Abstract
Low-dose metronomic chemotherapy is a promising therapeutic cancer treatment strategy thought to have an antiangiogenic basis. However, the advantages of reduced toxicity, increased efficacy in some cases, and ability to combine chemotherapy administered long term in this way with targeted therapies can be compromised by the empiricism associated with determining the optimum biologic dose (OBD). Using 4 distinct metronomic chemotherapy regimens in 4 different preclinical tumor models, including a hematologic malignancy, we established the OBD by determining the maximum efficacy associated with minimum or no toxicity. We then found each OBD to be strikingly correlated with the maximum reduction in viable peripheral blood circulating vascular endothelial growth factor receptor 2–positive (VEGFR-2+) endothelial precursors (CEPs). These results suggest that CEPs may serve as a pharmacodynamic biomarker to determine the OBD of metronomic chemotherapy regimens.
Introduction
The tumor vasculature has emerged as a clinically validated therapeutic target.1 In addition to rationally designed, molecularly targeted antiangiogenic drugs such as anti–vascular endothelial growth factor (anti-VEGF) antibodies,1 many conventional and new therapeutic agents may have antiangiogenic effects that can contribute to their treatment efficacy.2,3 These agents include chemotherapy drugs, the antiangiogenic efficacy of which appear to be optimized by “metronomic” dosing or the administration of relatively low, nontoxic doses at regular close intervals with no prolonged interruptions.3-5 Some metronomic regimens can have surprisingly potent antitumor effects in preclinical models compared with respective maximum tolerated dose regimens, despite being less toxic.4,6,7 This makes it possible to consider combining simultaneously such long-term “maintenance” chemotherapy with targeted antiangiogenic drugs8,9 or other agents, such as tumor vaccines.10 Some promising preliminary results have also begun to emerge in small clinical studies using mostly orally administered metronomic chemotherapy–based regimens,3,11,12 including those in the adjuvant setting for early-stage cancer.13 However, a significant disadvantage is the empiricism in establishing the optimal biologic dose (OBD) and in monitoring therapeutic activity early during the course of treatment.3 Using (1) previous observations showing significant and sustained declines in circulating VEGF receptor 2–positive (VEGFR-2+) endothelial progenitor cells (CEPs) induced by prolonged daily low-dose metronomic chemotherapy6 ; (2) preclinical validation of measuring levels of such cells as a surrogate blood–based marker of angiogenesis and targeted antiangiogenic drug activity, including optimal biologic dosing14 ; and (3) CEP potentially as a marker in the clinic of targeted antiangiogenic drug activity,15 we assessed whether determining OBD ranges of various chemotherapy drugs is possible using this cellular pharmacodynamic biomarker approach. Specifically, we addressed the question of whether the optimal metronomic dose correlates with the optimal antiangiogenic dose. An affirmative answer would also serve to strengthen the hypothesis that metronomic dosing indeed inhibits tumor growth primarily by an antiangiogenic mechanism.3,4
The approach we used consisted of empirically establishing the OBD for 4 metronomic chemotherapy regimens in 4 different tumor models, evaluating both treatment efficacy and host toxicity. Subsequently, we undertook a retrospective analysis of the viable CEPs to determine whether their values strictly correlated with the defined OBD.
Study design
Tumor models and drug scheduling
The following tumor models and drugs were tested: (1) MeWo, a human melanoma grown subdermally16 in nude mice, treated with 0 to 50 mg/kg cyclophosphamide (CTX) administered daily through the drinking water7 ; (2) MVB9, a multidrug resistant P-glycoprotein expressing a variant of the human MDA-MB-231 breast cancer cell line,17 grown in female SCID mice treated with 0 to 0.67 mg/kg vinblastine (Vbl) administered intraperitoneally 3 times a week; (3) orthotopic 231/LM2-4, an aggressive metastatic variant of the human MDA-MB-231 breast cancer cell line derived from 2 rounds of lung metastases selection in female SCID mice, treated with 0 to 12 mg/kg vinorelbine (Nvb) administered orally 3 times a week; and (4) spontaneous erythroleukemia model (induced by the Friend virus18 ) in BALB/cJ mice and treated with either 0 to 50 mg/kg CTX orally each day through drinking water or 0 to 3 mg/kg cisplatinum (CDDP) administered intraperitoneally twice a week.
