS, but this was not followed by the formation of thrombi in the presence of aPL-negative IgG.
The colocalization of C3 and C9 with aPL-positive IgG on the endothelium of the mesenteric microvessels in the areas of thrombotic occlusions suggests that rat C is activated by these antibodies in vivo as already shown for mouse C activated in murine models of aPL antibody-induced fetal loss32 and of tissue damage caused by ischemia reperfusion.33 C activation proceeds to completion with the assembly of the terminal complex as indicated by the deposition of rat C9. The specificity of this finding is confirmed by our failure to detect bound C9 in aPL IgG-treated C6-deficient rats, while the extent of C3 deposition was similar to that observed in C6-sufficient rats.
The negligible thrombogenic activity of aPL IgG in C6-deficient rats suggests that the process of vessel occlusion and thrombus formation induced by aPL antibodies is C-dependent in our experimental model. Holers et al32 have previously shown that C is involved in the fetal death caused by passive transfer of aPL IgG. More recently, Girardi et al34 have identified in C5a the C activation product responsible for fetal injury together with neutrophils. Our finding that the C6-deficient rats are protected from the prothrombotic effect of the aPL antibodies for the first time points to the terminal C complex as the mediator mainly implicated in this process. This is not surprising because both the sublytic membrane attack complex (MAC) and the noncytolytic terminal complex trigger the coagulation process by stimulating the endothelial cells to express tissue factor35,36 and, in the case of MAC, to shed vesicles that support the formation of a prothrombinase complex.37 In addition, sublytic MAC was shown to induce platelets to expose phosphatidylserine and to express procoagulant activity.38
The beneficial effects obtained with the anti-C5 miniantibody in preventing the prothrombotic activity of aPL antibodies further support the important role of the terminal complex in inducing thrombus formation. It is important to emphasize that this antibody is directed against the cleavage site of C5, blocking the splitting of this molecule into C5a and C5b and the subsequent assembly of the terminal complex. The miniantibody has the interesting feature of reacting with the C5 from human as well as other animals, including rats, mice, and rabbits, and thus can be tested in an animal model prior to being used as a therapeutic strategy. These findings do suggest that anti-C5 miniantibody could be useful in treating patients with the most aggressive variant of APS known as catastrophic APS (CAPS). The term “catastrophic” APS is used to define an accelerated form of APS characterized by recurrent and widespread thrombotic events and resulting in multiorgan failure.39 Anticoagulation, corticosteroids, plasma exchange, intravenous γ-globulin, and, if associated with lupus flare, cyclophosphamide are the most commonly used treatments for patients with CAPS. However, a significant proportion of patients do not respond to such treatment and may benefit from additional approaches that interfere with key pathogenic mechanisms of aPL antibody-mediated thrombosis.
In conclusion, the data presented in this study indicated that anti-β2GPI antibodies from patients with APS are able to trigger clotting in the presence of a priming proinflammatory factor, such as LPS, and that the terminal C complex is the main mediator of the coagulation process.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-03-1319.
Supported by grants from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) (Cofin 2003-06220 004 and FIRB 2001 RBAU01C3CJ) and the European Union (EU) 6th Framework Programme Network of Excellence (FP6 NoE; LSHM-CT-2400-512040; F.T.), and from the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Auxologico (Ricerca Corrente 2004; P.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.