Abstract
Regulatory T cells (Tregs) play a fundamental role in the suppression of different immune responses; however, compartments at which they exert suppressive functions in vivo are unknown. Although many groups have described the presence of Tregs within inflammatory sites, it has not been shown that inflamed tissues are, indeed, the sites of active suppression of ongoing immune reactions. Here, by using αE+ effector/memory-like Tregs from fucosyltransferase VII-deficient animals, which lack E/P-selectin ligands and fail to migrate into inflamed sites, we analyzed the functional importance of appropriate Treg localization for in vivo suppressive capacity in an inflammation model. Lack of suppression by Tregs deficient in E/P-selectin ligands demonstrates that immigration into inflamed sites is a prerequisite for the resolution of inflammatory reactions in vivo because these selectin ligands merely regulate entry into inflamed tissues. In contrast, control of proliferation of naive CD4+ T cells during the induction phase of the immune response is more efficiently exerted by the naive-like αE–CD25+ Treg subset preferentially recirculating through lymph nodes when compared with its inflammation-seeking counterpart. Together, these findings provide the first conclusive evidence that appropriate localization is crucial for in vivo activity of Tregs and might have significant implications for anti-inflammatory therapies targeting recruitment mechanisms.
Introduction
CD25+CD4+ regulatory T cells (Tregs) fulfill a central role in the maintenance of immunologic homeostasis and self-tolerance (for a review, see Sakaguchi1 ). Initial in vitro characterization of these cells revealed an anergic phenotype2,3 ; however, more recent in vivo studies considered their dynamic nature,4-7 indicating distinct in vitro and in vivo features of Tregs.8
Tregs have been detected in lymphoid sites including thymus, lymph nodes, spleen, and peripheral blood.1 Because numerous studies have demonstrated a capacity of Tregs to prevent the induction of immune responses9-11 and because suppression requires direct cell-to-cell contact with T cells or antigen-presenting cells (APCs),12,13 it is conceivable that Tregs act as central regulators within lymphoid tissues.
On the other side, Tregs were also detected in peripheral tissues and at sites of ongoing immune responses such as synovial fluid from rheumatoid arthritis patients,14 tumors,15,16 transplants,17,18 skin lesions in mice infected with Leishmania major,19 lungs from mice infected with Pneumocystis carinii,20 islets of Langerhans in diabetes models,21-23 or inflamed intestine in induced colitis.24 Moreover, a few studies demonstrated the capacity of Tregs to down-regulate established immune reactions,24-27 suggesting that regulation might also act locally in the inflammatory environment.
The resulting questions—in which compartment suppression by Tregs occurs and how critical is an appropriate localization of Tregs for their in vivo suppressive capacity—have not been addressed directly, as yet.
We have previously shown that by use of the marker αE (CD103) the Treg compartment can be subdivided into a lineage or differentiation stage of natural, naive-like αE–CD25+ Tregs and into that of αE+ Tregs (either CD25+ or CD25–) with the phenotype of effector/memory cells.28,29 Whereas αE–CD25+ Tregs express high levels of CD62L and CCR7 and recirculate through lymph nodes, effector/memory-like αE+ Tregs express high levels of inflammatory chemokine receptors and multiple adhesion molecules as well as selectin ligands and efficiently migrate into inflamed sites. Importantly, the integrin αEβ7 itself only seems to play a role for the retention within peripheral tissues,30,31 but is not involved in the extravasation process of CD4+ T lymphocytes.32 In the present study it rather serves as a surrogate marker to identify inflammation-seeking Tregs.
Migration of CD4+ effector T cells into inflamed skin has been previously reported to be critically dependent on the expression of selectin ligands,33,34 which bind to the partially redundantly acting E- and P-selectins, expressed almost exclusively on endothelium of inflamed tissues. Biosynthesis of functional selectin ligands is mediated by several glycosyltransferases.35 Among these enzymes, fucosyltransferase VII (FucTVII) has been shown to catalyze an essential step in the generation of selectin ligands in T cells.36,37 FucTVII-deficient T cells lack selectin ligand activity38 and display impaired migration into the skin.39
We here show that, analogous to effector cells, αE+ Tregs from FucTVII-deficient mice are unable to enter inflamed sites. This allowed us to analyze whether the capacity to migrate into the inflamed skin is a prerequisite for their suppressive function in vivo. Strikingly, only αE+ Tregs from wild-type (WT) mice, which are able to migrate into the inflamed skin, but neither the corresponding subset from FucTVII–/– mice nor naive-like αE–CD25+ Tregs efficiently suppressed the Th1-mediated delayedtype hypersensitivity (DTH) response. However, using the same model, naive-like αE–CD25+ Tregs turned out to be the most efficient subset preventing initial activation of T cells and their differentiation into effector cells within antigen-draining lymph nodes.
Materials and methods
Mice
BALB/c and DO11.10 mice were bred at the Bundesinstitut für Risikobewertung (BfR; Berlin, Germany) or purchased from Charles River (Sulzfeld, Germany) and used at 6 to 12 weeks of age. DO11.10 × FucTVII–/– mice were generated by backcrossing DO11.10 mice to FucTVII-deficient mice.38 All animal experiments were performed under specific pathogen-free conditions and in accordance with institutional, state, and federal guidelines.
Antibodies, staining, and sorting reagents
The following antibodies were produced in our laboratory: Anti-CD8 (Tib105), anti-CD11b (M1/70), anti–FcR II/III (2.4G2), anti-CD25 (PC6.1), anti-CD3 (145.2C11), anti-CD28 (37.51), anti–interleukin 4 (IL-4; 11B11), fluorescein isothiocyanate (FITC)–and cyanin 5 (Cy5)–labeled anti-CD4 (GK1.5), Cy5-labeled anti–ovalbumin (OVA) T-cell receptor (TCR; KJ1.26), FITC-labeled anti-CD4-F(ab) (GK1.5), biotinylated anti-αE (M290), and biotinylated anti–αE-F(ab)2 (M290). The recombinant P-selectin–human IgG fusion protein was kindly provided by D. Vestweber (Muenster, Germany). Phycoerythrin (PE)–labeled anti–human IgG antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). The following antibodies and secondary reagents were purchased from BD PharMingen (Heidelberg, Germany): anti-CD4 (RM4-5), anti-CD25 (7D4), anti-CD25 (PC6.1), anti-CD62L (Mel-14), anti-CD29 (Ha2/5), anti-CD11a (2D7), streptavidin, and appropriate isotype controls. The PE anti–mouse Foxp3 staining set was purchased from eBioscience (San Diego, CA). All microbeads were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany).
