Abstract
To obtain the large amount of T cells required for adoptive immunotherapy in a clinical setting, T-cell lifespan extension by human telomerase reverse transcriptase (hTERT) transduction is of particular interest. However, constitutive expression of hTERT is associated with malignant transformation and thus warrants a detailed evaluation of the safety of hTERT-transduced T cells before clinical application. In view of this, we performed an extensive cytogenetic analysis of hTERT-transduced MART-1 (melanoma antigen recognized by T cell 1)–and human papillomavirus type 16 (HPV16) E7–specific human CD8+ cytotoxic T lymphocytes (CTLs), reactive against melanoma and cervical carcinoma, respectively. Our results, obtained by (spectral) karyotyping and array comparative genomic hybridization, showed the development of minor chromosomal aberrations in an hTERT-transduced MART-1–specific CTL clone, whereas severe clonal aberrations were detected in an hTERT-transduced HPV16 E7–specific CTL clone. Furthermore, hTERT transduction did not protect CTLs from immunosenescence, because the HPV16 E7–specific, hTERT-transduced CTL clone showed a decreased functional activity on prolonged culture. Although the general frequency of major chromosomal aberrations in hTERT-transduced CTLs and the in vivo significance of our observations remain still unclear at this point, the currently available data suggest that clinical application of hTERT-transduced CTLs should proceed with caution.
Introduction
Adoptive immunotherapy using tumor-reactive T lymphocytes represents an alternative strategy to treat malignant disease. In different animal models, adoptive transfer of specific cytotoxic T lymphocytes (CTLs) has resulted in the eradication of established tumors.1-4 Also in patients with melanoma, adoptive CTL therapy has resulted in objective tumor regressions5,6 and thus holds promise as a treatment modality against malignant disease in a clinical setting.7 Given the poor immunogenicity of most tumor cells, the in vitro selection prior to adoptive transfer of high-avidity tumor reactive CTL clones could further improve the efficacy of in vivo tumor rejection.8,9 However, the expansion for therapeutic purposes of CTL clones with considerable in vitro replicative history can be severely hampered by replicative senescence.10,11 It has become clear that the catalytic subunit of the human telomerase complex, human telomerase reverse transcriptase (hTERT), can efficiently reverse replicative senescence (reviewed in Ducrest et al12 ). The ability of hTERT to elongate and stabilize telomeric ends can be exploited to restore the replicative potential of presenescent cells. Ectopic expression of hTERT has successfully mediated the in vitro lifespan extension of various human cell types, including fibroblasts, endothelial cells, muscle cells, and neuronal cells.13-16 Additionally, hTERT transduction of human T lymphocytes has resulted in restoration of their replicative potential in vitro. This has allowed the successful lifespan extension of bulk and clonal cultures consisting of cytotoxic, helper, and regulatory type T lymphocytes.10,11,17-19 As a result, hTERT-mediated lifespan extension would facilitate the generation of sufficient numbers of preselected high-savidity CTL clones for multiple rounds of clinical application.
Previous in vitro studies have indicated that hTERT-transduced human T cells may retain a normal phenotype, functional activity, growth requirements,10,11 and a normal karyotype as determined by G-banding analysis.17 Moreover, a recent in vivo study in immunodeficient mice has reported the safe and effective use of hTERT-transduced human CTLs for immunotherapy of melanoma.20 These results have raised enthusiasm for clinical evaluation of adoptive immunotherapy using hTERT-transduced T lymphocytes. However, constitutive expression of hTERT has been observed in greater than 85% of human tumor cells of various histologic origins to maintain their replicative potential.21,22 In combination with activation of 2 additional oncogenes, hTERT has been shown to mediate malignant transformation of normal human cells in vitro.23 Consequently, an important issue regarding potential clinical application is the safety of hTERT-mediated CTL lifespan extension.
To provide more insight into the potential side effects associated with hTERT-mediated lifespan extension, we performed a detailed cytogenetic analysis of hTERT-transduced CTLs. For this purpose karyotypes derived from hTERT-transduced MART-1 (melanoma antigen recognized by T cell 1)–and human papillomavirus type 16 (HPV16) E7–specific CD8+ CTLs, which represent potential candidates for adoptive immunotherapy of melanoma and cervical carcinoma, respectively, were subjected to DAPI (4′, 6-diamino-2-phenylindole) banding, spectral karyotyping (SKY), and microarraybased comparative genomic hybridization (array CGH). Our results showed that hTERT-transduced human CTLs can acquire chromosomal aberrations and provide evidence that genomic stability may be lost in hTERT-transduced human CD8+ T lymphocytes. We also showed that long-term cultures of hTERT-transduced CTLs, although protected from replicative senescence, can still enter a state of immunosenescence characterized by loss of functional activity, as previously documented for aged normal human T cells (reviewed in Pawelec et al24 ).
