Abstract
Aminoglycoside antibiotics exhibit their bactericidal effect by interfering with normal ribosomal activity. In this pilot study, we have evaluated the effect of the aminoglycoside antibiotic gentamicin on the factor VIII (FVIII) and IX levels of severe hemophiliacs with known nonsense mutations. Five patients were enrolled and each patient was given 3 consecutive days of gentamicin at a dose of 7 mg/kg intravenously every 24 hours. Two patients (patient no. 1: hemophilia A, Ser1395Stop; and patient no. 5: hemophilia B, Arg333Stop) showed a decrease in their activated partial thromboplastin time (aPTT), an increase in their FVIII (0.016 IU/mL, 1.6%) or FIX (0.02 IU/mL, 2%) levels, and an increase in thrombin generation. The remaining 3 patients (patient no. 2: hemophilia B, Arg252Stop; patient no. 3: hemophilia A, Arg2116Stop; and patient no. 4: hemophilia A, Arg427Stop) showed no response in the aPTTs or factor levels, but one (patient no. 2: hemophilia B, Arg252Stop) showed an increase in the factor IX antigen level (2%-5.5%) that persisted throughout the period of the study and was concordant with an increase in thrombin generation. Gentamicin is unlikely to be an effective treatment for severe hemophilia due to its potential toxicities and the minimal response documented in this report. This study, however, does provide a proof of principle, suggesting that ribosomal interference with a less toxic agent may be a potential therapeutic mechanism for severe hemophilia patients with nonsense mutations.
Introduction
Hemophilia A and B are inherited deficiencies of coagulation factor VIII (FVIII) and FIX, respectively, the genes for which are both located on the human X chromosome. Hemophilia A occurs at an incidence of 1 in 5000 live male births compared with 1 in 30 000 for hemophilia B.1 Both conditions occur in mild, moderate, and severe forms, corresponding to plasma clotting factor levels of 6% to 30%, 1% to 5%, and less than 1%, respectively. Mild hemophiliacs usually bleed only after trauma or surgery, however, those with severe hemophilia A or B bleed spontaneously into joints and muscles. Current treatment for correction of hemostasis involves the infusion of coagulation factor concentrates.
Advances in the understanding of the molecular genetics of hemophilia have allowed many innovations in management, including the production of recombinant clotting factor concentrates. Identification of the disease-causing hemophilic mutations is, in many cases, now possible and allows carrier detection and antenatal diagnosis as well as the tailoring of treatment in specific cases. In the past few years, studies of somatic cell gene therapy have also begun, and results from preclinical animal studies2-7 as well as initial phase 1/2 human trials8-11 have been promising, although evidence of long-term hemostatic benefit from this treatment strategy has still to be achieved. In addition to “classical” substitutive hemophilia gene therapy, other specific molecular therapies, such as the use of SMaRT (spliceosome-mediated RNA trans-splicing) to repair mutant DNA,12 and chimeric RNA/DNA oligonucleotide therapies13 are also being evaluated.
The molecular defects that cause hemophilia are highly variable. While about 45% of severe hemophilia A cases are caused by an inversion within intron 2214 and 3% by an inversion in intron 1,15 the vast majority of molecular defects causing hemophilia are a diverse array of either missense or nonsense mutations, deletions/insertions, or splicing abnormalities. Hemophilia B is also caused by a wide array of mutations, the majority of which are unique to each kindred. A review of the factor VIII HAMSTeRS database16 shows that of 952 reported hemophilia A mutations, 100 are nonsense mutations (∼9%). Similarly, review of the hemophilia B mutation database17 shows that of 622 reported mutations, 70 are nonsense mutations (∼9%).