Th1-mediated DTH model
Th1 cells were generated in vitro from DO11.10 mice as described previously.33 Briefly, CD4+ lymph node T cells were enriched by depletion of B cells, CD8+ T cells, macrophages, and CD25+ cells (clones: 2.4G2, Tib105, M1/70, PC6.1). Naive CD4+CD62Lhigh T cells were sorted using anti-CD62L microbeads and the AutoMACS magnetic separation system (Miltenyi Biotec). Sorted T cells were stimulated for 5 days with CD90-depleted, irradiated spleen cells (30 Gy) together with 5 μg/mL OVA323-339 peptide (synthesized at the Department of Biochemistry, Humboldt-University, Berlin, Germany), anti–IL-4 (5 μg/mL), interferon γ (IFN-γ; 20 ng/mL; R&D Systems, Wiesbaden, Germany), and IL-12 (5 ng/mL; R&D Systems). Cell culture was done with RPMI 1640 (Gibco, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS; Sigma, Taufkirch, Germany).
Th1 cells (5 × 105) were injected intravenously into naive BALB/c mice, and 24 hours later the DTH response was induced by subcutaneous injection of 250 ng OVA323-339 peptide together with incomplete Freund adjuvant (IFA; Sigma) into the left footpad. As a control the right footpad was injected with phosphate-buffered saline (PBS)/IFA. The inflammatory reaction was measured using an Oditest micrometer gauge in a blinded fashion (Kröplin Längenmesstechnik, Schlüchtern, Germany). For histologic evaluation, OVA-injected feet were fixed, decalcified in EDTA (ethylenediaminetetraacetic acid), and embedded in paraffin. Sections were stained with hematoxylin-eosin.
Sorting of Treg subsets for adoptive transfer and homing experiments
For all in vivo experiments, Treg subsets were isolated from lymphoid organs of DO11.10 or DO11.10 × FucTVII–/– mice using an isolation method with a combination of negative selection and preferential use of Fab fragments; CD4+ T cells were enriched by panning using anti-CD11b plus anti-CD8 and anti-Ig–coated plates (Dako, Hamburg, Germany). The subsets were separated after staining with FITC-labeled anti–CD4-F(ab), biotinylated αE-F(ab)2, PE-labeled streptavidin, and allophycocyanin-labeled anti-CD25 by fluorescence-activated cell sorting (FACS; DIVA, BD Biosciences, Heidelberg, Germany; and MoFlow, DakoCytomation, Hamburg, Germany). Control experiments demonstrated that the sorting procedure or the types of antibodies chosen for staining did not significantly influence the migration behavior of marker positive cells (K.S. and J.H., unpublished data, October 2002). Purity of the sorted subsets was generally 97% to 99% for αE–CD25–, 92% to 94% for αE–CD25+, and 87% to 92% for total αE+ cells. In some experiments sorted CD4+ T-cell subsets were preactivated for 18 hours with 3 μg/mL plate-bound anti-CD3 plus 10 μg/mL soluble anti-CD28 and 40 ng/mL recombinant murine IL-2 (R&D Systems) at 1 × 106 cells/mL. Cells were cultured as described (see “Th1-mediated DTH model”).
In vivo suppression of Th1 cells
Cultured Th1 cells (5-10 × 105) were injected intravenously together with preactivated Treg subsets isolated from DO11.10 or DO11.10 × FucTVII–/– mice into BALB/c mice. The ratio of Th1 cells to Tregs varied between 7:1 to 1:1 in different experiments. Twenty-four hours later the DTH response was induced and monitored as described (see “Th1-mediated DTH model”).
Flow cytometry
Cytometric analysis was performed as previously described29 using a FACSCalibur or an LSR (BD Biosciences) and the CellQuest software (BD Biosciences). Dead cells were excluded by propidium iodide (PI) or DAPI (4,6-diamido-2-phenylindole) staining (Sigma). Intracellular Foxp3 staining was performed with the PE anti–mouse Foxp3 staining set (eBioscience) according to the manufacturer's instructions.
Homing of adoptively transferred Treg subsets
Preactivated cells were labeled with 111In (Indiumoxin; Amersham Buchler, Freiburg, Germany; 1 × 108 cells/mL; 10 μCi/mL [0.37 MBq]) for 20 minutes at room temperature, followed by 1 hour of incubation at 37°C in fresh medium and removal of dead cells on Nycodenz (17.1% isotonic Nycodenz; Nyegaard, Oslo, Norway). The labeling of the Tregs did not change their capacity to suppress naive T-cell proliferation in vitro (K.S. and J.H., unpublished data, June 2005). Labeled cells were injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced using Th1 cells as described (see “Th1-mediated DTH model”). In all experiments a minimum of 104 cpm was injected. Mice were humanely killed 24 hours after transfer of labeled cells and the distribution of radioactivity in different organs, serum, and the remaining body was measured in a Wallac γ counter (Wallac, Turku, Finland).
Homing of Th1 cells
Th1 cells were labeled with 51Cr-chromate (Amersham Buchler) for 1 hour at 37°C (2 × 107 cells/mL; 20 μCi/mL [0.74 MBq]), followed by 1 hour of incubation at 37°C in fresh medium and removal of dead cells on Nycodenz (17.1% isotonic Nycodenz; Nyegaard). Labeled cells were injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced using nonlabeled Th1 cells as described (see “Th1-mediated DTH model”). In all experiments a minimum of 104 cpm was injected. At 24 hours after transfer of labeled cells mice were humanely killed and the distribution of radioactivity in different organs, serum, and the remaining body was measured in a Wallac γ counter.
In vivo suppression of naive CD4+ T-cell proliferation
A total of 5 × 105 ex vivo isolated, non-preactivated DO11.10 Treg subsets were adoptively transferred into BALB/c recipients, which were subcutaneously immunized with 25 μg OVA protein in IFA. Naive CD4+ T cells were isolated from lymphoid organs of DO11.10 mice. Briefly, single-cell suspensions were depleted of αE+ and CD25+ cells by magnetic-activated cell sorting (MACS; Miltenyi Biotec). CD4+ T cells were positively selected using FITC-labeled anti-CD4 and anti-FITC–multisort microbeads and the AutoMACS magnetic separation system (Miltenyi Biotec). After the beads were released according to the manufacturer's instructions, naive CD4+ T cells were sorted using anti-CD62L microbeads (Miltenyi Biotec). Sorted αE–CD25–CD62LhighCD4+ T cells were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Leiden, The Netherlands) as previously described.28 CFSE-labeled naive T cells (5 × 105) were adoptively transferred 2 days after mice had received sorted Treg subsets.
Three days after transfer of CFSE-labeled naive T cells, single-cell suspensions of the popliteal lymph node were prepared and stained for OVA-specific CD4+ T cells using anti-CD4 and the clonotypic antibody KJ1.26. Stained cells were analyzed by FACS as described (see “Flow cytometry”). Proliferation analysis is based on the CFSE geometric mean of total CFSE+CD4+KJ1.26+ T cells.
Sorting of Treg subsets for in vitro proliferation assay
T-cell subsets were isolated from lymphoid organs of DO11.10 or DO11.10 × FucTVII–/– mice. Erythrocyte-depleted cell suspensions were stained with anti-CD4–FITC, anti-CD25–allophycocyanin, and biotinylated anti-αE followed by PE-labeled streptavidin. Labeled cells were isolated using anti-PE and anti-allophycocyanin microbeads and the AutoMACS magnetic separation system (Miltenyi Biotec). Subsequently, αE–CD25+ and αE+CD4+ T-cell subsets were separated by FACS (DIVA; BD Biosciences). All sorted subsets were 96% to 98% pure on reanalysis.