Materials and methods
CTL culture and hTERT transduction
The MART-127-35–specific human CTL clone 4 has been described previously in detail.10 The HPV16 E711-20–specific human CTL clone A9 was obtained as described previously in detail.11 CTLs were maintained in vitro in Yssel medium25 supplemented with 1% human serum (HS; Perbio, Helsingborg, Sweden) and antibiotics (penicillin/streptomycin; Gibco, Paisley, Scotland) in 24-well plates (Nunc, Intermed, Denmark). CTLs (2-4 × 105) were stimulated biweekly with an irradiated (80 Gy) feeder mix consisting of 1 × 106 allogeneic peripheral blood mononuclear cells from 2 different donors and 1 × 105 JY cells per mL Yssel medium supplemented with 1% HS, 100 ng/mL phytoheamagglutinin (PHA; Murex Biotech, Dartford, United Kingdom), and 20 U/mL interleukin 2 (IL-2; Chiron, Amsterdam, The Netherlands). The Epstein-Barr virus (EBV)–transformed B-cell line JY was cultured in Iscoves medium (BioWhittaker, Verviers, Belgium) supplemented with 8% fetal calf serum (FCS; Perbio) and antibiotics. The retroviral vectors LZRS-hTERT-IRES-GFP and LZRS-hTERT-IRES-ΔNGFR were used for hTERT transduction of CTL clones 4 and A9, respectively, as reported previously.10,11 The used hTERT-encoding vectors only differ with respect to coexpression of either green fluorescent protein (GFP) or a truncated form of the low-affinity nerve growth factor receptor (ΔNGFR) as marker genes. The latter is signaling incompetent because of deletion of most (from residue 248) of its intracytoplasmic tail and has been used as a surface marker without adverse events in both animal models and clinical trials.26 Production of retroviral supernatant and transduction procedure have been described previously in detail.27 The hTERT-transduced CTL clones, designated 4-TG and A9-TN, were maintained as described for CTLs 4 and A9. Approval for these studies, according to the Declaration of Helsinki, was obtained from the VU University Medical Center Institutional Review Board.
DAPI banding
To produce metaphase spreads derived from the CTL clones used in this study, cells (1 × 106/mL) were stimulated for 4 days with 50 ng/mL anti-CD3 antibody OKT3 (Janssen-Cilag, Tilburg, The Netherlands) and 300 U/mL IL-2. During the final 20 minutes of stimulation 0.1 μg/mL colcemid (Invitrogen, Carlsbad, CA) was added. Cells were harvested, incubated for 20 minutes in a hypotonic 75 mM KCl solution (Merck, Darmstadt, Germany), and fixed using a 1: 3 methanol:acetic acid mixture (Merck). Metaphase spreads were prepared by dropping the fixed-cell suspension on glass slides. DAPI staining of metaphase slides was done by applying 30 μL Vectashield (Vector Laboratories, Burlingame, CA) containing 350 ng/mL DAPI (Sigma-Aldrich, Zwijndrecht, The Netherlands) and analyzed using Bandview software, version 1.7 (Applied Spectral Imaging, Migdal Haemek, Israel). Only metaphases that were properly spread and of average length were used for analysis.
Spectral karyotyping (SKY)
SKY was performed essentially as described previously.28 SKY hybridization was done using the human SkyPaint probe set (Applied Spectral Imaging). Metaphase slides were produced as described for DAPI banding. Pretreatment of the slides prior to hybridization was done according to the manufacturer's protocol. Following hybridization, the biotin moieties in the probe mixture were detected using avidin-cyanine 5 (Cy5; Amersham BioSciences, Roosendaal, The Netherlands), and digoxygenin moieties were detected with mouse antidigoxygenine (Sigma) followed by rabbit anti–mouse-Cy5.5 (Amersham BioSciences). Images were acquired with Spectral Imaging software (Applied Spectral Imaging) using a DMRA fluorescent microscope (Leica) equipped with the SpectraCube (Applied Spectral Imaging). Images were analyzed using the SkyView software (Applied Spectral Imaging).
Array comparative genomic hybridization (CGH)
DNA of the 1 Mb resolution Sanger Consortium BAC set,29-31 the OncoBac set,32 and in-house clones of interest, supplemented with clones from the Children's Hospital Oakland Research Institute (CHORI) adding up to a total of 4202 clones with known chromosomal position, was isolated according to the CHORI protocol.33 Amplification of the DNA was done by ligation-mediated polymerase chain reaction (PCR) according to Snijders et al,34 and PCR clones were purified using 96-well plate format Montage PCR (Millipore, Amsterdam, The Netherlands). Clones were spotted at a concentration of 1 μg/μL, in 150 mM sodium phosphate (pH 8.5), on CodeLink slides (Amersham BioSciences) using a Spot Array 72 robot (Perkin-Elmer Life Sciences, Zaventum, Belgium) and processed according to the manufacturer's protocol. Genomic DNA was isolated from CTLs using standard techniques. DNA (300 ng) was labeled by random priming (BioPrime DNA Labeling System; Gibco BRL) as reported previously34 and purified using ProbeQuant G50 microcolumns (Amersham). Hybridization was also done as reported previously,34 although prehybridization and hybridization were performed in a hybridization station (HybStation12; Perkin-Elmer). Before scanning slides (Scan Array Express; Perkin-Elmer) arrays were dried by centrifugation (141g) omitting DAPI staining. Images were acquired by Imagene 5.6 software (Biodiscovery, Marina del Rey, CA), using the default settings. Subtraction of local background was done for the signal median intensities of both test and reference DNAs. Normalization of the calculated ratios was done against the average of all ratios. As the clones were spotted in triplicate, average and standard deviations were calculated for each clone.