Aminoglycoside antibiotics are commonly used to treat Gram-negative infections. They exert their antimicrobial effect by interfering with the normal function of the ribosome, specifically with the process of “proofreading” that allows the discrimination against mismatched amino acyl-transfer RNA from becoming incorporated into the growing polypeptide chain.18 This mechanism has been shown to suppress premature termination codons by causing the disregard of the termination codon and the incorporation of another random amino acid into that position, thus allowing translation of the polypeptide chain to continue.19-21 Clinical studies exploiting this mechanism for therapeutic benefit have been performed in a number of conditions including cystic fibrosis,22 muscular dystrophy,23 Hurler syndrome,24 ataxia-telangiectasia,25 and late infantile neuronal ceroid lipofuscinosis.26 Furthermore, Srivastava et al27 treated 4 hemophilia B patients with gentamicin in an attempt to override their premature termination codons but did not see any increase in FIX levels. In this pilot study, we treated 5 patients (3 with severe hemophilia A and 2 with severe hemophilia B; Table 1), all with known nonsense mutations, with 3 consecutive days of gentamicin in an attempt to override their premature stop codons and increase their levels of FVIII or FIX and to increase overall thrombin generation.
Patients, materials, and methods
Patients and study protocol
Potential patients were identified through the Canadian National Hemophilia Genotyping Program (Kingston, ON, Canada) and invited to participate in the study by their local Hemophilia Comprehensive Care Clinic. Research Ethics Board approval was obtained from Queen's University (Kingston, ON, Canada) and from all treating centers. Investigational New Drug approval was obtained from Health Canada for this off-label use of gentamicin (control no. 079440). Patients were eligible if they were severe hemophiliacs older than 12 years of age with known nonsense mutations. Exclusion criteria included preexisting renal impairment, preexisting hearing impairment, presence of an inhibitor to FVIII or FIX, significant hepatic or cardiac impairment, or a known allergy to gentamicin or other aminoglycoside antibiotic. Patients were also excluded if they were taking furosemide, amphotericin B, vancomycin, or acyclovir or if they had been diagnosed with myasthenia gravis. Patients had to observe a 7-day washout for coagulation factor concentrates prior to the first treatment day of the study and were excluded from the study or the study was postponed if they had a bleed that required treatment within the 7-day period.
Patients underwent a number of prestudy investigations including FVIII or FIX levels and inhibitor studies, serum creatinine levels, electrolyte levels, liver enzyme levels and liver function tests, and audiometry. All patients gave informed consent. Each patient was treated with 3 consecutive days of gentamicin at a dose of 7 mg/kg intravenously every 24 hours. Pretreatment and posttreatment (1 hour and 6 hours) activated partial thromboplastin times (aPTTs), FVIII or FIX levels, thrombin generation assays, gentamicin levels, and serum creatinine levels were performed on each treatment day. Coagulation testing and a serum creatinine level were repeated on days 4 and 10. All patients had repeat audiometry within one month of completion of the study. Patients were monitored for bleeds throughout the study period and the protocol was terminated prematurely if a bleed developed that required treatment with a coagulation factor concentrate.
Coagulation testing
Blood for all coagulation testing was obtained by a 2-syringe technique and anticoagulated with 0.105 M buffered trisodium citrate at a ratio of 1:9 (anticoagulant-blood). After centrifugation at 10 000g for 15 minutes, the platelet-poor plasma was removed and stored at –70°C until testing was performed. All testing was carried out in a central reference laboratory after transfer of plasma samples on dry ice. Frozen plasma samples were thawed at 37°C for 5 minutes prior to testing. Testing was performed in such a way that the technologist was “blinded” to the identity of individual samples.
Functional FVIII and FIX studies were performed with one-stage assays employing aPTTs using the MDA Platelin L reagent from bio Merieux (St Laurent, QC, Canada) and factor-deficient plasmas from Precision Biologicals (Dartmouth, NS, Canada). Tests were performed on an MDA 180 automated coagulometer, and the sensitivity of the one-stage assays is 0.01 IU/mL (1%). A chromogenic FVIII assay was also performed using the Chromogenix COAMATIC assay (Diapharma, West Chester, OH) and is sensitive to a level of 0.005 IU/mL (0.5%). All assays were performed using a normal plasma pool from Precision Biologicals that is referenced to the appropriate World Health Organization (WHO) standards (FVIII WHO 97/586; FIX WHO 94/746). Factor VIII:Ag and factor IX:Ag assays were performed using polyclonal antibodies from Affinity Biologicals (Hamilton, ON, Canada). The level of sensitivity of the antigen assays is 0.01 IU/mL (1%). Factor VIII and IX inhibitor assays were performed using the Nijmegen modification of the original Bethesda method for FVIII28 and a standard Bethesda protocol for FIX.
