Abstract
In response to injury, monocytes migrate to the site of inflammation, where they differentiate into macrophages and participate in various biologic processes. However, their fate during the resolution of acute inflammation is not fully understood. Here, we show that inflammatory macrophages do not die locally by apoptosis; rather, they migrate across the peritoneal mesothelium to the lymphatics, through which they further migrate to the lymph nodes and to the blood circulation. Macrophage efflux is enhanced considerably on cell activation, and such accelerated macrophage migration is dependent specifically on integrin Mac-1, and can be blocked by addition of its antagonist. Thus, genetic inactivation of Mac-1 in mice inhibits the accelerated macrophage efflux from the inflammatory site to the lymphatics, but it does not compromise the accumulation of blood monocytes into the inflammatory site. Together, our study demonstrates that Mac-1 is involved specifically in the efflux of activated macrophages to the lymphatics, suggesting that Mac-1 may play an important role in the removal of local inflammatory macrophages and in their subsequent migration to the lymph nodes, a process that is critical to the development of the adaptive immunity.
Introduction
In response to acute inflammation, blood leukocytes roll on the endothelium via their selectins. Subsequent engagement of the integrins allows leukocytes to adhere and then migrate across the endothelial barrier into the site of injury, where they participate in innate immune responses, including phagocytosis of pathogens, production of cytotoxic enzymes and free oxygen radicals, and secretion of cytokines and growth factors.1 Function-blocking studies have identified the β1 (CD29) and β2 (CD18) integrins as the major players involved in leukocyte adhesion and migration,2-4 which are further confirmed, in the case of lymphocyte function-associated antigen-1 (LFA-1; a member of the β2 integrin subfamily along with Mac-1, CD11c/CD18, and CD11D/CD18), by genetic studies using LFA-1-/- mice.5-7 In contrast, although function-blocking studies strongly demonstrate an important role of Mac-1 (αMβ2, CD11b/CD18, and CR3) in neutrophil/monocyte adhesion and migration both in vitro and in vivo,3,8 targeted inactivation of Mac-1 in mice does not compromise neutrophil accumulation within the peritoneum of Mac-1-/- mice.9,10 Most surprisingly, deficiency of Mac-1 leads to dramatically enhanced accumulation of the peritoneal neutrophils, which in part is attributed to the defective apoptosis of the infiltrated neutrophils in the absence of Mac-1.10 Whether Mac-1 deficiency has similar effects on monocyte migration and apoptosis is currently unknown.
During the resolution phase of an acute inflammation, the apoptotic neutrophils are removed from the local inflammatory site via phagocytosis by the reticuloendothelial system.11 Although a similar reduction of inflammatory macrophages within the peritoneum occurs at the end of inflammation,12 the fate of the infiltrated monocytes/macrophages is not yet fully understood. Bellingan et al13 reported that the infiltrated monocytes/macrophages do not die locally; rather, they spontaneously migrate into the draining lymph nodes within a period of 4 days. Function-blocking studies suggest that such spontaneous macrophage migration to the lymph nodes depends primarily on the integrin β1.14 As genetic inactivation of the β1 integrin is embryonically lethal,15 its role in macrophage migration to the lymph nodes could not be independently verified using its corresponding deficient mice. Furthermore, as macrophages do undergo apoptosis under certain circumstances,16 the fate of the infiltrated monocytes remains uncertain.
In this work, we examined monocyte/macrophage migration both in vitro and in vivo using several different approaches. Our results demonstrate that, unlike neutrophils, the infiltrated monocytes/macrophages did not undergo apoptosis within the peritoneum; instead, they migrated across the peritoneal mesothelium into the lymphatics, which agrees well with the previous report.13 Furthermore, we found that a portion of the inflammatory macrophages that migrated into the lymphatics took residence within the lymph nodes, and the rest of them moved with the lymph flow back into the blood circulation. Most importantly, we found that genetic inactivation of Mac-1 does not comprise the early monocyte accumulation or its subsequent reduction within the peritoneum. Rather, Mac-1 is specifically required for the accelerated migration of the activated macrophages from the peritoneum to the lymphatics, suggesting that Mac-1 may play an important role during the resolution of acute inflammation. As failure of the inflammatory macrophages to egress from the site of injury could potentially lead to many chronic inflammatory diseases, for example, atherosclerosis,17 the information provided in this study may help us better understand the pathogenesis of these common diseases.
Materials and methods
Materials
All study protocols were approved by the Institutional Animal Care and Use Committee of the American Red Cross Holland Laboratory. Fluorescein isothiocyanate (FITC) conjugates of antibodies were purchased commercially: monoclonal antibody (mAb) F4/80 was from Serotec (Raleigh, NC) and mAb M1/70 for Mac-1 was from BD/Pharmingen (San Diego, CA). Human fibrin(ogen) (Fg) was from the Enzyme Research Labs (South Bend, IN). Other reagents were purchased from Sigma (St Louis, MO) at the highest purity unless otherwise noted.
Mice
The wild-type (WT) C57BL6 mice from 8 to 13 weeks old were purchased from NCI (Frederick, MD). Mac-1-/- mice were kindly provided by Dr Ballantyne, Baylor College of Medicine, and have been backcrossed to the C57BL6 background for more than 7 generations. The green fluorescent protein (GFP) transgenic mice were from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in a pathogen-free facility, and all procedures were performed in accordance with Institutional Animal Care and Use Committee approval.