In vitro suppression of naive CD4+ T-cell proliferation
Naive αE–CD25–CD62LhighCD4+ responder T cells were obtained and CFSE-labeled as described. APCs were prepared by depletion of CD90+ cells from BALB/c spleen cells using anti-CD90 microbeads and were irradiated (30 Gy) prior to culture. Unlabeled sorted Treg subsets were mixed with CFSE-labeled responder cells at a 1:1 ratio. T cells were cultured with APCs at a 1:2 ratio in round-bottom microtiter plates with addition of anti-CD3 (1 μg/mL) for 72 hours in triplicate. Cell culture was done as described. After incubation, cells were collected, stained for CD4, and analyzed by FACS. To exclude dead cells, propidium iodide (Sigma) was added. Proliferation analysis is based on the CFSE geometric mean of total CFSE+CD4+ T cells.
Statistics
Data are presented as mean plus or minus SD. Significance was determined by repeated measure analyses (footpad thickness), the Mann-Whitney test (homing), and the 2-tailed unpaired Student t test (CFSE; normal distribution was tested by the Kolmogorov-Smirnov test). Differences were considered statistically significant at a P value of .05 and highly significant at a P value of .01. In case of comparison of multiple samples, overall significance was tested in advance with the Kruskall-Wallis test.
Results
αE+ Tregs suppress Th1-mediated skin inflammation
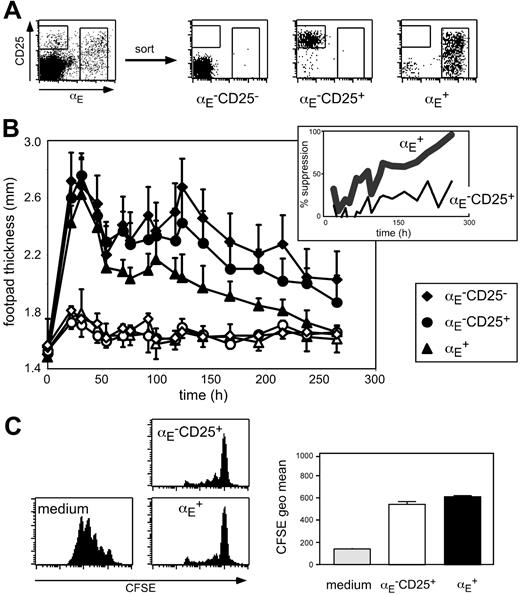
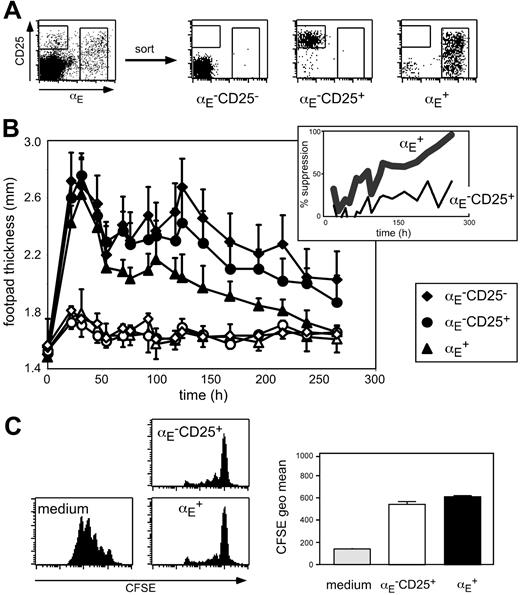
To investigate the suppressive capacity of Treg subsets (Figure 1A) with different in vivo migration properties, we have established an antigen-specific DTH model that is based on adoptive transfer of 5 × 105 in vitro generated OVA-specific Th1 cells from TCR-transgenic DO11.10 mice followed by antigen injection in IFA into the footpad. The oil emulsion was chosen to keep the antigen localized at the injection site. Typically, 1 day after subcutaneous immunization, footpad swelling reached its maximum followed by a gradual decline (Figure 1B). Histologic analysis of footpad sections revealed that during acute inflammation (24 hours after antigen injection) the cellular infiltrate was dominated by granulocytes, whereas at later time points predominantly lymphocytes could be detected in the antigen-injected footpad (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Next, we coadoptively transferred in vitro preactivated Treg subsets isolated from DO11.10 mice together with the inflammation-driving Th1 cells. Interestingly, only αE+ Tregs, but not αE–CD25+ cells significantly suppressed the Th1-induced footpad swelling (Figure 1B; Table S1; αE+: P < .001, αE–CD25+: P = .124, compared to control cells). No suppressive effect was observable during the acute phase. However, 50% reduction of the footpad swelling was reached at day 4 and the suppression increased up to more than 90% in the late phase of inflammation, suggesting an important role of Tregs especially in the resolution of immune reactions. In mice receiving αE–CD25– control cells, the course of footpad swelling was similar to PBS controls (Figure 1B; Table S1; K.S. and M.F., unpublished data, October 2003).
The αE+ Tregs efficiently suppress committed Th1 cells in vivo. (A) FACS analysis of pooled spleen and lymph node CD4+ T cells before and after subset sorting. (B) In vitro generated Th1 cells (5 × 105) were injected intravenously together with 5 × 105 FACS-sorted and preactivated T cells into naive BALB/c mice. Twenty-four hours later the DTH response was induced by OVA peptide injection into the footpad. Shown is progression of the inflammatory response monitored by the thickness of footpads injected with OVA/IFA (♦, •, ▴) and control footpads injected with PBS/IFA (♦,○, ▵; mean ± SD; n = 6). The αE+ Tregs showed a significantly higher suppressive capacity than αE–CD25+ cells (P < .01, repeated measure analyses). The insert shows the same data set expressed as percent suppression by indicated Treg subsets, in relation to the footpad swelling (OVA/IFA-injected footpad minus PBS/IFA-injected footpad) of mice adoptively transferred with αE–CD25– control cells. (C) In vitro suppressive capacity of indicated T-cell subsets on naive T-cell proliferation was determined after 72 hours of coculture with CFSE-labeled responder cells at a 1:1 ratio. Culture of CFSE-labeled responder cells alone (medium) served as control. Representative histogram plots of CFSE profiles from CFSE+CD4+ T cells are shown. Quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments).