Fluorogenic thrombin generation test
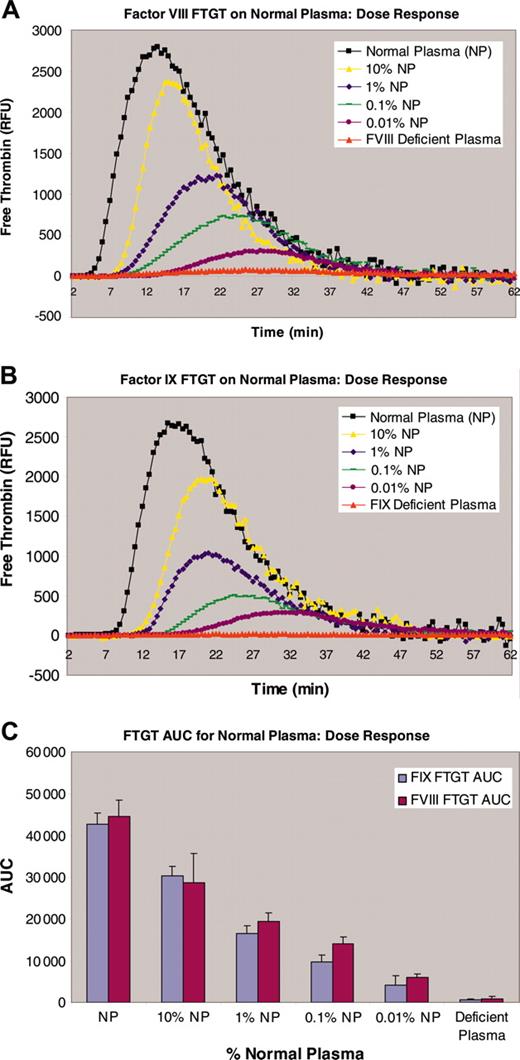
The fluorogenic thrombin generation test (FTGT) is based on the use of a fluorogenic thrombin substrate that allows thrombin generation to be determined continuously in a more physiologic system than prior TGT assays, without the need for subsampling or defibrination of plasma samples. This method is sensitive to low levels of FVIII (< 0.001 IU/mL),29 significantly below the level that is detectable by current coagulation factor assays (Figure 4). Thrombin generation is initiated by the addition of one part nondefibrinated plasma (40 μL) to 2 parts substrate mixture (80 μL; fluorogenic substrate, 0.238 mM; FIXa, 5 nM; phospholipids, 3 μg/mL; and Ca2+, 7 mM) in a black microtiter plate (Greiner, Frickenhausen, Germany). The plate is read in a Spectramax Gemini XS Fluorimeter (Molecular Devices, Sunnyvale, CA) at 30°C at 30-second intervals for 1 hour with excitation at 390 nm and reading at 460 nm. The data are exported into an Excel file and the amount of thrombin generated is calculated according to the method of Hemker and Begiun.30 This method allows determination of free thrombin in a continuous thrombin generation test with chromogenic and fluorogenic substrates.27 For hemophilia B plasma samples, the trigger is via contact activation. Factor IXa is omitted from the substrate mixture and the reaction is first carried out in a glass tube, prior to transfer to a microtiter plate. The data were analyzed and quantified for the area under the curve (AUC) parameter.
Results
Five patients were recruited and treated under the study protocol: 3 with hemophilia A (Ser1395Stop, Arg2116Stop, and Arg427Stop) and 2 with hemophilia B (Arg333Stop and Arg252Stop; Table 1). Patient no. 1 (hemophilia A: Ser1395Stop) revealed that he had prophylactically treated himself with recombinant FVIII 7 days prior to the start of the trial. Based on half-life calculations, we estimated that the exogenous factor would have disappeared from the plasma by the start of the trial and we therefore proceeded. One patient (hemophilia A: exon 9 Arg427Stop) did not continue past the day-2 6-hours posttreatment sample, due to the development of hematuria that required treatment. None of the patients showed any signs of renal or otoxicity or any other adverse events during or after the treatment protocol.