Macrophage migration to the lymph nodes
WT and Mac-1-/- mice were injected intraperitoneally with 1 mL 5% sterile thioglycollate (TG) broth. Four days later, mature macrophages were stimulated by intraperitoneal injection of 200 μL of 5 μg/mL lipopolysaccharide (LPS). In certain experiments, 200 μL mAbs (0.5 mg/mL), neutrophil inhibitory factor (NIF; 100 nM), or ethylenediaminetetraacetic acid (EDTA; 5 mM) were injected prior to LPS injections. Four hours later, the cell number in the peritoneal lavage was determined, after lysis of red blood cells, using a Coulter counter, and the percentages of macrophages were assessed by fluorescence-activated cell sorting (FACS) analysis with mAb F4/80, and by morphologic examination of Hema3 (Fisher Scientific, Suwannee, GA)-stained Cytospin (Shandon, Pittsburgh, PA) smears.
Macrophage migration on Fg
Peritoneal macrophages (0.5 × 106) in 100 μL Dulbecco modified Eagle medium (DMEM), stimulated with 10 ng/mL LPS, in the presence or absence of anti-Mac-1 mAb M1/70 (50 μg/mL), control rat immunoglobulin G (IgG; 50 μg/mL), or EDTA (5 mM) were added onto the upper chambers of a 24-well transwell plate (5-μm pore; Costar, Acton MA) and 500 μL 5% fetal bovine serum (FBS)/DMEM was placed in the lower chambers. The inserts were precoated on both sides with Fg (20 μg/mL) and then blocked with bovine serum albumin. After 4 hours at 37°C, the number of migrated cells was determined by hemocytometer, and cell differential was done by Cytospin as described in “Macrophage migration to the lymph nodes.”
Macrophage apoptosis
Total peritoneal macrophages, obtained as above from either phosphate-buffered saline (PBS)- or LPS-treated mice were either analyzed immediately for apoptosis (see below), or were further cultured in 6-well plates at 37°C and 5% CO2 for 4 and 20 hours in either DMEM/10% FBS alone or with the addition of 100 ng/mL LPS or 100 ng/mL LPS plus 10 μM SB202190. To determine apoptosis by DNA fragmentation, total genomic DNA was prepared with the genomic DNA preparation kit from Roche (Indianapolis, IN) and separated on 1.5% agarose gel. To determine apoptosis by dual-color FACS analysis, total peritoneal cells were stained with phycoerythrin (PE)-annexin V (for apoptosis) and 7-aminoactinomycin D (7-AAD; for necrosis), using the Apoptosis Detection Kit from BD/Pharmingen, according the manufacturer's instructions.
Passive macrophage transfer
GFP+ macrophages were obtained from the TG-treated (4 days) GFP-transgenic mice by peritoneal lavage, verified by FACS analysis using mAb F4/80 and by morphologic examination, and then injected intraperitoneally into WT mice (5 × 106 GFP+ cells per mouse) that have been preconditioned by intraperitoneal injection of TG for 4 days. In certain experiments, 200 μL mAbs (0.5 mg/mL) or NIF (100 nM) were coinjected with the cells. For passive transfer of the Mac-1-/- macrophages or a mixture of WT and Mac-1-/- cells, WT or Mac-1-/- mice were injected intraperitoneally with 5% TG for 4 days, and then peritoneal macrophages were labeled with a red (PKH26) or green (PKH67) fluorescence dye (both are from Sigma), based on the products' instruction. The labeled peritoneal cells were washed and then injected intraperitoneally into the TG-preconditioned Mac-1-/- mice as described above. After 30 minutes, mice were further stimulated by intraperitoneal injections of 500 μL PBS or LPS (1 μg). At different time points (4 minutes to 4 hours), 100 μL total blood was collected by retro-orbital bleeding, and the peritoneal cells were retrieved by lavage. In addition, the peritoneal membranes were collected and fixed in 4% paraformaldehyde, and the draining lymph nodes were collected, part of which were processed for frozen sectioning, and the rest were digested with 1 mg/mL Collagenase D to yield single-cell suspension. The number of GFP+ cells were determined manually under a fluorescence microscope, using 4 randomly picked fields (at 100 × magnification) for the frozen sections (5 μm thick), and with a hemocytometer for the single-cell suspensions. Fluorescent images were obtained using a Nikon Eclipse TE2000U (Nikon, Melville, NY) system equipped with a Nikon Plan Fluor 10 × objective lens (numeric aperture 0.30) and a Nikon DXM1200F digital camera. The MetaMorph imaging system (Molecular Devices, Downingtown, PA) was used for image acquisition, and Adobe Photoshop (Adobe Systems, San Jose, CA) was used for image processing.
Cytokine ELISA
Peritoneal macrophages were stimulated with different concentrations of LPS at 37°C for 4 hours. The amount of tumor necrosis factor-α (TNFα) secreted into the conditioned media was determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Results
Mac-1 deficiency does not compromise monocyte infiltration in vivo
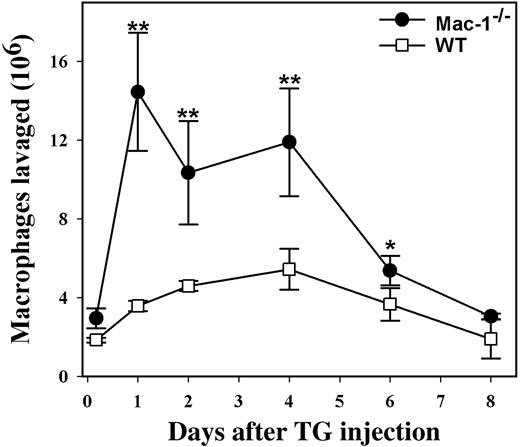
Both in vitro and in vivo studies have strongly implicated a critical role for Mac-1 in leukocyte adhesion and migration.3,8 However, genetic inactivation of Mac-1 did not compromise neutrophil migration through the vascular endothelium.9 Rather, neutrophil accumulation within the peritoneal cavity was unexpectedly enhanced in Mac-1-/- mice, which was attributed in part to the defective apoptosis of infiltrated neutrophils in the absence of Mac-1.10 To determine whether Mac-1 deficiency has similar effects on monocyte migration and apoptosis, we evaluated monocyte infiltration into the peritoneum, using a TG-induced peritonitis model. Similar to the results observed for neutrophil migration,10 we found that significantly more monocytes/macrophages accumulated within the peritoneum of the Mac-1-/- mice when compared with WT controls. In addition, the peritoneal monocyte/macrophage number peaked earlier in Mac-1-/- mice than in the WT mice (Figure 1). Thus, Mac-1 deficiency does not compromise monocyte recruitment into the peritoneum.