The αE+ Tregs efficiently suppress committed Th1 cells in vivo. (A) FACS analysis of pooled spleen and lymph node CD4+ T cells before and after subset sorting. (B) In vitro generated Th1 cells (5 × 105) were injected intravenously together with 5 × 105 FACS-sorted and preactivated T cells into naive BALB/c mice. Twenty-four hours later the DTH response was induced by OVA peptide injection into the footpad. Shown is progression of the inflammatory response monitored by the thickness of footpads injected with OVA/IFA (♦, •, ▴) and control footpads injected with PBS/IFA (♦,○, ▵; mean ± SD; n = 6). The αE+ Tregs showed a significantly higher suppressive capacity than αE–CD25+ cells (P < .01, repeated measure analyses). The insert shows the same data set expressed as percent suppression by indicated Treg subsets, in relation to the footpad swelling (OVA/IFA-injected footpad minus PBS/IFA-injected footpad) of mice adoptively transferred with αE–CD25– control cells. (C) In vitro suppressive capacity of indicated T-cell subsets on naive T-cell proliferation was determined after 72 hours of coculture with CFSE-labeled responder cells at a 1:1 ratio. Culture of CFSE-labeled responder cells alone (medium) served as control. Representative histogram plots of CFSE profiles from CFSE+CD4+ T cells are shown. Quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments).
Interestingly, suppression of the Th1-mediated inflammatory reaction was only achieved if in vitro preactivated antigen-specific Tregs isolated from DO11.10 mice were adoptively transferred. A requirement for preactivation has also been shown in other studies40-42 and might relate to the slower activation kinetics observed for Tregs (see “αE-CD25+ Tregs are specialized to control expansion of naive CD4+ T cells in antigen-draining lymph nodes”). Tregs isolated from BALB/c mice had no suppressive effect (unpublished data), indicating that in this Th1-mediated DTH model further in vivo triggering of the antigen-specific TCR is required to maintain full suppressive capacity. However, we could not observe any significant difference in the expression of the clonotypic OVA-specific TCR (KJ1.26) between αE–CD25+ and αE+ Treg subsets isolated from DO11.10 mice (Figure S2), which was lower than in naive αE–CD25– control cells due to the preferential expression of endogenous TCR-α chains in Tregs from TCR-transgenic mice.43 Furthermore, no major differences between αE–CD25+ and αE+ cells were found with regard to Foxp3 expression as well as in vitro suppressive capacity (Figure 1C and K.S., unpublished data, November 2004). Together, these findings strongly suggest a role of other properties, such as homing, for the exclusive suppressive capacity of αE+ Tregs in the DTH model.
Preventing Treg migration into the inflamed site abrogates suppressive activity in vivo
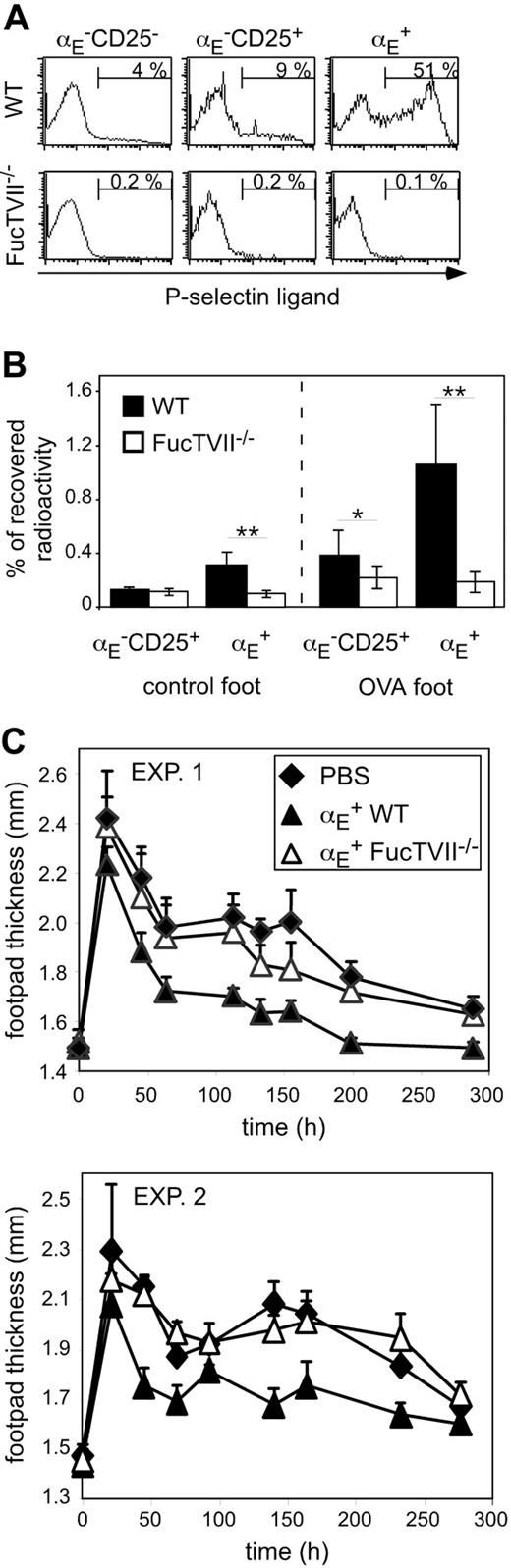
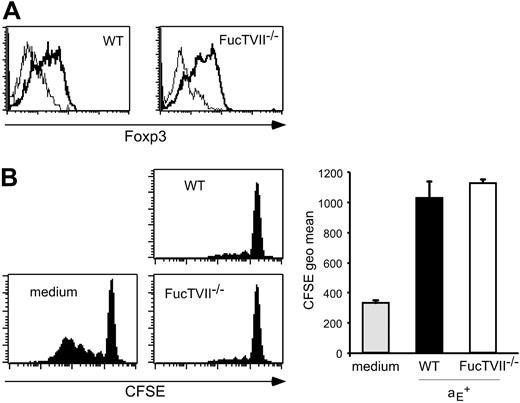
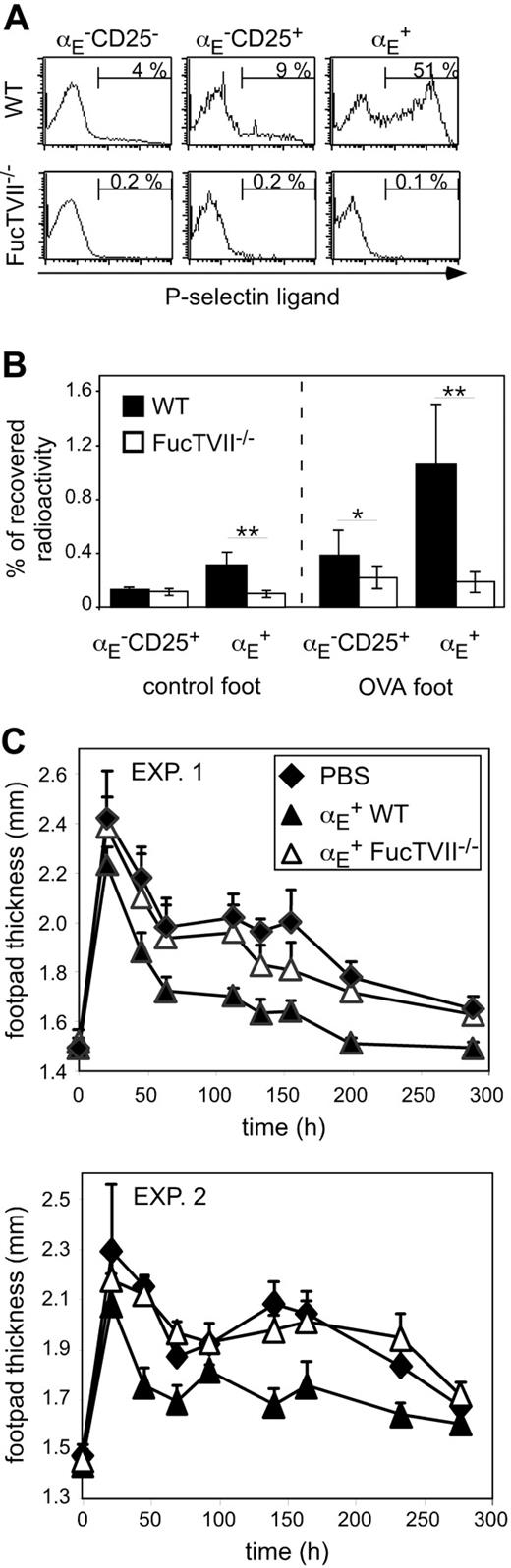
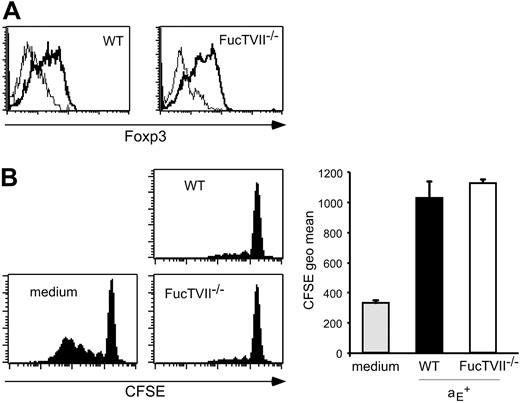
To clarify the role of migration for the in vivo suppressive capacity, we analyzed homing pattern as well as functional capacities of Treg subsets from DO11.10 × FucTVII–/– mice. FucTVII deficiency results in a lack of functional selectin ligands, which are required for the migration of effector T cells into inflamed skin.39 As expected, αE+ Tregs from FucTVII–/– mice lacked E- and P-selectin ligands, whereas roughly 50% of αE+ Tregs from WT mice expressed P-selectin ligands (Figure 2A and K.S., unpublished data, October 2003). FucTVII deficiency influenced neither the expression of other homing receptors such as CD62L, β1-integrin, and lymphocyte function-associated antigen 1 (LFA-1) nor expression of the clonotypic OVA-specific TCR (Figure S2). Furthermore, staining for intracellular Foxp3 revealed comparable expression levels in αE+ Tregs from WT and FucTVII–/– mice (Figure 3A). This corresponded to similar in vitro suppressive activity indicating that FucTVII deficiency did not result in a mere reduction of regulatory potential (Figure 3B).