Antibodies, tetramers, and flow cytometry
Fluorescein isothiocyanate (FITC)–or phycoerythrin (PE)–labeled antibodies directed against human CD2, CD3, CD4, CD8α, CD11a, CD25, CD54, CD56, T-cell receptor αβ (TCRαβ) (all from BD Biosciences, Mountain View, CA), and CD8β (Beckman Coulter, Marseille, France) were used for flow cytometric analysis to determine CTL phenotype. Allophycocyanin (APC)–labeled HLA-A2.1 tetramers (T A2) presenting the HPV16 E711-20 and influenza A virus MP58-66 epitopes were prepared in house as described previously.27 Tetramer and/or antibody staining of cells was performed in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] supplemented with 0.1% bovine serum albumin [BSA] and 0.01% azide) for 15 minutes at 37°C and/or 20 minutes on ice, followed by washing with FACS buffer. Stained cells were analyzed on a FACScalibur (BD Biosciences) using CellQuest software. To exclude dead cells, all flow cytometric analyses were performed in the presence of 0.5 μg/mL propidium iodide (ICN Biomedicals, Zoetermeer, The Netherlands).
CTL function assays
Cytolytic activity of CTLs was determined using a standard chromium release assay. Target cells were labeled with 100 μCi [3.7 MBq] Na2[51Cr]O4 (Amersham BioSciences) for 1 hour at 37°C and washed extensively. Effector CTLs were added to 2 × 103 target cells, at the indicated effector-to-target (E/T) ratios in triplicate wells of a round-bottom 96-well plate (Nunc). After a 4-hour incubation at 37°C, radioactive content was measured in the supernatant to determine the percentage of specific lysis. Flow cytometric analysis of CTL degranulation was determined by cumulative activation-dependent CD107a expression as described recently.35,36 Stimulations were performed for 4 hours at 37°C in a round-bottom 96-well plate (Nunc) containing 1 × 105 responder CTLs and 5 × 104 target cells per well in the presence of 4 μM monensin (Sigma) and 10% vol/vol PE-labeled CD107a-specific antibody (BD Biosciences), followed by tetramer-APC staining as described for flow cytometry. Samples were subsequently analyzed by flow cytometry. Production of interferon γ (IFNγ) by stimulated CTLs was determined using intracellular staining of permeabilized CTLs with a FITC-labeled IFNγ-specific antibody according to the manufacturer's instructions (CytoFix/CytoPerm kit with GolgiStop; BD Biosciences). Stimulations were performed for 4 hours at 37°C in a round-bottom 96-well plate (Nunc) containing 1 × 105 responder CTLs and 1 × 104 targets cells per well, followed by tetramer-APC staining as described for flow cytometry, and intracellular IFNγ staining. Samples were subsequently analyzed by flow cytometry. In all CTL function assays, the used targets were HLA-A2.1–positive JY cells loaded with 1 μM E711-20 or MP58-66 peptide for 60 minutes at 37°C. Peptides were synthesized with a free carboxy-terminus by solid-phase strategies on an automated Syro II multiple peptide synthesizer (MultiSyntech, Witten, Germany) using Fmoc chemistry. Peptides were greater than 90% pure as analyzed by reverse-phased high-performance liquid chromatography (HPLC), dissolved in dimethyl sulfoxide (DMSO; 10 mg/mL) and stored at –20°C.
Results
Lifespan extension of human CTL clones 4 and A9
CTL clone 4, specific for the MART-127-35 epitope, was obtained as reported previously after stimulation of melanoma patient-derived peripheral T cells with autologous melanoma cells genetically engineered to produce IL-7.10 The healthy donor-derived HPV16 E711-20–specific CTL clone A9 was obtained as reported previously by in vitro primary CTL induction using autologous E711-20 peptide–loaded dendritic cells.11 Both CTL clones were isolated by limiting dilution cloning and subsequently transduced with hTERT using similar procedures and retroviral constructs. The resulting CTLs, 4-TG and A9-TN, displayed similar phenotype, functional activity, and growth requirements as compared with their untransduced counterparts.10,11 The hTERT-mediated lifespan extension of CTLs 4 and A9 has allowed their continuous in vitro expansion for 12 and 18 months, respectively, at an average growth rate of 3 and 2 population doublings per week, respectively. In contrast, the in vitro life span of untransduced T cells and, importantly, T cells transduced with a control retroviral vector (same vector without hTERT insert) was limited to 3 to 4 months, as reported previously by us and others.10,11,17-19
Cytogenetic analysis of CTL 4-TG
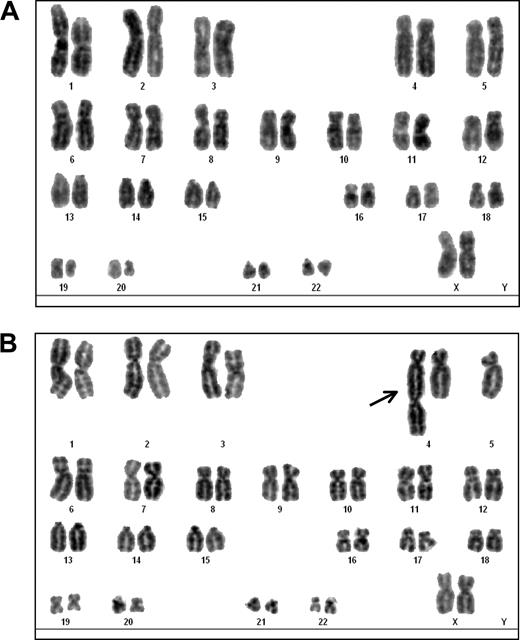
We performed an extensive karyotype analysis of CD8+ T cells transduced with hTERT. Analysis of CTL 4-TG was performed at 2 and 10 months after hTERT transduction. Detailed review of multiple DAPI-banded metaphases (n = 28) derived from CTL 4-TG at 2 months after hTERT transduction showed a normal karyotype without abnormalities, including the absence of chromatid breaks/gaps or chromosomal rearrangements, in all cases as represented by Figure 1A. Karyotype analysis at 10 months after hTERT transduction revealed a dicentric chromosome, consisting of a complete chromosome 4 fused to a centromere containing 5q, found in 1 cell of 24 cells analyzed (Figure 1B). Subsequently, potential DNA copy number changes were investigated in CTL 4-TG by genome-wide array CGH analysis. Genomic DNA isolated from CTL 4-TG at 2 and 10 months after hTERT transduction did not show DNA copy number changes, indicating genomic integrity in general (data not shown). The untransduced CTL 4 was not available for analysis as a consequence of replicative senescence, however, is not expected to contain any cytogenetic abnormalities given the results described in this section. Collectively, the results obtained with CTL 4-TG indicate minor chromosomal abnormalities, suggesting low-level genomic instability in hTERT-transduced CTLs expanded continuously for 10 months.