Enhanced monocyte accumulation within the peritoneum of Mac-1-/- mice. WT (□, n = 4) and Mac-1-/- mice (•, n = 4) were injected intraperitoneally with sterile TG to elicit monocyte infiltration into the peritoneum. At different times after TG injection, peritoneal lavages were performed. The total number of leukocytes in the lavage was determined with a Coulter counter, and the percentage of monocytes/macrophages was determined by FACS analysis using mAb F4/80 and by Hema3 staining of Cytospin smears. Monocyte accumulation in the peritoneal cavity was enhanced in Mac-1-/- mice (•) compared to the control WT mice (□), whereas the reduction in peritoneal macrophages, possibly due to their spontaneous efflux out of the peritoneum, between day 4 and 8 was similar. The data represent the mean ± SD of 3 mice. WT versus Mac-1-/-: **P < .005; *P < .02.
Enhanced monocyte accumulation within the peritoneum of Mac-1-/- mice. WT (□, n = 4) and Mac-1-/- mice (•, n = 4) were injected intraperitoneally with sterile TG to elicit monocyte infiltration into the peritoneum. At different times after TG injection, peritoneal lavages were performed. The total number of leukocytes in the lavage was determined with a Coulter counter, and the percentage of monocytes/macrophages was determined by FACS analysis using mAb F4/80 and by Hema3 staining of Cytospin smears. Monocyte accumulation in the peritoneal cavity was enhanced in Mac-1-/- mice (•) compared to the control WT mice (□), whereas the reduction in peritoneal macrophages, possibly due to their spontaneous efflux out of the peritoneum, between day 4 and 8 was similar. The data represent the mean ± SD of 3 mice. WT versus Mac-1-/-: **P < .005; *P < .02.
Infiltrated monocytes/macrophages do not undergo apoptosis
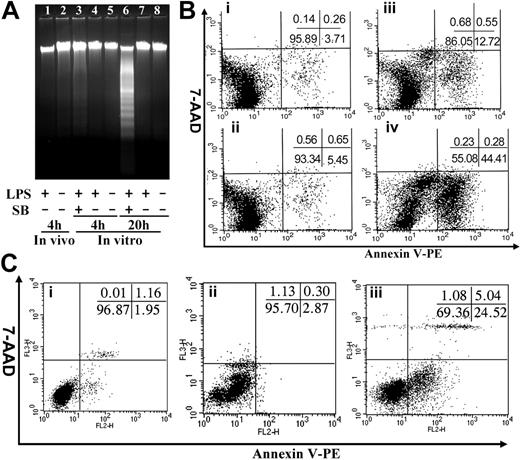
Mac-1 deficiency results in defective neutrophil apoptosis.10 A similar defect in apoptosis of the Mac-1-/- monocytes/macrophages could also contribute to the enhanced accumulation of peritoneal monocytes/macrophages within the Mac-1-/- mice. To determine whether the monocytes/macrophages within the peritoneal cavity undergo apoptosis, we induced monocyte infiltration into the peritoneal cavity of WT and Mac-1-/- mice by TG injection, and apoptosis of the infiltrated cells was evaluated at different time points (day 4, 6, and 8) after TG injection by annexin V staining (Figure 2; Table 1). In addition, on day 4, the macrophages within WT and Mac-1-/- mice were also stimulated by intraperitoneal injection of LPS. Four hours later, the total peritoneal cells were collected by lavage and analyzed for apoptosis, using DNA fragmentation (Figure 2A) and annexin V staining (Figure 2B). The results showed that the infiltrated macrophages within the peritoneum of WT and Mac-1-/- mice on day 4, 6, or 8 after TG injections did not undergo significant apoptosis (Figure 2; Table 1), suggesting that the observed reduction in peritoneal macrophages on days 6 and 8 was not caused by macrophage apoptosis. In addition, LPS stimulation in vivo did not lead to significant apoptosis, evidenced by the lack of DNA fragmentation (Figure 2A, lane 1) or annexin V staining (Figure 2Bi) of the lavaged macrophages. A low percentage of apoptotic cells were observed (Figure 2B), which is likely attributed to the presence of a small number of neutrophils (5%) within the lavaged peritoneal cells (data not shown). These results indicate that unlike neutrophils, the infiltrated monocytes/macrophages, with or without LPS treatment, did not undergo substantial apoptosis in vivo.