Tumor growth, toxicity, and CEP measurements
Mice were monitored regularly, and when solid tumor volumes reached 150 to 200 mm3, administration of the various chemotherapy regimens was initiated. In the (spontaneous) erythroleukemia model that did not involve transplantation, treatment was initiated in 4-week-old erythroleukemic (late-stage disease18 ) mice. After 1 week of treatment, tumors (and spleens in the erythroleukemia model only) were measured, mice were killed, and tissues were removed for evidence of host toxicity19 and viable CEPs.6 In all cases, a parallel experiment to evaluate longer-term treatment efficacy was performed: drugs were administered until solid tumor volumes reached 1000 to 1500 mm3 or, in the case of the erythroleukemia model, severe anemia developed as a result of erythrocyte replacement by malignant erythroleukemic cells that home to the spleen.18,20
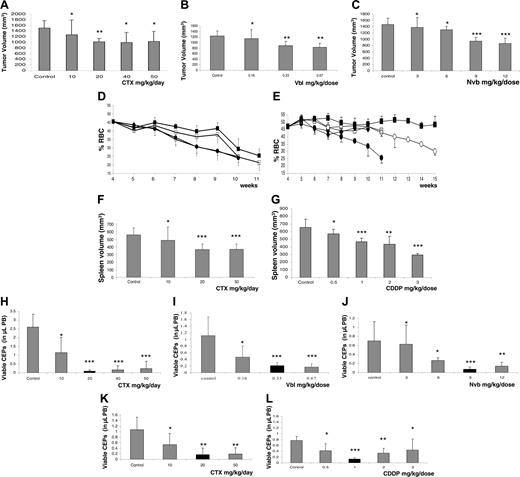
Correlation between dose-dependent treatment efficacy and reduction in viable CEPs in various tumor models. Tumor growth delays or tumor volumes at the end of treatment. (A) MeWo human melanoma treated with CTX administered through drinking water on a daily basis. (B) MDA-MB-231/MVB9 human breast cancer–derived multidrug-resistant variant tumors treated with Vbl, injected intraperitoneally 3 times a week. (C) MDA-MB-231/LM2-4 human breast cancer treated with Nvb administered by gavage 3 times a week, at the indicated doses. In addition, 4-week-old erythroleukemic mice were treated with either (D, F) CTX through drinking water or (E, G) CDDP by intraperitoneal injection twice a week; tumor growth in this model is represented by reduction in hematocrit levels and changes in spleen volume at end point. For both drugs, groups are designated as follows: control untreated (•), 10 mg/kg CTX daily or 0.5 mg/kg CDDP (○), 20 mg/kg CTX daily or 1 mg/kg CDDP (▪), 50 mg/kg CTX daily or 2 mg/kg CDDP (□) and 3 mg/kg CDDP (♦). Parallel experiments were performed to test for viable CEPs by flow cytometry, as previously described6 (H-L). Black columns represent the optimal therapeutic doses in each case that induce the most significant decline in viable CEP levels and a reduction in tumor volumes, with minimal or no toxicity, as summarized in Table 1. Significant differences from control: *P > .05; **.05>P > .01; and *** P < .01.
Correlation between dose-dependent treatment efficacy and reduction in viable CEPs in various tumor models. Tumor growth delays or tumor volumes at the end of treatment. (A) MeWo human melanoma treated with CTX administered through drinking water on a daily basis. (B) MDA-MB-231/MVB9 human breast cancer–derived multidrug-resistant variant tumors treated with Vbl, injected intraperitoneally 3 times a week. (C) MDA-MB-231/LM2-4 human breast cancer treated with Nvb administered by gavage 3 times a week, at the indicated doses. In addition, 4-week-old erythroleukemic mice were treated with either (D, F) CTX through drinking water or (E, G) CDDP by intraperitoneal injection twice a week; tumor growth in this model is represented by reduction in hematocrit levels and changes in spleen volume at end point. For both drugs, groups are designated as follows: control untreated (•), 10 mg/kg CTX daily or 0.5 mg/kg CDDP (○), 20 mg/kg CTX daily or 1 mg/kg CDDP (▪), 50 mg/kg CTX daily or 2 mg/kg CDDP (□) and 3 mg/kg CDDP (♦). Parallel experiments were performed to test for viable CEPs by flow cytometry, as previously described6 (H-L). Black columns represent the optimal therapeutic doses in each case that induce the most significant decline in viable CEP levels and a reduction in tumor volumes, with minimal or no toxicity, as summarized in Table 1. Significant differences from control: *P > .05; **.05>P > .01; and *** P < .01.