DAPI-banding analysis of MART-127-35–specific, hTERT-transduced CTL 4-TG. (A) DAPI-banded karyogram of a representative metaphase (n = 28) derived from CTL 4-TG at 2 months after hTERT transduction, indicating a normal 46, XX karyotype. (B) DAPI-banded karyogram of a metaphase derived from 4-TG at 10 months after hTERT transduction, showing a dicentric chromosome (arrow), consisting of a complete chromosome 4 fused to a centromere containing 5q, found in 1 cell of 24 cells analyzed.
DAPI-banding analysis of MART-127-35–specific, hTERT-transduced CTL 4-TG. (A) DAPI-banded karyogram of a representative metaphase (n = 28) derived from CTL 4-TG at 2 months after hTERT transduction, indicating a normal 46, XX karyotype. (B) DAPI-banded karyogram of a metaphase derived from 4-TG at 10 months after hTERT transduction, showing a dicentric chromosome (arrow), consisting of a complete chromosome 4 fused to a centromere containing 5q, found in 1 cell of 24 cells analyzed.
Cytogenetic analysis of CTL A9-TN
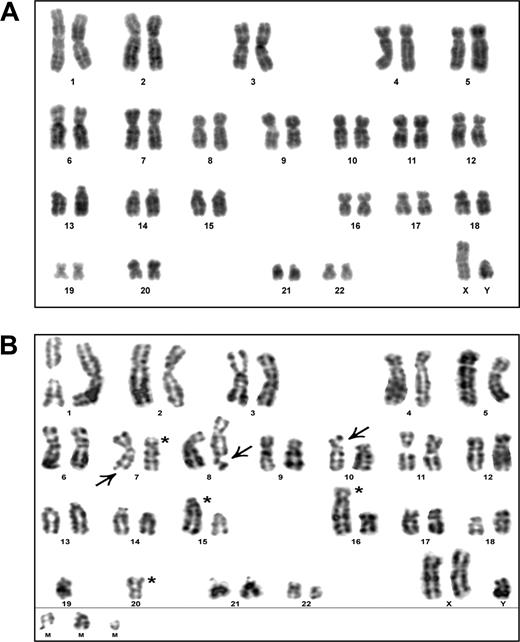
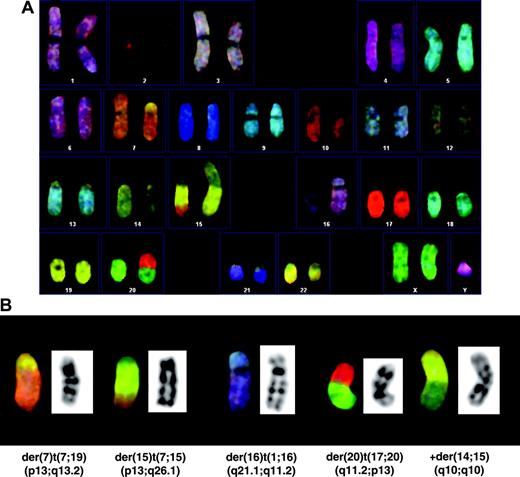
CTL A9-TN was analyzed at 2, 7, and 10 months after hTERT transduction. We could include the untransduced CTL A9 in the analysis, because it was still available. The majority of DAPI-banded metaphases derived from untransduced A9 showed a normal karyotype without chromosomal damage (Figure 2A). A single chromatid break was found in 13 untransduced A9-derived metaphases. Furthermore, no chromosomal aberrations were found in 5 metaphases derived from presenescent A9 (data not shown). At 2 months after hTERT transduction similar results were obtained, one chromatid break was detected in 9 A9-TN–derived metaphases (data not shown). In contrast, we detected chromosomal aberrations in all DAPI-banded metaphases derived from A9-TN at 7 months after hTERT transduction. In total, 13 chromatid breaks and gaps were present in 10 analyzed metaphases, and the majority of metaphases indicated the presence of chromosomal rearrangements. A representative A9-TN karyotype at this time point clearly shows structural damage of chromosome arms 7q, 8q, and 10p and rearrangements involving chromosomes 7, 15, 16, and 20 (Figure 2B). Surprisingly, at 10 months after transduction only one chromatid break was observed in 10 metaphases analyzed, suggesting that structural chromosomal damage had resolved on further culturing of A9-TN. However, the majority of metaphases did still indicate the presence of chromosomal rearrangements (data not shown). To confirm the presence of translocations in the A9-TN sample taken 10 months after hTERT transduction, we performed SKY analysis. Indeed, at 10 months after transduction A9-TN was found to have clonal chromosomal translocations, including translocations that were not detected before by DAPI-banding (Figure 3A). All cells analyzed (n = 8) contained 4 clonal translocations involving multiple chromosomes (Figure 3B). Furthermore, a small majority of samples (5 of 8) contained a whole arm 14q translocation (Figure 3B). A minority of samples (3 of 8) showed a loss of chromosome 21 (data not shown) and/or an extra X chromosome (Figure 3A). As a control, SKY analysis was performed on untransduced A9 CTLs and showed a normal 46, XY karyotype without aberrations (data not shown).