It is possible that the infiltrated monocytes/macrophages did undergo apoptosis; however, the apoptotic cells were quickly eliminated via phagocytosis by the reticuloendothelial system, and thus could not be observed by our apoptosis assays. To exclude this possibility, we cultured the above peritoneal cells (obtained on day 4) from both WT and Mac-1-/- mice in vitro for 4 and 20 hours in the presence or absence of LPS and analyzed the degree of macrophage apoptosis as described above. Additionally, we cultured the macrophages in the presence of both LPS and the MAPK inhibitor SB202190, which in combination was shown to induce macrophage apoptosis after extended incubation.16 Among these different culture conditions, only extended incubation with both LPS and SB202190 promoted substantial macrophage apoptosis (44.4% after 20 hours; Figure 2A, lane 6, and Figure 2Biv), which agrees well with the published study.16 In the absence of SB202190 treatment, the primary macrophages, regardless of their genotypes (WT or Mac-1-/-), or LPS stimulation, did not undergo significant apoptosis after 4- or 20-hour incubation. Even under stressful conditions (ie, treated with both LPS and SB202190), short incubation (4 hours) did not induce significant apoptosis of peritoneal macrophages (12.7%; Figure 2A, lane 3; Figure 2Bii), among which 5% of the apoptotic cells were likely attributed to the presence of neutrophils in the lavaged cells. Interestingly, when macrophages were stressed for an extended period of time (20 hours) by treatments with LPS and SB202190, Mac-1 deficiency exhibited some protective effects against apoptosis (Figure 2C; Table 1). Together, these data demonstrate that the infiltrated monocytes/macrophages did not undergo considerable apoptosis. Thus, the enhanced accumulation of monocytes/macrophages in the peritoneum of the Mac-1-/- mice cannot be caused by the decreased apoptosis of the monocytes/macrophages in the absence of Mac-1.
Spontaneous reduction of peritoneal macrophages is normal in Mac-1-deficient mice
The above results suggested that unlike neutrophils, mature macrophages do not die locally via apoptosis. Indeed, Bellingan et al13 reported that the infiltrated monocytes/macrophages migrate from the peritoneum to the local lymph nodes during the resolution phase of the acute inflammation. Thus, it is possible that Mac-1 deficiency inhibited the spontaneous efflux of macrophages out of the peritoneum, resulting in an increased accumulation of monocytes/macrophages in Mac-1-/- mice. To assess this possibility, we followed the efflux of monocytes/macrophages during the resolution phase of the acute inflammation, as described in the above study.13 We found that the number of peritoneal macrophages peaked between day 1 and day 4 after TG injection and then decreased gradually to its normal level on day 8 for both WT and the Mac-1-/- mice (Figure 1). These data suggest that the reduction of peritoneal macrophages, possibly due to their spontaneous efflux out of the peritoneum, does not depend on Mac-1, and thus other integrin receptors, such as integrin β1,14 may be involved in this process.
The infiltrated monocytes/macrophages do not die by apoptosis. WT and Mac-1-/- macrophages were injected intraperitoneally with TG for 4 days and then were injected intraperitoneally with PBS or LPS. Four hours later, the peritoneal macrophages were assessed for apoptosis by DNA fragmentation (A) and by dual-color FACS analysis using annexin V and 7-AAD (B-C), where annexin V stains apoptotic cells and the cell-impermeable fluorescent dye 7-AAD stains necrotic cells. Additionally, the lavaged WT (B) and Mac-1-/- (C) macrophages were cultured in vitro for 0 (B, Ci) and 20 (B, Cii) hours in the absence or 4 (Biii) and 20 (Biv, Ciii) hours in the presence of LPS plus the mitogen-activated protein kinase (MAPK) inhibitor SB202190, and then analyzed for apoptosis. The inserts shown are the percentages of different populations. The data shown are representative of 2 independent experiments.
The infiltrated monocytes/macrophages do not die by apoptosis. WT and Mac-1-/- macrophages were injected intraperitoneally with TG for 4 days and then were injected intraperitoneally with PBS or LPS. Four hours later, the peritoneal macrophages were assessed for apoptosis by DNA fragmentation (A) and by dual-color FACS analysis using annexin V and 7-AAD (B-C), where annexin V stains apoptotic cells and the cell-impermeable fluorescent dye 7-AAD stains necrotic cells. Additionally, the lavaged WT (B) and Mac-1-/- (C) macrophages were cultured in vitro for 0 (B, Ci) and 20 (B, Cii) hours in the absence or 4 (Biii) and 20 (Biv, Ciii) hours in the presence of LPS plus the mitogen-activated protein kinase (MAPK) inhibitor SB202190, and then analyzed for apoptosis. The inserts shown are the percentages of different populations. The data shown are representative of 2 independent experiments.
Adhesion/migration of activated macrophages across the mesothelium is dependent on Mac-1
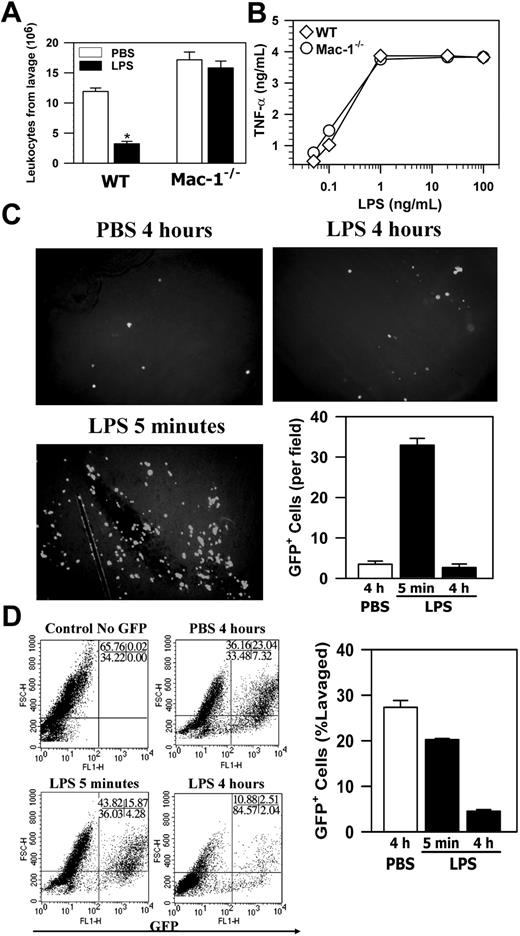
Szaba et al18 reported that stimulation of macrophages by antigen activation or by LPS enhances their adhesion/migration across the mesothelium in a fibrin-dependent manner. Since Fg is a ligand of Mac-1,19 we tested the effect of Mac-1 deficiency on macrophage adhesion/migration in vivo using LPS as the activator, and macrophage adhesion/migration was measured indirectly by the loss of macrophages within the peritoneal cavity, as described in their study.18 Briefly, monocyte infiltration into the peritoneum was induced by TG injection, and the infiltrated monocytes were allowed to mature into macrophages over 4 days. Macrophages were then activated by intraperitoneal injection of LPS, and 4 hours later the total peritoneal cells were retrieved by lavage. Macrophage adhesion/migration was demonstrated by the difference in the total peritoneal leukocyte number between the LPS-treated mice and the control PBS-treated mice. Figure 3A shows that for WT mice, LPS treatment led to a 3.7-fold reduction in total peritoneal leukocytes (Figure 3A). In contrast, similar LPS treatment of Mac-1-/- mice did not have any effect. Considering that LPS treatment did not induce significant macrophage apoptosis (Figure 2), the above results indicate that loss of peritoneal macrophages, which is likely due to the adhesion/migration of activated macrophages across the mesothelial layer, is dependent on Mac-1.