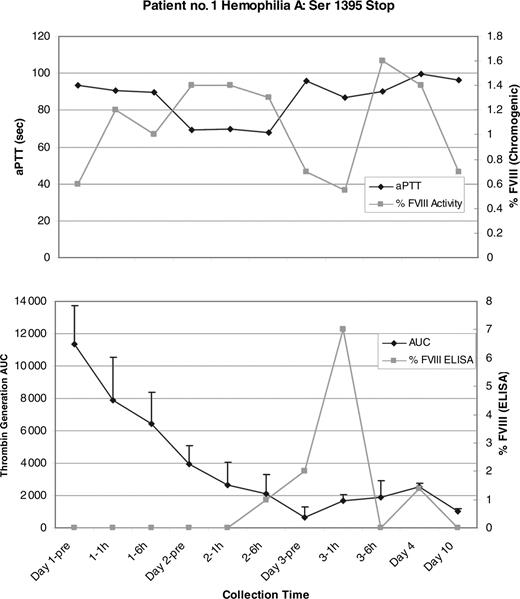
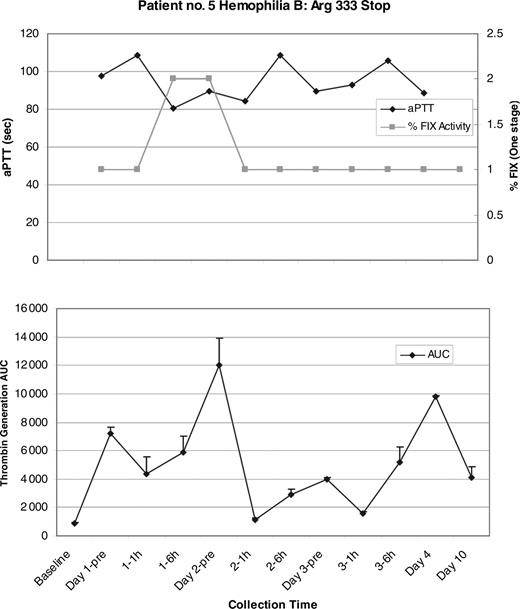
Two patients showed a change in the aPTTs and factor levels during the gentamicin treatment period. Patient no. 1 (hemophilia A: Ser1395Stop) showed a 25-second decrease in the aPTT at the day-2 6-hours posttreatment sample that coincided with an increase in the FVIII level from a baseline of 0.006 IU/mL to 0.014 IU/mL (or 1.4%) by chromogenic FVIII assay. On day 3, the aPTT returned to baseline however the FVIII level peaked at 0.016 IU/mL (1.6%; Figure 1). Patient no. 5 (hemophilia B: Arg333Stop) showed a similar decrease in the aPTT of approximately 20 seconds that started at the day-1 6-hours sample and continued into day 2. These results coincided with an increase in the FIX level to 0.02 IU/mL (2%) by one-stage FIX assay. Both the aPTT and FIX level returned to the baseline on day 3 (Figure 2). Significant changes in the factor levels or aPTTs were not seen in patients no. 2 (hemophilia B: Arg252Stop), no. 3 (hemophilia A: Arg2116Stop), or no. 4 (hemophilia A: Arg427Stop).
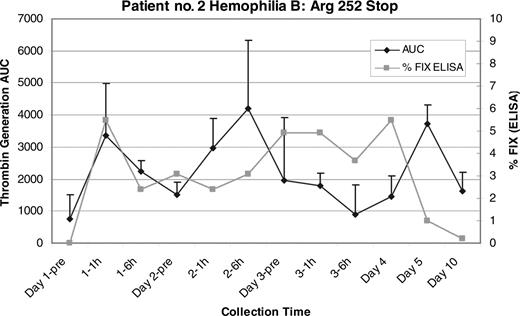
The FVIII enzyme-linked immunosorbent assay (ELISA) results for patient no. 1 (hemophilia A: Ser1395Stop) showed an increase in FVIII antigen above baseline to a peak of 0.07 IU/mL (7%), which is higher than the 1.6% detected by chromogenic assay, suggesting the production of a mixture of functional and nonfunctional proteins (Figure 1). Likewise, the FIX ELISA results for patient no. 2 (hemophilia B: Arg252Stop) showed a persistent increase in FIX antigen during the study between 0.02 (2%) and 0.055 IU/mL (5.5%; Figure 3). Interestingly, patient no. 2 showed no response in either the aPTT or the FIX level, suggesting that in this case any new FIX protein synthesized in response to the gentamicin possessed insufficient procoagulant function to register an effect in the aPTT-based clotting assay.