DAPI-banding analysis of HPV16 E711-20–specific CTL A9 and its hTERT-transduced counterpart CTL A9-TN. (A) DAPI-banded karyogram of a representative metaphase (n = 13) derived from untransduced A9, indicating a normal 46, XY karyotype. (B) DAPI-banded karyogram of a representative metaphase (n = 10) derived from A9-TN at 7 months after hTERT transduction, showing structural damage of chromosome arms 7q, 8q, and 10p (arrows) and rearrangements involving chromosomes 7, 15, 16, and 20 (asterisks). M indicates marker, unidentified chromosomes, or chromosome parts.
DAPI-banding analysis of HPV16 E711-20–specific CTL A9 and its hTERT-transduced counterpart CTL A9-TN. (A) DAPI-banded karyogram of a representative metaphase (n = 13) derived from untransduced A9, indicating a normal 46, XY karyotype. (B) DAPI-banded karyogram of a representative metaphase (n = 10) derived from A9-TN at 7 months after hTERT transduction, showing structural damage of chromosome arms 7q, 8q, and 10p (arrows) and rearrangements involving chromosomes 7, 15, 16, and 20 (asterisks). M indicates marker, unidentified chromosomes, or chromosome parts.
Spectral karyotyping (SKY) of hTERT-transduced HPV16 E711-20–specific CTL A9-TN. (A) SKY karyogram of a representative metaphase (n = 8) derived from A9-TN, 10 months after hTERT transduction, showing a 47, XY, +X, der(7)t(7;19)(p13;q13.2),+der(14;15)(q10;q10),–15, der(15)t(7;15)(p13;q26.1), der(16)t(1;16)(q21.1;q11.2), der(20)t(17;20)(q11.2;p13) karyotype containing chromosomal translocations. (B) Representative enlargements of the 4 indicated clonal translocations detected in 8 of 8 metaphases, and 1 nonclonal translocation detected in 5 of 8 metaphases, respectively, present at 10 months after hTERT transduction.
Spectral karyotyping (SKY) of hTERT-transduced HPV16 E711-20–specific CTL A9-TN. (A) SKY karyogram of a representative metaphase (n = 8) derived from A9-TN, 10 months after hTERT transduction, showing a 47, XY, +X, der(7)t(7;19)(p13;q13.2),+der(14;15)(q10;q10),–15, der(15)t(7;15)(p13;q26.1), der(16)t(1;16)(q21.1;q11.2), der(20)t(17;20)(q11.2;p13) karyotype containing chromosomal translocations. (B) Representative enlargements of the 4 indicated clonal translocations detected in 8 of 8 metaphases, and 1 nonclonal translocation detected in 5 of 8 metaphases, respectively, present at 10 months after hTERT transduction.
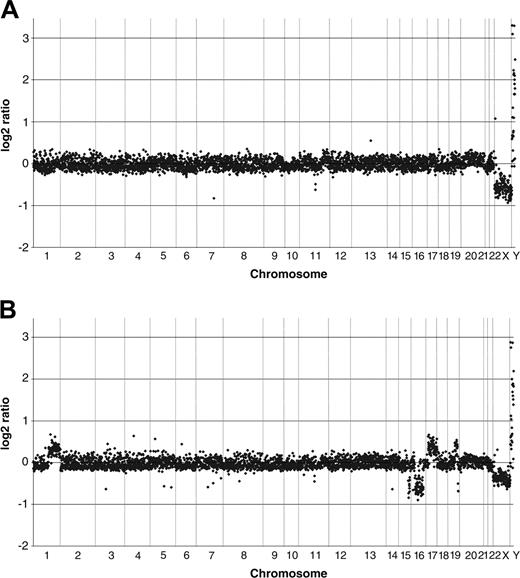
To investigate potential DNA copy number changes in CTL A9-TN as a result of the observed chromosomal translocations, we performed genome-wide array CGH. As expected, genomic DNA isolated from untransduced A9 CTLs did not show DNA copy number changes (Figure 4A). Similar results were obtained with A9-TN at 2 months after hTERT transduction (data not shown). In contrast, we found gains and losses of DNA at 10 months after hTERT transduction (Figure 4B). The gains and losses of DNA observed at 10 months after transduction were also observed at 7 months after hTERT transduction (data not shown) and were in agreement with the chromosomal translocations detected by the SKY analysis, as confirmed by analysis of the array CGH profiles of individual chromosomes. Although chromosome 7 was involved in the translocations detected by SKY, no copy number changes of DNA derived from this chromosome were observed (Figure 4B). The array CGH profile of chromosome 14 suggested a gain of 14q (Figure 4B), in agreement with the whole arm 14q translocation observed in part (5 of 8) of the cells analyzed by SKY (Figure 3B). Furthermore, the appearance of the DNA copy number changes in the array CGH analysis, starting at 7 months after hTERT transduction, coincided with the detection of chromosomal instability by DAPI banding. Table 1 summarizes the detailed analysis of the gains and losses detected by the SKY/array CGH analysis of A9 and A9-TN. Despite the observed chromosomal aberrations, A9-TN cells did not acquire the autonomous growth potential normally associated with malignant transformation. In contrast, they remained dependent on feeder mix stimulation during their continuous in vitro culture period. Collectively, the results obtained with CTL A9-TN show major chromosomal abnormalities, indicating severe genomic instability in hTERT-transduced CTLs expanded continuously for 10 months.