One caveat of the above interpretation is that because Mac-1 can also bind to LPS,20 it is possible that Mac-1-/- macrophages were unable to respond to the LPS stimulation, resulting in defective cell signaling. To exclude this possibility, we assessed the activation of Mac-1-/- macrophages by examining their ability to produce TNFα in response to LPS.20 These studies indicated that there was no significant difference in the response to LPS between the Mac-1-/- macrophages and the wild-type macrophages (Figure 3B). This is consistent with results reported for the targeted deletion of CD18,20 which destroys expression of all CD18-containing integrins, including Mac-1. Thus, the defective adhesion/migration of Mac-1-/- macrophages was not due to their inability to respond to LPS, and, taken together, these data demonstrate that, although Mac-1 is not required for monocyte recruitment into the peritoneum or the spontaneous efflux of macrophages, it is necessary for adhesion/migration of the activated macrophages out of the peritoneum.
Macrophage efflux to the lymphatic system and to the circulation
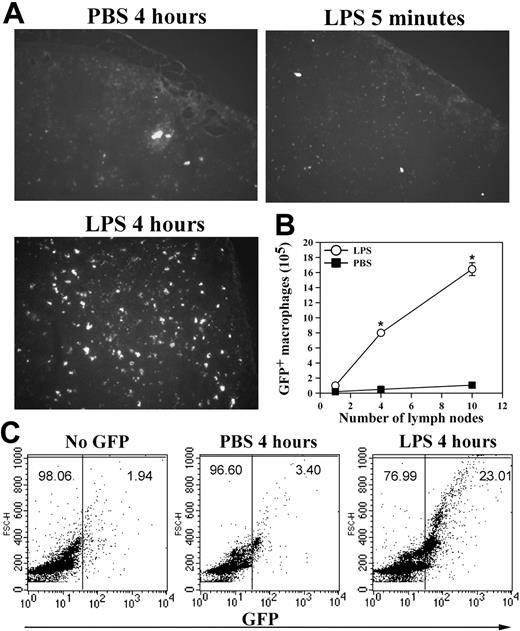
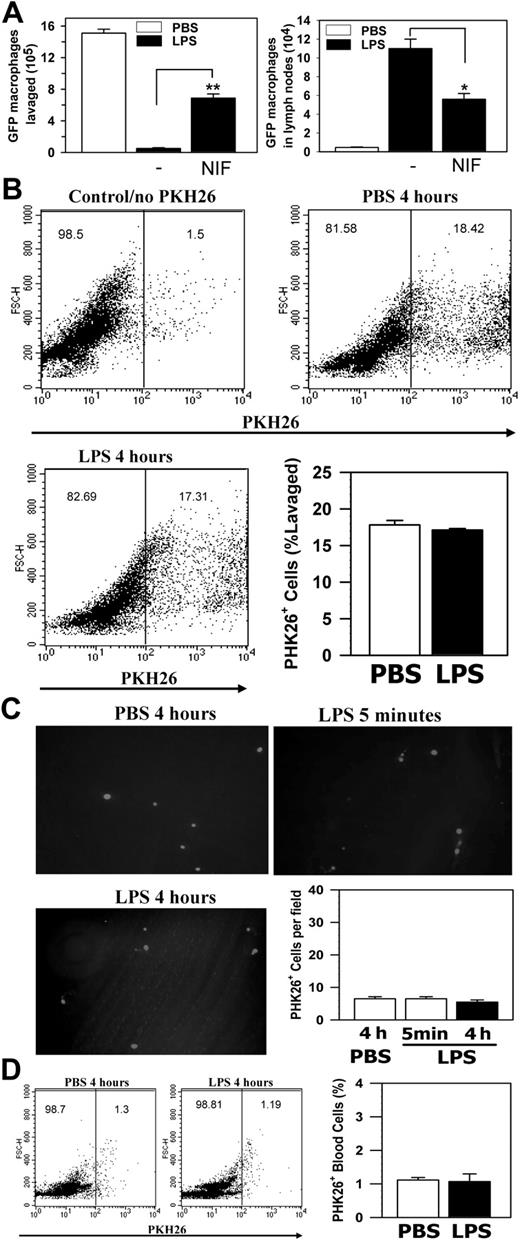
In addition to apoptosis, cell adhesion to the peritoneal membrane and/or cell migration through the mesothelial layer into the lymphatic system can also contribute to the loss of macrophages from the peritoneum. Furthermore, the enhanced neutrophil accumulation in Mac-1-/- mice10 could mask the decrease of their peritoneal macrophages in response to LPS stimulation. To assess these possibilities and, more importantly, to determine the fate of the LPS-stimulated macrophages within the peritoneal cavity, we conducted passive cell transfer experiments using the GFP+ macrophages. In these experiments, the green GFP+ macrophages, isolated from the GFP transgenic mice21 on day 4 after TG injection, were transferred into the peritoneum of TG-preconditioned WT mice as described in “Materials and methods,” which resembles the inflammatory state of the mice used in the in vivo adhesion/migration experiments (Figure 3A). Following cell transfer, the recipient mice were injected intraperitoneally with either PBS or LPS, and, at different time points, the number of the GFP+ macrophages that remained within the peritoneal cavity, adhered to the mesothelium, migrated into the local draining lymph nodes or into the blood circulation was determined. Figure 3C shows that significant numbers of the GFP+ macrophages could be seen on the peritoneal membrane at 5 minutes after stimulation, a majority of which disappeared at 4 hours after stimulation. The disappearance of these adherent GFP+ macrophages was not due to their release from the peritoneal membrane back into the peritoneal cavity, as the number of the GFP+ macrophages in the peritoneum was similarly reduced at 4 hours after stimulation (Figure 3D). Note that the number of the endogenous macrophages was similarly reduced after LPS stimulation, as evidenced by the reduction of the F4/80+ macrophages (cells with larger forward scatter [FSC] values; Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article) within the GFP-negative population of the FACS analysis (Figure 3D, bottom right). A small but significant reduction in the number of the GFP+ peritoneal macrophages at 5 minutes was observed, which is likely due to their adhesion to the peritoneal membrane (Figure 3C). Consistent with macrophage efflux to the lymphatics, visualization of the frozen sections (5-μm thick) of the retrieved draining lymph nodes under a fluorescence microscope demonstrated abundant GFP+ macrophages within the lymph nodes at 4 hours but not 5 minutes after LPS stimulation (Figure 4A), which parallels the disappearance of the GFP+ cells from the peritoneal mesothelium (Figure 3C-D). No significant number of GFP+ macrophages was seen on the peritoneal membrane (Figure 3C) or within the lymph nodes of the PBS-treated mice, even at 4 hours after cell transfer (Figure 4A). Given the large number of lymph nodes within a mouse, it is difficult to recover all of