On injection of in vitro preactivated radioactively labeled Treg subsets from WT and FucTVII–/– mice, only αE+ Tregs from WT mice could efficiently enter the inflamed footpad. The αE+ Tregs from FucTVII–/– mice showed only poor migration into the inflamed skin comparable with that of αE–CD25+ Tregs (Figure 2B). Preactivation, as previously shown,29 did not influence the general migration behavior of Treg subsets, in contrast to that of naive T cells. Thus, FucTVII is required to generate the unique inflammation-seeking potential of αE+ Tregs.
FucTVII–/ – αE+ Tregs cannot enter the inflamed footpad and show no suppression of the Th1-mediated DTH reaction. (A) Expression of P-selectin–binding ligands on indicated CD4+ T-cell subsets from DO11.10 (WT) and DO11.10×FucTVII–/–mice is shown. Representative histogram plots from 3 independently analyzed mice were selected. Numbers indicate frequency of P-selectin–binding cells. (B) In vitro preactivated Treg subsets from DO11.10 (WT) and DO11.10×FucTVII–/– mice were radioactively labeled with 111In and injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced, followed by the determination of radioactivity in the indicated organs after 24 hours using a γ counter. Percentage of total recovered radioactivity is shown (n = 12; mean ± SD; data pooled from 2 independent experiments; **P < .01). (C) In vitro generated Th1 cells (5 × 105) were injected intravenously together with 2.5 × 105 FACS-sorted and preactivated αE+ Tregs from DO11.10 (WT) and DO11.10 × FucTVII–/– mice. Twenty-four hours later the DTH response was induced. Shown is the progression of the inflammatory response monitored by the thickness of footpads injected with OVA/IFA (mean ± SD; n = 6). Two individual experiments are depicted. The αE+ Tregs from FucTVII–/– mice showed a significantly reduced suppressive capacity compared to αE+ Tregs from WT mice (P < .01, repeated measure analyses).
FucTVII–/ – αE+ Tregs cannot enter the inflamed footpad and show no suppression of the Th1-mediated DTH reaction. (A) Expression of P-selectin–binding ligands on indicated CD4+ T-cell subsets from DO11.10 (WT) and DO11.10×FucTVII–/–mice is shown. Representative histogram plots from 3 independently analyzed mice were selected. Numbers indicate frequency of P-selectin–binding cells. (B) In vitro preactivated Treg subsets from DO11.10 (WT) and DO11.10×FucTVII–/– mice were radioactively labeled with 111In and injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced, followed by the determination of radioactivity in the indicated organs after 24 hours using a γ counter. Percentage of total recovered radioactivity is shown (n = 12; mean ± SD; data pooled from 2 independent experiments; **P < .01). (C) In vitro generated Th1 cells (5 × 105) were injected intravenously together with 2.5 × 105 FACS-sorted and preactivated αE+ Tregs from DO11.10 (WT) and DO11.10 × FucTVII–/– mice. Twenty-four hours later the DTH response was induced. Shown is the progression of the inflammatory response monitored by the thickness of footpads injected with OVA/IFA (mean ± SD; n = 6). Two individual experiments are depicted. The αE+ Tregs from FucTVII–/– mice showed a significantly reduced suppressive capacity compared to αE+ Tregs from WT mice (P < .01, repeated measure analyses).
The αE+ Tregs from WT and FucTVII–/– mice show similar Foxp3 expression and in vitro suppressive capacity. (A) Intracellular expression of Foxp3 in αE+ Tregs from DO11.10 (WT) and DO11.10 × FucTVII–/– mice is shown. Representative histogram plots from 3 independently analyzed mice were selected. (B) In vitro suppressive capacity of αE+ Treg subsets from DO11.10 and DO11.10 × FucTVII–/– mice on naive T-cell proliferation was determined after 72 hours of coculture with CFSE-labeled responder cells at a 1:1 ratio. Culture of CFSE-labeled responder cells alone (medium) served as control. Representative histogram plots of CFSE profiles from CFSE+CD4+ T cells are shown. Quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments).