Statistical analysis
Results are reported as mean plus or minus SD. Statistical significance of differences was assessed by one-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls test or the 2-tailed Student t test (where indicated) using PRISM (version 4.00; GraphPad, San Diego, CA) and Excel (2000; Microsoft Office, Santa Rosa, CA). The level of significance was set at P less than .05.
Results and discussion
The results in Figure 1A-G show that with escalating doses of CTX, Vbl, Nvb, or CDDP, significant reductions in tumor/spleen volumes or tumor growth delays were observed (except in the case of the MeWo tumor model, in which differences that were significant from control group were observed only with the 20-mg/kg daily dose). Regarding toxicity, as summarized in Table 1, a significant reduction (compared with untreated controls) in neutrophil count, bone marrow proliferation, or total white blood cell (WBC) count was observed with 50 mg/kg CTX daily (in both models), 0.66 mg/kg Vbl, and 9 to 12 mg/kg Nvb (P < .05). With CDDP, a significant reduction in body weight (more than 15%) was observed in only the 2- to 3-mg/kg dose range, necessitating the humane killing of the mice according to institutional guidelines. Therefore, we empirically defined OBD as that dose causing maximum reduction in the tumor volume with no or minimal toxicity. This was assessed to be 20 mg/kg CTX daily (for both tumor models), 0.33 mg/kg Vbl, 9 mg/kg Nvb, or 1 mg/kg CDDP in the various tumor models used. Of note, we chose 9 mg/kg Nvb as the OBD even though this dose revealed moderate hematotoxicity by a slight reduction in WBC count (Table 1), in contrast to the 6 mg/kg dose; however, the latter dose was not efficacious. Whether a dose between 6 and 9 mg/kg is the OBD requires further investigation.
In parallel experiments for each model and drug, we analyzed the levels of viable CEPs in peripheral blood (PB), after only 1 week of treatment, to test whether OBD was strictly associated with the observed maximum antiangiogenic activities. The results in Figure 1H-1L show that a significant reduction in the viable CEPs was observed with 20 mg/kg CTX daily (in the MeWo tumor model), 0.33 mg/kg Vbl, 9 mg/kg Nvb, or 1 mg/kg CDDP. Of note, in an erythroleukemia model, CTX did not show a significant reduction in CEPs using one-way ANOVA. However, the 20- to 50-mg/kg daily doses were significantly different from those of the untreated control group according to 2-tailed Student t test. The various treatments were found to have a significant dose-dependent reduction in viable CEPs, the nadir of which coincided exactly with the previously determined OBD. Moreover, the decline in levels of viable CEP values we detected in non–tumor-bearing BALB/cJ mice treated with 0 to 50 mg/kg CTX daily or 0 to 12 mg/kg Nvb were consistent with the pattern we observed in tumor-bearing mice (data not shown), which rules out a possible impact of changes in tumor volume on CEP levels after 1 week of treatment. We also observed that when the dose of CDDP was escalated above the 1 mg/kg, an increase in viable CEPs was actually observed. These results suggest that with some chemotherapeutic drugs administered in a metronomic regimen, the therapeutic window may be narrow. Reduced CEP levels may have the potential to predict the optimal dose range of such drugs.