High-resolution genome-wide array CGH analysis of HPV16 E711-20–specific CTL A9 and its hTERT-transduced counterpart A9-TN. The array CGH log2 ratios, indicating changes in DNA copy number, obtained using genomic DNA isolated from (A) untransduced A9 and (B) A9-TN at 10 months after hTERT transduction, are depicted for all clones included in the analysis. Gains and losses of genomic sequences are illustrated by an increased or decreased log2 ratio of the respective clones, above or below the average ratio, respectively. The loss of an X chromosome and gain of the Y chromosome served as an internal control.
High-resolution genome-wide array CGH analysis of HPV16 E711-20–specific CTL A9 and its hTERT-transduced counterpart A9-TN. The array CGH log2 ratios, indicating changes in DNA copy number, obtained using genomic DNA isolated from (A) untransduced A9 and (B) A9-TN at 10 months after hTERT transduction, are depicted for all clones included in the analysis. Gains and losses of genomic sequences are illustrated by an increased or decreased log2 ratio of the respective clones, above or below the average ratio, respectively. The loss of an X chromosome and gain of the Y chromosome served as an internal control.
Phenotype and functional activity of CTL A9-TN after prolonged culture
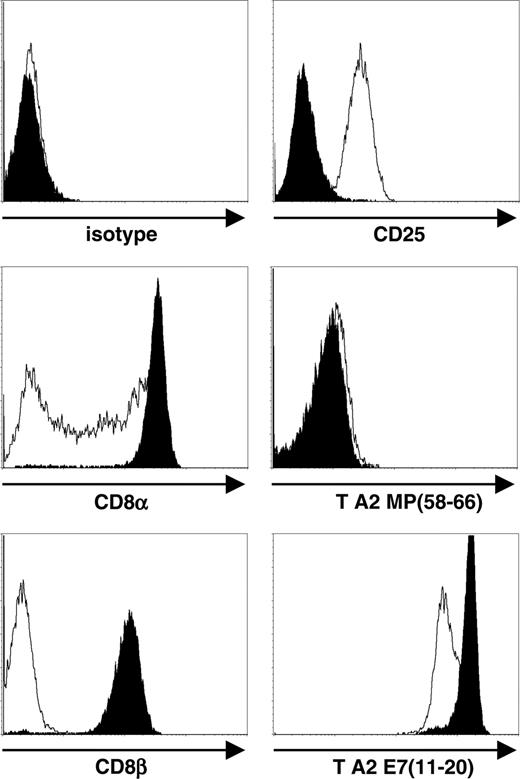
Our previous results showed that the phenotype, growth requirements, and functional activity of HPV16 E711-20–specific CTLs are retained on hTERT transduction.11 Regarding A9-TN, these results can now be extended from 6 months, as reported previously, to the first 12 months of continuous culture after hTERT transduction. However, we observed marked phenotypic changes on prolonged culture of A9-TN beyond 12 months. As compared with the expression levels observed during the first 12 months, expression of CD8α and CD8β was down-regulated, whereas expression of CD25 was up-regulated (Figure 5). The expression levels of CD2, CD3, CD4, CD11a, CD54, CD56, and TCR αβ remained unaltered (data not shown). We observed a minor decrease in staining with tetramers presenting the relevant E711-20 epitope, whereas no change in staining with tetramers presenting an irrelevant epitope was observed (Figure 5). This minor decrease in relevant tetramer binding is likely to be caused by a decrease in CD8α coreceptor availability. Additionally, beyond 12 months of culture both the growth rate and the survival in time after feeder mix stimulation of A9-TN were elevated (data not shown). The latter most probably resulted from chronic expression of CD25, the high-affinity receptor for IL-2, allowing more efficient IL-2 responsiveness upon feeder mix stimulation of A9-TN.
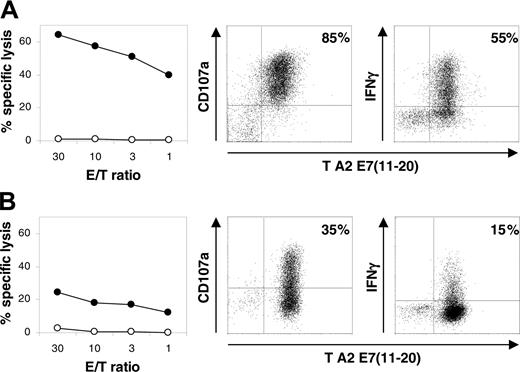
The functional activity of A9-TN remained unaltered during the first 12 months of continuous culture after hTERT transduction. Target cells loaded with the relevant E711-20 epitope were efficiently lysed, stimulated the expression of CD107a, a molecule shown recently to be expressed on CTL degranulation,35,36 and stimulated the production of IFNγ (Figure 6A). In contrast, prolonged culture beyond 12 months resulted in a substantial decrease of antigendependent lytic activity, CD107a expression, and IFNγ production by A9-TN (Figure 6B). The observed decrease in functional activity of A9-TN was not associated with decreased tetramer binding, but it may involve the down-regulated CD8α and CD8β coreceptor expression. We did not detect changes in the morphology of A9-TN during the 18 months of continuous expansion (determined by phase-contrast light microscopy, data not shown).