The αE+ Tregs from WT and FucTVII–/– mice show similar Foxp3 expression and in vitro suppressive capacity. (A) Intracellular expression of Foxp3 in αE+ Tregs from DO11.10 (WT) and DO11.10 × FucTVII–/– mice is shown. Representative histogram plots from 3 independently analyzed mice were selected. (B) In vitro suppressive capacity of αE+ Treg subsets from DO11.10 and DO11.10 × FucTVII–/– mice on naive T-cell proliferation was determined after 72 hours of coculture with CFSE-labeled responder cells at a 1:1 ratio. Culture of CFSE-labeled responder cells alone (medium) served as control. Representative histogram plots of CFSE profiles from CFSE+CD4+ T cells are shown. Quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments).
The functional importance of migratory competence was tested by comparing the suppressive capacity of in vitro preactivated αE+ Tregs from WT and FucTVII–/– mice in the DTH model. Strikingly, αE+ Tregs from FucTVII–/– mice, in contrast to their counterparts from WT mice, were not capable of significantly inhibiting the footpad swelling (Figure 2C). These findings demonstrate that immigration of αE+ Tregs into inflamed sites is essential for their in vivo suppressive activity.
αE–CD25+ Tregs are specialized to control expansion of naive CD4+ T cells in antigen-draining lymph nodes
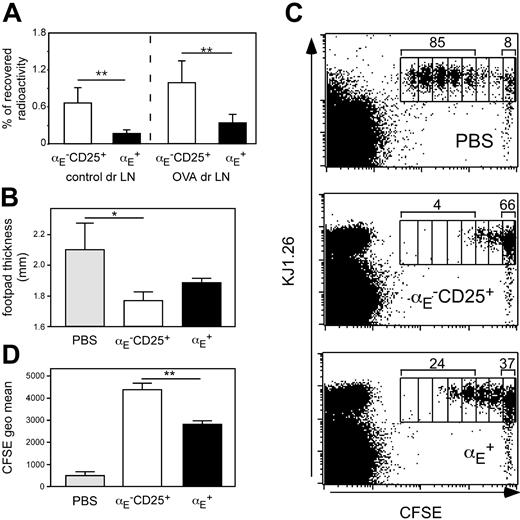
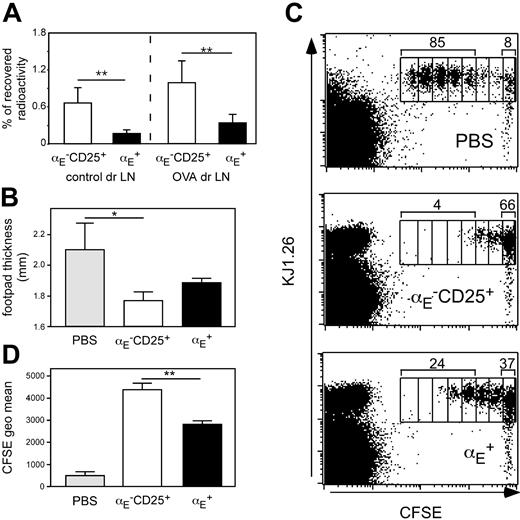
Priming of naive CD4+ T cells has been shown in numerous studies to occur in lymph node draining sites of inflammation.44,45 We previously have shown that αE–CD25+ Tregs, in contrast to αE+ cells, display a naive-like phenotype and recirculate through lymph nodes.29 Extending our previous findings we here show that αE–CD25+ Tregs also more efficiently enter antigen-draining reactive lymph nodes in the Th1-mediated footpad inflammation model as compared to αE+ Tregs (Figure 4A). Albeit ineffective in suppressing ongoing inflammations, it could be hypothesized that αE–CD25+ Tregs might inhibit the initial activation and expansion of naive CD4+ T cells occurring within this lymphoid compartment.
To test this, we modified the DTH model by using DO11.10-derived naive CD62LhighCD4+ responder T cells (depleted of αE+ and CD25+ Tregs) instead of predifferentiated Th1 cells. The degree of footpad swelling is somewhat lower under these conditions (Figure 4B). The naive responder cells were labeled with CFSE to monitor their proliferation in vivo. Cells (5 × 105) were adoptively transferred into BALB/c recipients, which had already received sorted Treg subsets (5 × 105) and OVA-footpad immunization 2 days before. Three days after transfer of responder cells, draining popliteal lymph nodes were removed, and the proliferation profile of CFSE-labeled T cells was analyzed by flow cytometry.
The majority of CFSE-labeled naive T cells had undergone 3 to 7 cell divisions if no Treg subsets had been adoptively transferred (Figure 4C). However, when αE–CD25+ Tregs were transferred proliferation of naive T cells was strongly suppressed because the majority of CFSE-labeled cells remained undivided and only few cells had undergone 3 or more cell divisions. The αE+ Tregs showed some suppressive activity but were clearly inferior to αE–CD25+ Tregs in suppressing T-cell proliferation because a marked number of CFSE-labeled cells had completed 3 or more cell divisions. Quantification of the CFSE intensity of total CFSE+CD4+KJ1.26+ T cells revealed a significantly higher capacity of αE–CD25+ Tregs to suppress naive T-cell proliferation in vivo compared to αE+ Tregs (Figure 4D; P < .01). The partial, although weak, in vivo inhibition of naive T-cell proliferation by αE+ Tregs can be explained by the presence of about 25% CD62Lhigh cells in this subset (Figure S2) corresponding to the low, but significant, entry into the draining lymph node (Figure 4A). The αE–CD25– cells injected as controls differentiated into effector cells and thereby modulated the DTH response and responder cell proliferation to some degree (Table S2).
Interestingly, suppression of naive T-cell proliferation was only observable in this system when the Tregs had contact with specific antigen 2 days in advance, whereas simultaneous adoptive transfer of Tregs and CFSE-labeled responder cells did not result in suppression of naive T-cell proliferation during the 3-day investigation period (unpublished data). A similar delay in the activity of Tregs has been described in other models4,42 fitting to a scenario in which Treg function is delayed to allow for an unimpeded acute reaction but limiting the chronic phase of the immune response.