the injected GFP+ cells that have migrated into the lymphatics. To gauge the degree of macrophage efflux into the lymph nodes, we determined the number of GFP+ macrophages as a function of the lymph nodes collected from the same mouse. Figure 4B shows that recovery of the GFP+ cells is directly proportional to the number of the mesenteric lymph nodes retrieved, and the number of GFP+ macrophages (1.6 × 106 cells) obtained from 10 lymph nodes could account for approximately 30% of the injected GFP+ macrophages (5 × 106 cells). Moreover, as leukocytes within the lymphatics, if not taking residence in the lymph nodes, ultimately flow with the lymph into the blood circulation, we also determined the number of GFP+ cells in the blood at different time points after stimulation. FACS analysis of whole blood taken at different time points after LPS stimulation shows that approximately 25% of the blood leukocytes were GFP+ 4 hours after stimulation, whereas very few GFP+ macrophages could be observed at 5 minutes after stimulation or within the blood taken from the PBS-treated mice (Figure 4C). Assuming that a mouse (∼ 20 g body weight) has 1.4 mL total blood,22 and its leukocyte count is approximately 3 to 8 × 106 cells/mL,23 the number of GFP+ macrophages in the circulation at 4 hours after stimulation would be approximately 2 × 106, which represents approximately 40% of the 5 × 106 injected GFP+ cells. Altogether, these results demonstrate that on stimulation, a majority (∼ 70%) of the transferred GFP+ macrophages migrated out of the peritoneal cavity and could be recovered directly or indirectly from the lymphatic system, thus definitively establishing the lymphatics as the major destination of the macrophage efflux.
Mac-1 deficiency inhibits the loss of leukocytes from the peritoneum. (A) The TG-conditioned WT and Mac-1-/- mice were injected intraperitoneally with either PBS or LPS. Four hours later, the total leukocytes in peritoneum were determined. The data represent the mean ± SD (n = 6) and are representative of 3 independent experiments. (B) WT or Mac-1-/- peritoneal macrophages were stimulated with different concentrations of LPS. Four hours later, the amount of TNFα produced was determined by ELISA. The data shown are representative of 2 independent experiments. (C-D) The GFP+ peritoneal macrophages were injected intraperitoneally into TG-preconditioned WT mice, followed with intraperitoneal injections of PBS or LPS. At different time points, the peritoneal membrane was washed, fixed, and photographed using a fluorescence microscope (objective × 100) (C), and the percentage of GFP+ cells in total peritoneal cells was analyzed by FACS (D). The number of adherent GFP+ cells on the peritoneal mesothelium was counted manually based on 4 randomly picked fields. The data represent the mean ± SD of 3 mice and are representative of 3 independent experiments. *PBS versus LPS, P < .001.
Mac-1 deficiency inhibits the loss of leukocytes from the peritoneum. (A) The TG-conditioned WT and Mac-1-/- mice were injected intraperitoneally with either PBS or LPS. Four hours later, the total leukocytes in peritoneum were determined. The data represent the mean ± SD (n = 6) and are representative of 3 independent experiments. (B) WT or Mac-1-/- peritoneal macrophages were stimulated with different concentrations of LPS. Four hours later, the amount of TNFα produced was determined by ELISA. The data shown are representative of 2 independent experiments. (C-D) The GFP+ peritoneal macrophages were injected intraperitoneally into TG-preconditioned WT mice, followed with intraperitoneal injections of PBS or LPS. At different time points, the peritoneal membrane was washed, fixed, and photographed using a fluorescence microscope (objective × 100) (C), and the percentage of GFP+ cells in total peritoneal cells was analyzed by FACS (D). The number of adherent GFP+ cells on the peritoneal mesothelium was counted manually based on 4 randomly picked fields. The data represent the mean ± SD of 3 mice and are representative of 3 independent experiments. *PBS versus LPS, P < .001.