Flow cytometric analysis of surface phenotype of hTERT-transduced, HPV16 E711-20–specific CTL A9-TN. Fluorescence intensity is shown for isotype control, anti-CD8α, anti-CD8β, and anti-CD25 antibody stainings, and for HLA-A2.1/MP58-66 (T A2 MP58-66) and HLA-A2.1/E711-20 (T A2 E711-20) tetramer stainings. Filled histograms represent A9-TN during the first 12 months after hTERT transduction, and open histograms represent A9-TN beyond 12 months after hTERT transduction. Flow cytometric analysis was performed 3 weeks after feeder mix stimulation.
Flow cytometric analysis of surface phenotype of hTERT-transduced, HPV16 E711-20–specific CTL A9-TN. Fluorescence intensity is shown for isotype control, anti-CD8α, anti-CD8β, and anti-CD25 antibody stainings, and for HLA-A2.1/MP58-66 (T A2 MP58-66) and HLA-A2.1/E711-20 (T A2 E711-20) tetramer stainings. Filled histograms represent A9-TN during the first 12 months after hTERT transduction, and open histograms represent A9-TN beyond 12 months after hTERT transduction. Flow cytometric analysis was performed 3 weeks after feeder mix stimulation.
Functional activity analysis of hTERT-transduced, HPV16 E711-20–specific CTL A9-TN. Lytic activity, determined in a chromium release assay using HLA-A2.1–positive JY target cells loaded with MP58-66 peptide (○) and E711-20 peptide (•) at the indicated E/T ratios, CD107a expression in response to E711-20 peptide–loaded JY cells and intracellular IFNγ staining in response to E711-20 peptide–loaded JY cells, respectively, are shown for (A) A9-TN during the first 12 months after hTERT transduction and (B) A9-TN beyond 12 months after hTERT transduction. CD107a and IFNγ staining are shown in combination with HLA-A2.1/E711-20 tetramer (T A2 E711-20) staining. No CD107a and IFNγ staining above background was observed in response to MP58-66 peptide–loaded JY cells. The percentage of double-positive cells is shown in the top right quadrant. The indicated experiment was performed twice with similar results.
Functional activity analysis of hTERT-transduced, HPV16 E711-20–specific CTL A9-TN. Lytic activity, determined in a chromium release assay using HLA-A2.1–positive JY target cells loaded with MP58-66 peptide (○) and E711-20 peptide (•) at the indicated E/T ratios, CD107a expression in response to E711-20 peptide–loaded JY cells and intracellular IFNγ staining in response to E711-20 peptide–loaded JY cells, respectively, are shown for (A) A9-TN during the first 12 months after hTERT transduction and (B) A9-TN beyond 12 months after hTERT transduction. CD107a and IFNγ staining are shown in combination with HLA-A2.1/E711-20 tetramer (T A2 E711-20) staining. No CD107a and IFNγ staining above background was observed in response to MP58-66 peptide–loaded JY cells. The percentage of double-positive cells is shown in the top right quadrant. The indicated experiment was performed twice with similar results.
Discussion
Lifespan extension of human T lymphocytes via hTERT transduction has been proposed to allow expansion to the large numbers required for adoptive antitumor immunotherapy. Especially the expansion potential of well-characterized CTL clones with considerable replicative history in vitro can be rescued by hTERT transduction, otherwise severely restricted by replicative senescence.10,11 Such well-characterized CTL clones are expected to provide substantial clinical benefit in a therapeutic setting, further illustrating the value of hTERT-mediated lifespan extension. Different in vitro studies have shown that hTERT-transduced T cells retain the characteristics of untransduced counterparts.10,11,17 More importantly, a recent in vivo study has shown that hTERT-transduced CTLs have the potential to suppress the growth of human tumors in immunocompromised mice, as do untransduced CTLs.20 Furthermore, no adverse effects were observed in treated mice despite in vivo persistence of hTERT-transduced CTLs up to 6 months. These results have raised enthusiasm regarding the clinical application of hTERT-transduced T cells for tumor immunotherapy. However, the in vitro and in vivo studies mentioned lack a detailed analysis of hTERT-transduced CTLs at the cytogenetic level, warranted by the apparent association of hTERT with malignant transformation.21-23 Two human CD8+, hTERT-transduced CTL clones, regarded as potential candidates for adoptive immunotherapy of human cancer, were subjected to such analysis in the current study. In both cases, the analysis was performed after a substantial in vitro expansion period to obtain the large numbers of CTLs required for clinical application. The MART-127-35–specific CTL clone 4-TG displayed only minor chromosomal instability. In contrast, we clearly detected multiple chromosomal abnormalities in the HPV16 E711-20–specific CTL clone A9-TN, starting in intermediate term cultures of hTERT-transduced CTLs, and persisting as clonal aberrations in long-term cultures. Consequently, in combination with the observed potential of hTERT-transduced CTLs to persist in vivo up to 6 months,20 adoptive transfer of hTERT-transduced CTLs into patients could result in the in vivo persistence of cells harboring potentially harmful chromosomal abnormalities. Importantly, the chromosomal aberrations observed in the hTERT-transduced CTL A9-TN did not directly result in either phenotypic or functional alterations. As a consequence, retention of normal phenotype and function on hTERT transduction of CTLs may not guarantee their safe application, since it can coexist with genomic instability.