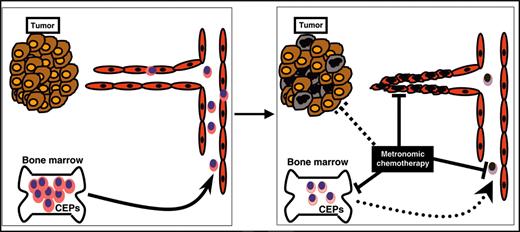
Although our results do not discount other possible mechanisms for the antitumor effects of metronomic chemotherapy, they do strengthen the hypothesis that a significant contributing mechanism is the inhibition of angiogenesis, likely mediated by selective killing of endothelial cells21 and interference with the mobilization or function of circulating VEGFR-2+ PB cells, outlines in Figure 2. In this regard, the fact that dose-dependent declines in CEPs, which correlate with the OBD, can be detected in non–tumor-bearing mice means that such changes do not occur only as a secondary consequence of inhibition of tumor growth. Rapid decline—that is, preceding tumor responses in tumor-bearing hosts—may make it easier to translate the approach in the clinical setting as a surrogate marker for antiangiogenic activity and optimal dosing for metronomic chemotherapy regimens. Encouraging in this regard is that our results were obtained only after a single week of treatment. However, other parameters should be taken into consideration with respect to the anticipated greater difficulty of reliably assessing CEPs in the clinical setting. The use of homogeneous (genetically identical) mice that underwent transplantation with xenograft tumors or syngeneic spontaneous hematologic malignancy are clearly easier to evaluate. The evaluation of CEPs in a heterogeneous population, such as humans, may be more complicated. Moreover, it is noteworthy that the overall levels of circulating endothelial cells also rapidly decline (within days) in patients with rectal cancer receiving an infusion of bevacizumab, the humanized anti-VEGF antibody,15 and in patients with multiple myeloma treated with thalidomide.22
Model for various mechanisms of metronomic chemotherapy. Metronomic chemotherapy may have different mechanisms for eradicating tumors. For example, it may directly affect tumor cells (brown and black ovals), it may cause direct endothelial cell death or growth inhibition (red ovals), or it may decrease the mobilization or viability of bone marrow–derived CEPs, which contribute to the tumor vasculature (pink ovals). Suppression in the levels of viable CEPs detected in PB may serve as a biomarker to define the OBD of chemotherapy drugs given in a metronomic chemotherapy regimen.
Model for various mechanisms of metronomic chemotherapy. Metronomic chemotherapy may have different mechanisms for eradicating tumors. For example, it may directly affect tumor cells (brown and black ovals), it may cause direct endothelial cell death or growth inhibition (red ovals), or it may decrease the mobilization or viability of bone marrow–derived CEPs, which contribute to the tumor vasculature (pink ovals). Suppression in the levels of viable CEPs detected in PB may serve as a biomarker to define the OBD of chemotherapy drugs given in a metronomic chemotherapy regimen.
Although the biologic effects of CEPs in hematologic malignancies are unclear, several investigators have reported that increases in the levels of CEPs are observed in various preclinical hematologic tumor models.6,14 The fact that metronomic chemotherapy treatment prolonged the survival of erythroleukemic mice suggests the efficacy of this treatment strategy not only for solid tumors but also for hematologic malignancies, at least in part through the inhibition of CEP levels. Patients with refractory non-Hodgkin lymphoma indeed showed a decline in CEP levels after receiving low-dose daily metronomic cyclophosphamide and a cyclooxygenase-2 (COX-2) inhibitor, celecoxib.23 These observations strengthen the possibility of successfully translating this approach when testing metronomic chemotherapy protocols for hematologic malignancies.3
Prepublished online as Blood First Edition Paper, July 5, 2005; DOI 10.1182/blood-2005-04-1422.
Supported by grants from the National Cancer Institute of Canada (NCIC), the Canadian Institute of Health Research (CIHR), and the National Institutes of Health (CA-41233) (R.S.K.); the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Istituto Superiore di Sanita (ISS), and the Sixth European Union Framework Programme (Integrated Project “Angiotargeting”; contract no. 504743) in the area of Life sciences, genomics, and biotechnology for health (F.B.); CIHR and the Ontario Cancer Research Network (OCRN) (Y.B.D.); and the Swiss National Science Foundation and the Swiss Cancer League/Oncosuisse (BIL SKL 1237–02-2002) (U.E.). R.S.K. holds a Canada Research Chair in Molecular Medicine. Y.S. is a recipient of a postdoctoral fellowship award from the CIHR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Cassandra Cheng for her excellent secretarial assistance and Dr. Liat Korn for assistance with the statistical analysis tests.