Patient no. 1 aPTT, chromogenic FVIII results, mean thrombin generation area under the curve (AUC), and FVIII antigen level (ELISA). (Top) There is good correlation between the drop in aPTT and rise in FVIII in the day-2 pretreatment (pre), day-2 1-hour, and day-2 6-hour samples. There is a peak in FVIII activity at the day-3 6-hour sample, and both the aPTT and FVIII return to baseline by the day-10 sample. (Bottom) There is a peak in FVIII:Ag at the day-3 1-hour sample to a level of 0.07 IU/mL (7%). The mean thrombin generation AUC is also shown and begins with the greatest AUC in the baseline sample that most likely reflects the exogenous recombinant FVIII that the patient self-administered 7 days before the start of the study. The AUC steadily declines from that time point on and it is possible that this exogenous factor masked any contribution to overall thrombin generation that the endogenous factor synthesized under the influence of gentamicin could have made. Error bars indicate the standard error of the mean (SEM).
Patient no. 1 aPTT, chromogenic FVIII results, mean thrombin generation area under the curve (AUC), and FVIII antigen level (ELISA). (Top) There is good correlation between the drop in aPTT and rise in FVIII in the day-2 pretreatment (pre), day-2 1-hour, and day-2 6-hour samples. There is a peak in FVIII activity at the day-3 6-hour sample, and both the aPTT and FVIII return to baseline by the day-10 sample. (Bottom) There is a peak in FVIII:Ag at the day-3 1-hour sample to a level of 0.07 IU/mL (7%). The mean thrombin generation AUC is also shown and begins with the greatest AUC in the baseline sample that most likely reflects the exogenous recombinant FVIII that the patient self-administered 7 days before the start of the study. The AUC steadily declines from that time point on and it is possible that this exogenous factor masked any contribution to overall thrombin generation that the endogenous factor synthesized under the influence of gentamicin could have made. Error bars indicate the standard error of the mean (SEM).
Patient no. 5 aPTT, one-stage FIX results and mean thrombin generation AUC. (Top) There is good correlation between the drop in aPTT and the rise in FIX in the day-1 6-hours and the day-2 pretreatment samples, however the changes are not sustained, and both the aPTT and FIX essentially return to baseline by the day-2 1-hour sample. The greatest mean thrombin generation (bottom) also correlates with the peak FIX and drop in the aPTT seen in the day-2 pretreatment sample. Error bars indicate SEM.
Patient no. 5 aPTT, one-stage FIX results and mean thrombin generation AUC. (Top) There is good correlation between the drop in aPTT and the rise in FIX in the day-1 6-hours and the day-2 pretreatment samples, however the changes are not sustained, and both the aPTT and FIX essentially return to baseline by the day-2 1-hour sample. The greatest mean thrombin generation (bottom) also correlates with the peak FIX and drop in the aPTT seen in the day-2 pretreatment sample. Error bars indicate SEM.