Macrophage efflux to the lymphatic system and to the blood circulation. (A) The GFP+ peritoneal macrophages, obtained from the GFP transgenic mice, were injected into the peritoneum of TG-preconditioned WT mice (5 × 106 GFP+ cells per mouse). At different time points after LPS stimulation, the draining lymph nodes were collected and cyrofixed. The presence of the GFP+ macrophages within the frozen sections of the fixed lymph nodes was visualized by fluorescence microscopy (objective lens 100 ×). (B) Additionally, leukocytes were recovered from different lymph nodes of the same mice that were treated with PBS (▪) or LPS (○) for 4 hours, and the number of the GFP+ cells retrieved was determined by hemocytometer. The data shown are the mean ± SD (n = 3). (C) Total blood samples were taken at different time points from mice in panel A and analyzed for the presence of the GFP+ macrophages by FACS analysis. Representative data of 2 independent experiments are shown. *PBS versus LPS P < .001.
Macrophage efflux to the lymphatic system and to the blood circulation. (A) The GFP+ peritoneal macrophages, obtained from the GFP transgenic mice, were injected into the peritoneum of TG-preconditioned WT mice (5 × 106 GFP+ cells per mouse). At different time points after LPS stimulation, the draining lymph nodes were collected and cyrofixed. The presence of the GFP+ macrophages within the frozen sections of the fixed lymph nodes was visualized by fluorescence microscopy (objective lens 100 ×). (B) Additionally, leukocytes were recovered from different lymph nodes of the same mice that were treated with PBS (▪) or LPS (○) for 4 hours, and the number of the GFP+ cells retrieved was determined by hemocytometer. The data shown are the mean ± SD (n = 3). (C) Total blood samples were taken at different time points from mice in panel A and analyzed for the presence of the GFP+ macrophages by FACS analysis. Representative data of 2 independent experiments are shown. *PBS versus LPS P < .001.
Macrophage migration to the lymphatics is dependent on Mac-1
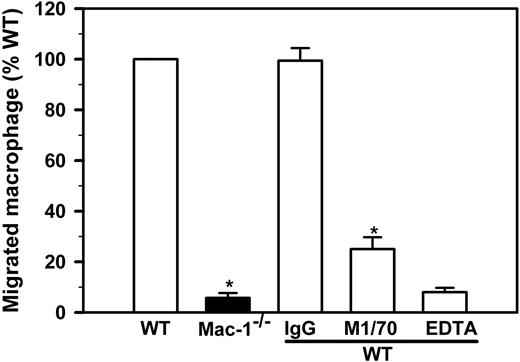
Our observation that LPS stimulation failed to reduce the number of peritoneal leukocytes in Mac-1-/- mice (Figure 3A) suggests that macrophage migration to the lymphatics may depend on Mac-1. To directly test this hypothesis, we conducted in vitro migration experiments in a well-defined system, using Boyden chamber-type transwell plates with a pore size of 5 μm. We used immobilized Fg, a major physiologic ligand of Mac-1 that is present within the site of inflammation because of increased vascular permeability. In a typical experiment, 4 × 105 freshly isolated and LPS-stimulated macrophages are added to the upper chamber, among which 1.3 × 104 (32.5%) cells migrate into the lower chamber after a 4-hour incubation. Using this assay format, we found that Mac-1-/- macrophages exhibited a 17-fold reduction in cell migration, thus supporting a critical role of Mac-1 in macrophage migration (Figure 5). Moreover, addition of a Mac-1-specific function-blocking mAb (M1/70) or EDTA reduced WT macrophage migration by 75%, whereas addition of nonimmune IgG had no effect. These data demonstrate that in vitro migration of the LPS-stimulated macrophages depends on both Mac-1 and the divalent cations.
To demonstrate that Mac-1 is involved in the accelerated migration of activated macrophages in vivo, we conducted similar passive transfer experiments using WT mice, in which the ligand binding functions of Mac-1 were blocked by intraperitoneal administration of its high-affinity antagonist NIF.24 We found that administration of NIF in the peritoneum of WT mice significantly inhibited both the efflux of GFP+ macrophages from the peritoneum and their corresponding increase in the lymph nodes (Figure 6A), confirming a critical role of Mac-1 in macrophage migration to the draining lymph nodes. As an ultimate proof, we injected the PKH26 (a red phagocytic fluorescent dye)-labeled Mac-1-/- macrophages, obtained from the 4-day TG-treated Mac-1-/- mice by peritoneal lavage, into the peritoneum of TG-preconditioned Mac-1-/- mice. After cell transfer, these mice received single intraperitoneal injection of PBS or LPS, and 4 hours after injection the number of fluorescent Mac-1-/- macrophages that remained in the peritoneal cavity, adherent on the peritoneal membrane, and migrated into the lymph nodes and the blood circulation were determined. No significant reduction in the number of PKH26+ Mac-1-/- macrophages were observed at 4 hours after LPS stimulation (Figure 6B), and no significant number of Mac-1-/- macrophages adhered to the peritoneal membrane at either 5 minutes or 4 hours after LPS injection (Figure 6C), indicating that the Mac-1-/- macrophages are defective both in cell adhesion and in the subsequent migration across the peritoneal membrane. Consistent with the defective efflux of the Mac-1-/- macrophages, no significant increase in the number of fluorescent cells was observed within either the lymph nodes (data not shown) or in the blood circulation after 4 hours of LPS stimulation (Figure 6D).