The αE–CD25+ Tregs efficiently prevent naive T-cell proliferation in vivo. (A) In vitro preactivated Treg subsets from DO11.10 mice were radioactively labeled with 111In and injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced, followed by the determination of radioactivity in the control and the antigen-draining lymph node (dr LN) after 24 hours using a γ counter. Percentage of total recovered radioactivity is shown (n = 12; mean ± SD; data pooled from 2 independent experiments; **P < .01). (B-D) CFSE-labeled naive CD4+ T cells (5 × 105) derived from DO11.10 mice were adoptively transferred into BALB/c recipients, which 48 hours before had received 5 × 105 non-preactivated FACS-sorted Tregs followed by subcutaneous OVA immunization. Three days later the effect of the transferred Tregs on naive T-cell proliferation was assessed by measurement of footpad swelling and by FACS. (B) Measurement of footpad thickness shows the suppressive effect of indicated Treg subsets on the development of effector cells. Significance was determined by one-tailed unpaired Student t test (n = 3; mean ± SD; 1 representative of 2 independent experiments; *P < .05). (C) Representative FACS plots of gated CD4+ cells from antigen-draining popliteal lymph nodes show the effect of indicated Treg subsets on the proliferation of CFSE-labeled KJ1.26+ responder cells. Numbers indicate the frequency of CFSE+KJ1.26+ cells, which have undergone 3 or more cell divisions, and the frequency of CFSE+KJ1.26+ cells in the undivided fraction. Interestingly, during the 5-day in vivo period more than 95% of adoptively transferredαE–CD25+ Tregs (CFSE–KJ1.26+) did not acquire αE expression, indicating relatively stable phenotypes under these conditions. (D) The quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+KJ1.26+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments; **P < .01).
The αE–CD25+ Tregs efficiently prevent naive T-cell proliferation in vivo. (A) In vitro preactivated Treg subsets from DO11.10 mice were radioactively labeled with 111In and injected intravenously into BALB/c mice, in which 24 hours before a DTH response had been induced, followed by the determination of radioactivity in the control and the antigen-draining lymph node (dr LN) after 24 hours using a γ counter. Percentage of total recovered radioactivity is shown (n = 12; mean ± SD; data pooled from 2 independent experiments; **P < .01). (B-D) CFSE-labeled naive CD4+ T cells (5 × 105) derived from DO11.10 mice were adoptively transferred into BALB/c recipients, which 48 hours before had received 5 × 105 non-preactivated FACS-sorted Tregs followed by subcutaneous OVA immunization. Three days later the effect of the transferred Tregs on naive T-cell proliferation was assessed by measurement of footpad swelling and by FACS. (B) Measurement of footpad thickness shows the suppressive effect of indicated Treg subsets on the development of effector cells. Significance was determined by one-tailed unpaired Student t test (n = 3; mean ± SD; 1 representative of 2 independent experiments; *P < .05). (C) Representative FACS plots of gated CD4+ cells from antigen-draining popliteal lymph nodes show the effect of indicated Treg subsets on the proliferation of CFSE-labeled KJ1.26+ responder cells. Numbers indicate the frequency of CFSE+KJ1.26+ cells, which have undergone 3 or more cell divisions, and the frequency of CFSE+KJ1.26+ cells in the undivided fraction. Interestingly, during the 5-day in vivo period more than 95% of adoptively transferredαE–CD25+ Tregs (CFSE–KJ1.26+) did not acquire αE expression, indicating relatively stable phenotypes under these conditions. (D) The quantification of suppressive capacity is based on the CFSE geometric mean of total CFSE+CD4+KJ1.26+ T cells (n = 3; mean ± SD; 1 representative of 2 independent experiments; **P < .01).
The aforementioned suppression of naive T-cell proliferation by αE–CD25+ Tregs was directly related to a significantly reduced effector response (footpad swelling) at the site of immunization (Figure 4B; P < .05 compared to PBS control). This indicates that both inhibition of the effector phase by αE+ Tregs and inhibition of the induction phase by αE–CD25+ Tregs are complementary pathways by which these subsets of Tregs contribute to control of immune reactions. Under these conditions, when the DTH response develops from naive precursors, αE+ Tregs also led to a certain, albeit not significant, reduction of the footpad swelling (P = .078 compared to PBS control). It is not clear so far whether αE+ Tregs are acting in this setting by inhibiting the induction phase or whether they suppress the Th1-mediated inflammation within the footpad once effector cells have developed from naive precursors.
Because both αE–CD25+ and αE+ Tregs were able to inhibit the in vitro proliferation of naive T cells (Figure 1C), these findings suggest that the preferential migration of αE–CD25+ Tregs into the antigen-draining lymph node correlates with the more efficient suppression of naive T-cell proliferation at this site.
Discussion
Tregs have been found in both lymphoid organs and sites of ongoing immune responses (for reviews, see Sakaguchi1 and Huehn et al46 ). Despite increasing knowledge about the development and function of Tregs, conclusive data on the site of their immunoregulatory action are not available. Based on phenotypic properties, we previously have proposed that the appropriate localization of Tregs is of functional significance for their in vivo suppressive capacity, and that both, functional role and migratory properties, differ for distinct subsets of Tregs.29,46 Our current data now provide the first experimental evidence that migration of effector/memory-like Tregs into inflamed sites is indeed essential for their regulatory function in vivo and, furthermore, demonstrate that specialized Treg subsets fulfill divergent tasks by controlling different stages of immune reactions.
To address this issue, we here used a footpad DTH model based on adoptive transfer of predifferentiated DO11.10 TCR-transgenic Th1 cells together with transgenic Tregs. The αE+ Tregs, which represent a subset of Tregs phenotypically resembling effector/memory cells,29 more efficiently entered the inflamed paw as compared to naive-like αE–CD25+ Tregs, as shown previously for other models of inflammation.29 Among Tregs, only the subset of αE+ cells was able to suppress the Th1-mediated inflammation. We crossed DO11.10 animals with mice deficient in FucTVII, a key enzyme involved in the generation of ligands for endothelial selectins. FucTVII–/– effector T cells were unable to enter most inflamed sites, especially skin, as has been shown previously.39 This also applied to Tregs isolated from FucTVII–/– animals, which otherwise are indistinguishable from WT Tregs, providing direct evidence that the selectin pathway is of major importance for the migration of Tregs into the inflamed site.
Strikingly, the failure of FucTVII-deficient αE+ Tregs to migrate into sites of inflammation was connected with an almost complete lack of in vivo suppressive activity. No effect of FucTVII deficiency was observed on in vitro suppressive activity and, furthermore, αE–CD25+ Tregs from FucTVII–/– mice showed a comparable inhibition of naive T-cell proliferation in vivo (K.S. and J.H., unpublished data, July 2004) indicating that FucTVII deficiency per se did not result in a reduction of regulatory potential.
These findings provide unequivocal evidence that the immunoregulatory activity of αE+ effector/memory-like Tregs in ongoing immune reactions requires their trafficking into the inflamed tissue, although we cannot formally exclude that this Treg subset acts in the draining lymph node, accessed via the afferent lymph after passing the inflamed tissue. But, it is unlikely that this accounts for the lack of suppression by FucTVII-deficient Tregs, because many more naive-like αE–CD25+ Tregs, regardless whether WT or FucTVII-deficient, are recovered from the draining lymph nodes (Figure S3). However, no suppression of the Th1-mediated DTH response is observed with such naive-like Tregs.