The thrombin generation assay (FTGT) for patient no. 1 (hemophilia A: Ser1395Stop) showed a peak in the day-1 pretreatment sample, which steadily declined from that point on (Figure 5A). As mentioned above, this patient had given himself a prophylactic infusion of recombinant FVIII concentrate 7 days prior to the start of the trial. The effects of this treatment were not detectable by either the FVIII chromogenic assay or the aPTT in the day-1 pretreatment sample (Figure 1). FTGT for patient no. 5 (hemophilia B: Arg333Stop) showed a peak of thrombin generation in the day-2 pretreatment sample, followed by the day-4 sample, followed by the day-1 6-hours sample (Figure 5B). The changes seen in the day-1 6-hours and day-2 pretreatment sample correlated with the increase in the FIX level and the decrease in the aPTT, but the thrombin generation seen in the day-4 sample did not. FTGT for patient no. 2, in whom FIX antigen values between 0.02 and 0.055 IU/mL were documented throughout the 3-day study, showed a peak of thrombin generation in the day-2 6-hours posttreatment sample. Moreover, minor but definite increases in thrombin generation were seen at every time point during the study period in patient no. 2 (Figure 5C). The results in this patient suggest that low levels of FIX synthesis were stimulated by gentamicin administration sufficient to generate low levels of thrombin but insufficient to influence aPTT-based coagulant assays. Increased thrombin generation was not observed in patient no. 3 (hemophilia A: Arg2116Stop) or patient no. 4 (hemophilia A: Arg427Stop).
Patient no. 2 FIX ELISA and mean thrombin generation AUC. There is a sustained increase in the FIX:Ag throughout the study period that returns to baseline by the day-10 sample. This correlates with a sustained increase in the mean thrombin AUC that is seen throughout the study period as well. There was no change seen in the aPTT or the FIX:C level in this patient (not shown). Error bars indicate SEM.
Patient no. 2 FIX ELISA and mean thrombin generation AUC. There is a sustained increase in the FIX:Ag throughout the study period that returns to baseline by the day-10 sample. This correlates with a sustained increase in the mean thrombin AUC that is seen throughout the study period as well. There was no change seen in the aPTT or the FIX:C level in this patient (not shown). Error bars indicate SEM.
FTGT on normal plasma (NP). (A) The thrombin generation dose response for normal plasma with differing FVIII concentrations. (B) The thrombin generation dose response for normal plasma with differing FIX concentrations. (C) The calculated area under the thrombin generation curve (AUC) for different dilutions of both FVIII and FIX plasma.
FTGT on normal plasma (NP). (A) The thrombin generation dose response for normal plasma with differing FVIII concentrations. (B) The thrombin generation dose response for normal plasma with differing FIX concentrations. (C) The calculated area under the thrombin generation curve (AUC) for different dilutions of both FVIII and FIX plasma.
Discussion
In this pilot study, we show a change in standard functional hemostatic parameters (chromogenic and aPTT-based coagulant assays) in 2 patients with severe hemophilia caused by nonsense mutations and changes in antigen level and thrombin generation in one additional patient during treatment with the aminoglycoside antibiotic gentamicin. Despite this interesting difference between those who showed hemostatic changes and those who did not, the magnitude of hemostatic response documented in this study was small. Based on our results, the potential for gentamicin to be an effective treatment for severe hemophiliacs with nonsense mutations appears limited, given the minimal hemostatic benefit, the potential toxicities, and the intravenous route of administration of this agent. Additionally, there are a number of factors to consider in terms of the changes in hemostatic parameters that we saw, including the lack of complete correlation between the aPTTs, clotting factor assay results, and the FTGT results.
In patient no. 1 the nonsense mutation Ser1395Stop is located at the position of a nonconserved amino acid. However, in the other 4 patients the mutation is at a conserved position. It can be speculated that in patient no. 1, the insertion of a random amino acid would be more likely to produce a functional protein, given the potential lesser importance of that amino acid position. This hypothesis cannot be extended to explain the positive hemostatic changes seen in patient nos. 5 and 2, however. We assume that the amino acid that is inserted under the influence of gentamicin is randomly selected, however, this is not certain, and the true sequence and structure of the resultant protein is unknown.