We have provided an in-depth cytogenetic analysis of 2 hTERT-transduced CD8+ CTL clones, and we found minor genomic instability in one clone, whereas severe genomic instability was observed in the other clone. Reports on the genomic stability of hTERT-transduced cells so far are somewhat inconsistent. Whereas hTERT transduction did not mediate genomic instability in muscle and neuronal cells,15,16 it did so in epithelial cells.37 In hTERT-transduced fibroblasts both absence and presence of chromosomal aberrations have been reported.38,39 In a recent study, genomic instability consisting of binucleated cells with connected nuclei was reported in CD4+, hTERT-transduced T lymphocytes.40 Collectively, these and our results indicate that cells constitutively expressing hTERT can acquire genomic abnormalities that may persist on extended culture. At this point it is important to note that the genomic instability observed so far in hTERT-transduced cells occurred irrespective of the marker genes present in the hTERT constructs used for transduction, including neomycin resistence,37 GFP,39,40 and ΔNGFR (current study). Although the safety of ΔNGFR as a cell-marking molecule has been the subject of some concern, cumulative results from both animal models and clinical trials clearly support the safety of retroviral gene marking with the truncated (Δ) form of NGFR.26 Formally, it cannot be excluded that the ΔNGFR insert in the construct used in the current study to generate CTL clone A9-TN may have contributed to the development of genetic instability. However, CTLs transduced with a construct containing the ΔNGFR insert only, so without the hTERT insert, do not acquire an extended life span and are thus unavailable for cytogenetic analysis on extended culture. The underlying mechanisms of genomic instability associated with hTERT transduction are poorly understood and beyond the scope of the current study. Possible explanations may involve the mechanisms by which dysfunctional telomeres can initiate genomic instability and the ability of hTERT to stabilize genomic instability and facilitate forced cell-cycle progression.41-43 Additionally, chromosomal aberrations, considered as biomarkers of aging in human cells, may represent an epiphenomenon of hTERT-mediated lifespan extension. Several studies have shown an increase in stable chromosomal aberrations as a function of age, through accumulation of mutations and failure of apoptotic pathways besides telomere dysfunction.41,44 Finally, insertion into the genome of the hTERT-encoding retrovirus may have initiated or contributed to genomic instability. However, although retroviral insertion has recently been implicated in oncogene activation through enhancer activities,45 evidence for a direct correlation with chromosomal rearrangement has not been provided so far.
At the immunologic level, aging of human lymphocytes is characterized by immunosenescence (reviewed in Pawelec et al24 ). In agreement with this phenomenon, we showed that prolonged culture of hTERT-transduced CTLs beyond 12 months results in an altered surface phenotype and a decreased functional activity. Although ectopic expression of hTERT effectively protects human CTLs from replicative senescence, it apparently does not confer protection against immunosenescence. Nevertheless, CTL A9-TN retained a wild-type phenotype and functional activity during the first 12 months of continuous culture.
Ectopic hTERT expression has resulted in the generation of large numbers of melanomaand cervical carcinoma–specific CTL clones, which would have been severely hampered by the lack of sufficient expansion of wild-type CTLs. However, we show that the hTERT-mediated lifespan extension of these 2 CTL clones is accompanied by minor to severe genomic instability. In general only few hTERT-transduced CTLs are available for a similar time-consuming as well as labor-intensive cytogenetic follow-up. Given the restricted number of CTL clones analyzed so far, the development of genomic instability in hTERT-transduced CTLs cannot be regarded as a general phenomenon at this point. Nevertheless, it cannot be predicted on forehand either, and the mere chance that severe genomic instability develops in hTERT-transduced CTLs following clinical application represents a substantial risk. Consequently, the use of hTERT-transduced CTL for adoptive immunotherapy in the near future requires incorporation of a safety measure. For this purpose, a suicide gene may be included in the hTERT-encoding retrovirus, shown recently to inhibit the in vitro growth of hTERT-transduced CTLs.46 Although malignant transformation has not been observed so far in hTERT-transduced cells, gene expression analysis of hTERT-transduced fibroblasts has indicated the development of a premalignant phenotype, including activation of genes involved in tumorigenesis and overexpression of oncogenes.47,48 Whether the observed genomic instability in the hTERT-transduced CTLs results in a premalignant phenotype and may ultimately mediate malignant transformation remains to be established. In favor of this hypothesis is the recent finding that Lck-Tert transgenic mice that constitutively express hTERT in their thymocytes display an increased incidence of T-cell lymphomas.49 In conclusion, although the general frequency of major chromosomal aberrations in hTERT-transduced CTLs and the in vivo significance of our observations remain still unclear at this point, the currently available data suggest that clinical application of hTERT-transduced CTLs should proceed with caution.
Prepublished online as Blood First Edition Paper, July 7, 2005;DOI 10.1182/blood-2004-09-3742.
Supported by the Dutch Cancer Society (grant VUMC2001-2503), the Netherlands Organization for Scientific Research (grant 901-10-124), and the Maurits and Anna de Kock Foundation.
M.A.J.A.H. and R.I.K.G. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr B. Carvalho and E. Hopmans for expert contributions regarding the array CGH analysis, J. Ruizendaal for CTL culture assistance, and the Mapping Core and Map Finishing groups of the Wellcome Trust Sanger Institute for initial clone supply and verification.