Immigration of αE–CD25+ cells into inflamed sites is poor compared to αE+ Tregs. Having shown that expression of selectin ligands and trafficking into the inflamed tissue is crucial, it is obvious that the absence of naive-like Tregs from the inflamed tissues alone would be sufficient to preclude a major contribution to the control of an ongoing local inflammation. Furthermore, αE–CD25+ and αE+ Tregs displayed a similar in vitro suppressive activity, ruling out a mere difference in regulatory potential per se. Although the mechanism of in vivo suppression in general and of an ongoing immune reaction in particular is still unclear and we therefore cannot exclude further functional differences between naive-like and effector/memory-like Treg subsets besides homing behavior, our findings strongly suggest that Tregs have to interact directly with proinflammatory effector cells in the affected tissue.
Complementary evidence for the critical role of appropriate localization of Tregs for their in vivo suppressive capacity has recently been provided in a contact hypersensitivity model, where unseparated hapten-specific Tregs induced by ultraviolet radiation were only capable of inhibiting the induction phase of the skin inflammation, but not their effector phase.47 Suppressive activity was mediated by Tregs expressing high levels of L-selectin (CD62L), but lacking ligands for E- and P-selectin, suggesting that this population of Tregs only has access to lymph nodes, but not to inflamed tissues. Interestingly, when these Tregs were injected directly into the inflamed skin, suppression of the effector phase was obtained, suggesting that inappropriate localization within the inflamed tissue was the main obstacle for a suppression of the ongoing inflammation.47
Inhibition of naive T-cell proliferation by CD25+CD4+ Tregs has recently been shown to occur within the antigen-draining lymph node.5,10,48 We here demonstrate that naive-like αE–CD25+ Tregs are more potent suppressors of naive T-cell proliferation in vivo, coinciding with a more than 3-fold higher migration rate into this site as compared to αE+ Tregs. The high migration rate into the antigen-draining reactive lymph node was noticeable even after in vitro preactivation of αE–CD25+ Tregs corresponding to the exceptionally stable expression of CD62L following TCR triggering on Tregs.29
Interestingly, a high and exclusive in vivo suppressive capacity of CD62LhighCD25+CD4+ Tregs, which mainly correspond to αE–CD25+ Tregs, has recently been observed in diabetes models9,21,49 as well as in models of graft-versus-host disease,50 predicting that in these models immune control occurs primarily in lymph nodes and operates during the initiation phase of the immune reaction.
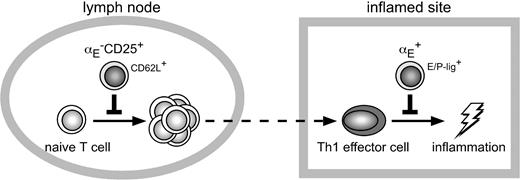
Compartmentalization of immune regulation by distinct migration behavior of αE–CD25+ and αE+ Tregs. High expression levels of CD62L enable both αE–CD25+ Tregs and naive T cells to efficiently enter the antigen-draining lymph node from the bloodstream. Due to preferential recirculation of αE–CD25+ Tregs through lymph nodes these naive-like Tregs most efficiently inhibit activation and expansion of naive T cells. In contrast, both Th1 effector cells and αE+ Tregs efficiently enter inflamed sites via binding of E/P-selectin ligands (E/P-Lig) to E/P-selectin being up-regulated on inflamed endothelial cells. Due to the preferential accumulation of inflammation-seeking αE+ Tregs the Th1-mediated inflammatory reaction is most efficiently controlled by these effector/memory-like Tregs.
Compartmentalization of immune regulation by distinct migration behavior of αE–CD25+ and αE+ Tregs. High expression levels of CD62L enable both αE–CD25+ Tregs and naive T cells to efficiently enter the antigen-draining lymph node from the bloodstream. Due to preferential recirculation of αE–CD25+ Tregs through lymph nodes these naive-like Tregs most efficiently inhibit activation and expansion of naive T cells. In contrast, both Th1 effector cells and αE+ Tregs efficiently enter inflamed sites via binding of E/P-selectin ligands (E/P-Lig) to E/P-selectin being up-regulated on inflamed endothelial cells. Due to the preferential accumulation of inflammation-seeking αE+ Tregs the Th1-mediated inflammatory reaction is most efficiently controlled by these effector/memory-like Tregs.
The findings of the present study provide further evidence for a functional specialization among subsets or differentiation stages of Tregs. The αE–CD25+ cells seem to represent the proposed “natural” Tregs51 involved in homeostasis of the immune system, and this population preferentially migrates into lymph nodes to control both activation and expansion of naive T cells and their differentiation into effector cells (Figure 5). In contrast, effector/memory-like αE+ Tregs appear to represent a second line of control primarily tasked when recirculating “natural” Tregs have failed or when immune reactions are going out of control and become chronic. This “fail-safe” system appears to act directly at the effector site and, therefore, inflammation-seeking Tregs are a prerequisite. For the first time, our findings demonstrate in one and the same model a clear division of labor between Treg subsets controlling distinct stages of CD4+ T cell–dependent immune responses, either the induction phase occurring in lymph nodes or the effector phase dominated by local processes.
Inhibition of migratory functions of effector cells is currently explored as anti-inflammatory therapy for a number of autoimmune diseases. Our observation that highly potent αE+ Tregs shared several adhesion molecules and chemokine receptors with pathogenic effector cells has important implications for the use of such molecules as targets for anti-inflammatory therapies as not only the infiltration of inflammation-driving effector cells, but also that of beneficial Tregs might be prevented. This issue was recently addressed in a model of collagen-induced arthritis, where blockade of CCR2 during the later phase of arthritis progression significantly aggravated clinical and histologic signs of arthritis.52 The authors postulated that this effect was most likely due to interference with the proper in vivo localization of CCR2+CD25+CD4+ Tregs, which seemed to be essential for the self-limitation of the inflammatory response. CCR2 expression on CD25+CD4+ Tregs strongly correlated with the expression of the integrin αEβ7,52 supporting the notion that this subset of effector/memory-like Tregs is important for the control of established inflammatory reactions. It has to be noticed that the issue of Treg localization might also be of relevance for the question, to what extent tumor rejection is prevented by the recruitment of Tregs into the tumor, for example, by tumor-produced chemokines.16
Our findings indicate that migratory capacities of Tregs and their subdivision into populations with different homing properties have to be considered when therapeutic concepts based on the adoptive transfer of Tregs are devised for clinical application or when trafficking mechanisms are considered as a therapeutic target of anti-inflammatory strategies.
Prepublished online as Blood First Edition Paper, July 12, 2005; DOI 10.1182/blood-2005-05-1864.
Supported by the Deutsche Forschungsgemeinschaft (SFB366, SFB421, SFB650, and Scho565/5-1), the Bundesministerium fuer Bildung und Forschung (BMBF) (Nationales Genomforschungsnetzwerk [NGFN] II), and the National Institutes of Health (1P01CA71932 and GM 62116).
U.S. and J.H. are joint senior authors. K.S. and M.F. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank T. Kaiser and K. Raba for FACS sorting, K. Eulenburg and A. Schrage for help in establishing the DTH model, and D. Huscher for statistical analysis.