In both patients who showed a response in their aPTTs and clotting factor levels, there was a lack of complete correlation between the shortening of the aPTT and the increase in factor levels, and both patients showed the changes early in the treatment protocol. This change was not sustained through to the end of the protocol in either patient no. 1 or patient no. 5. This may be related to specific nonsustained interactions between gentamicin and the ribosome in the hemophiliac system, and, certainly, fluctuations in responses to aminoglycosides have been observed in other conditions.31,32 However, the lack of correlation may simply reflect the relative sensitivity of the assays to small changes in the determinants of coagulation. In this study, all of the more routine tests of hemostasis are being conducted at their lower limits of detection. Standard clotting assays such as the aPTT achieve their end point when approximately 4% of the total thrombin has been generated33 and may not be the most reflective measure of the true hemostatic response. Much work has been done recently in order to more accurately characterize global hemostatic responses including evaluating the contribution that a number of procoagulation factors including FVII/VIIa, FIX, FX, FII, FV, FVIII, and the anticoagulants tissue factor pathway inhibitor (TFPI) and antithrombin (AT) make to overall thrombin generation in in vitro experiments and in computer-generated active thrombin profiles.34,35 In the paper by Brummel-Ziedins et al,34 data are presented on 4 severe hemophiliacs, showing significant variability in the ability to generate thrombin in both the untreated and treated states. It may be that small changes in determinants of coagulation other than FVIII and FIX in our patients are responsible for the variable and discordant responses seen both within and between individuals. In light of these additional complicating factors, we performed the FTGT in an attempt to evaluate global hemostasis.
The thrombin generation data for patient no. 1 may reflect, at least in part, the exogenous factor that was self-administered 7 days prior to the start of the trial because of the clear peak in the day-1 pretreatment sample and the subsequent, progressive decline. Although calculations of the conventionally accepted half-life for FVIII would suggest that no residual coagulant activity should be detectable after a 7-day period, there is growing evidence from hemophilia prophylaxis studies to question this fact.36 It is possible that this exogenous factor masked any contribution to overall thrombin generation that the endogenous factor synthesized under the influence of gentamicin could have made. The thrombin generation data for patient no. 5 correlated, although not completely, with the results of the FIX assays and the aPTTs, and although there was no correlation between the thrombin generation data in patient no. 2 and the clotting factor or aPTT results, there was good correlation between the FTGT and the FIX ELISA results. This suggests that the FTGT may be capable of detecting levels of FIX protein that possess partial functionality below the level of detection of the FIX one-stage assay or the aPTT. Another difference that merits comment is the discrepancy between the time-to-peak thrombin generation as seen in the dose-response curves for normal plasma (Figure 5) and the time to peak seen in our patients. In patient no. 5 and patient no. 2, the increase in thrombin generation is likely reflecting the endogenous production of FIX under the influence of gentamicin; however, it may be that differences between this endogenously produced protein and normal protein are responsible for the increased time to peak.
In addition to the potential differences in other determinants of the global hemostatic response, there are other factors that may contribute to the variability of response seen in this study. Both the specific stop codon and the DNA sequence context of that stop codon have been shown to have an effect on the efficiency of translation termination,37-39 however, these factors did not appear important in our patients.
The identification of therapeutic methods for overriding nonsense mutations would be beneficial for approximately 10% of hemophiliacs and for individuals with other inherited conditions caused by nonsense mutations. In this proof-of-principle study, we have documented small positive effects on the clotting factor levels in 2 of 5 patients, with additional benefits seen in the FIX antigen level and thrombin generation in another patient. However, our results do not support the use of gentamicin as a clinical therapy to suppress nonsense mutations in severe hemophiliacs. The changes documented in 3 of these patients support the proof of principle that ribosomal interference with a less toxic, more efficient, and potentially orally administered agent may present a viable therapy for severe hemophiliacs with nonsense mutations in the future.
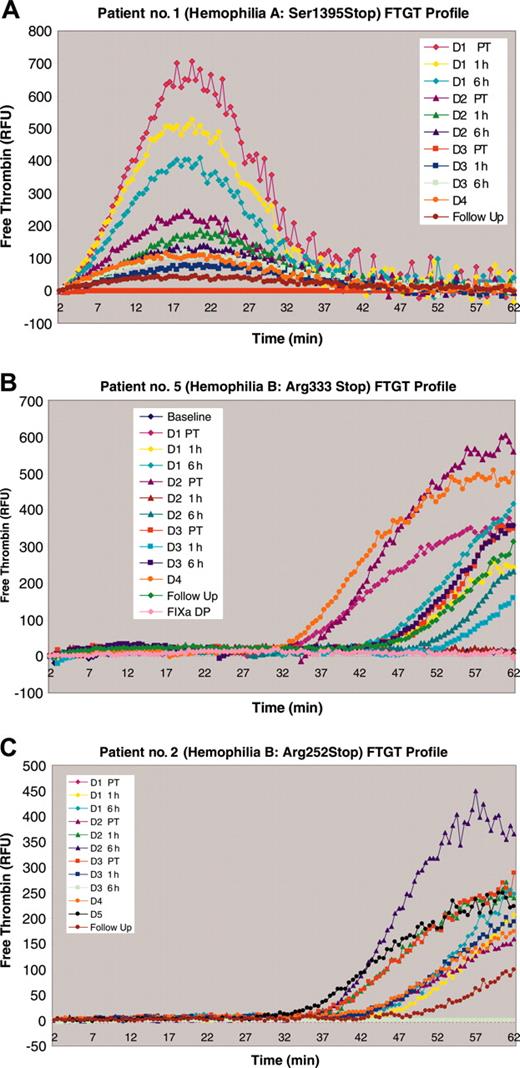
FTGT results on patient nos. 1, 5, and 2. (A) The fluorogenic thrombin generation curve for patient no. 1 before and throughout the study period. There is a clear high peak in the baseline sample that most likely reflects the exogenous recombinant FVIII that the patient self-administered 7 days before the start of the study. The peak of thrombin generation steadily declines from that time point on and it is possible that this exogenous factor masked any contribution to overall thrombin generation that the endogenous factor synthesized under the influence of gentamicin could have made. (B) The FTGT for patient no. 5 before and throughout the study period. There is a peak of thrombin generation in the day-2 pretreatment (PT) sample that correlates with the drop in aPTT and rise in FIX level seen in Figure 2. (C) The FTGT for patient no. 2 before and throughout the study period. There is an increase in thrombin generation throughout the study period that correlates with the rise in the FIX antigen level (Figure 3) but is not reflected in the aPTT or FIX one-stage assay. RFU indicates relative fluorescence units.
FTGT results on patient nos. 1, 5, and 2. (A) The fluorogenic thrombin generation curve for patient no. 1 before and throughout the study period. There is a clear high peak in the baseline sample that most likely reflects the exogenous recombinant FVIII that the patient self-administered 7 days before the start of the study. The peak of thrombin generation steadily declines from that time point on and it is possible that this exogenous factor masked any contribution to overall thrombin generation that the endogenous factor synthesized under the influence of gentamicin could have made. (B) The FTGT for patient no. 5 before and throughout the study period. There is a peak of thrombin generation in the day-2 pretreatment (PT) sample that correlates with the drop in aPTT and rise in FIX level seen in Figure 2. (C) The FTGT for patient no. 2 before and throughout the study period. There is an increase in thrombin generation throughout the study period that correlates with the rise in the FIX antigen level (Figure 3) but is not reflected in the aPTT or FIX one-stage assay. RFU indicates relative fluorescence units.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-03-1307.
Supported by a Canadian Hemophilia Society Care Until Cure Research Grant and an operating grant from the Canadian Institutes for Health Research (MOP-10912). P.D.J. held the Aventis-Behring–Canadian Hemophilia Society—Association of Hemophilia Clinic Directors of Canada (CHS-AHCDC) Fellowship in Hemophilia at the time of this study. D.L. holds a Canada Research Chair in Molecular Hemostasis and a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
D.L. and P.D.J. designed research, analyzed data, and wrote the paper; S.R. performed research, contributed vital investigations, analyzed data, and contributed to final manuscript; G.E.R. and M.-C.P. performed research, analyzed data, and contributed to final manuscript; M.W. performed research; S.M. designed research, and J.L. performed research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge the invaluable contributions of Francine Derome, Morna Brown, Colleen Notley, and Caroline Hensman. D.L. is a Career Investigator of the Heart and Stroke Foundation of Ontario, and holds a Canada Research Chair in Molecular Hemostasis.