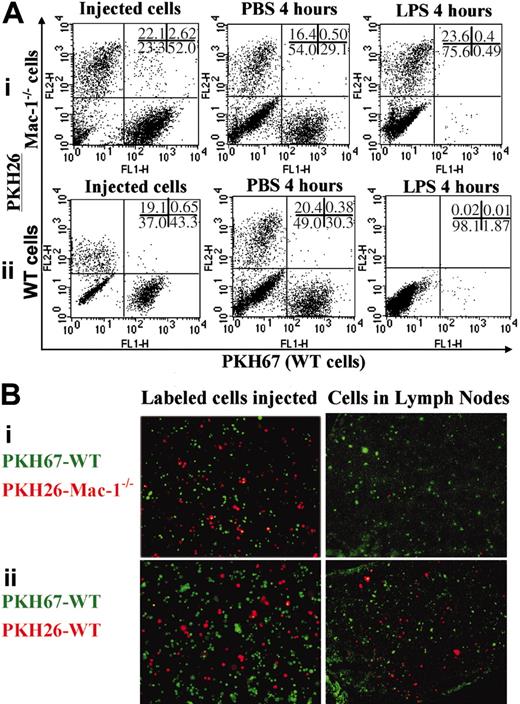
To further confirm the importance of Mac-1 in macrophage migration, we labeled WT and Mac-1-/- cells separately with red (PHK26) and green (PHK67) fluorescent dyes. A mixture of green WT and red Mac-1-/- cells (group I) or green and red WT cells (group II) were injected intraperitoneally into the same WT mice, followed by intraperitoneal injections of either PBS or LPS. Four hours later, the cells within the peritoneum were analyzed by dual-color FACS, and the frozen sections from their corresponding lymph nodes were evaluated under a fluorescence microscope. When a mixture of WT (in green) and Mac-1-/- (in red) cells were injected in the peritoneal cavity (Figure 7A,Bi), WT but not the deficient macrophages disappeared from the peritoneum (Figure 7Ai) and were found in the lymph nodes (Figure 7Bi) in response to LPS stimulation. When a mixture of green and red WT cells (Figure 7A,Bii) was injected, both green and red cells disappeared from the peritoneum (Figure 7Aii) and appeared in the lymph nodes (Figure 7Bii). Also, very small percentages (0.5%) of the cells within the peritoneum (the double-positive cells in Figure 7A) and in the lymph nodes (Figure 7B) were yellow. Altogether, these data demonstrate that (1) Mac-1 is critical to macrophage migration from the peritoneum to the lymph nodes, (2) the fluorescence dyes used in this study had no effect on cell migration, and (3) the fluorescent dyes were not released from the labeled cells and then picked up by cells within the lymph nodes.
Macrophage migration in vitro requires Mac-1. Migration of LPS-stimulated WT (open bars) and Mac-1-/- (filled bar) peritoneal macrophages was determined using transwell plates with a 5-μm pore insert in the presence or absence of anti-Mac-1 (M1/70), control IgG (IgG) or EDTA. The total number of macrophages within the lower chamber was determined manually by hemocytometer and normalized for the percentage of macrophages based on Hema3-staining of Cytospin smears. The number of migrated WT macrophages is assigned to 100%. The data represents mean ± SD of 3 to 5 independent experiments. *P < .001 for WT versus Mac-1-/- and M1/70 versus IgG.
Macrophage migration in vitro requires Mac-1. Migration of LPS-stimulated WT (open bars) and Mac-1-/- (filled bar) peritoneal macrophages was determined using transwell plates with a 5-μm pore insert in the presence or absence of anti-Mac-1 (M1/70), control IgG (IgG) or EDTA. The total number of macrophages within the lower chamber was determined manually by hemocytometer and normalized for the percentage of macrophages based on Hema3-staining of Cytospin smears. The number of migrated WT macrophages is assigned to 100%. The data represents mean ± SD of 3 to 5 independent experiments. *P < .001 for WT versus Mac-1-/- and M1/70 versus IgG.
Mac-1 is critical to macrophage migration in vivo to the lymphatics. The GFP+ WT peritoneal macrophages were injected intraperitoneally into the TG-preconditioned WT mice with and without NIF, followed with intraperitoneal injections of PBS or LPS. (A) The number of GFP+ cells within the peritoneum (left) or the draining lymph nodes (right) was determined 4 hours later. The data represent the mean ± SD of 2 independent experiments. (B-D) PKH26-labeled Mac-1-/- peritoneal macrophages were injected intraperitoneally into the TG-preconditioned Mac-1-/- mice, followed by intraperitoneal injections of PBS or LPS. The number of fluorescent Mac-1-/- macrophages within the peritoneum (B), adherent on the peritoneal membrane (C), or within the blood circulation (D) was determined 4 hours later. The data represent the mean ± SD of 2 independent experiments. **P < .01; *P < .05.
Mac-1 is critical to macrophage migration in vivo to the lymphatics. The GFP+ WT peritoneal macrophages were injected intraperitoneally into the TG-preconditioned WT mice with and without NIF, followed with intraperitoneal injections of PBS or LPS. (A) The number of GFP+ cells within the peritoneum (left) or the draining lymph nodes (right) was determined 4 hours later. The data represent the mean ± SD of 2 independent experiments. (B-D) PKH26-labeled Mac-1-/- peritoneal macrophages were injected intraperitoneally into the TG-preconditioned Mac-1-/- mice, followed by intraperitoneal injections of PBS or LPS. The number of fluorescent Mac-1-/- macrophages within the peritoneum (B), adherent on the peritoneal membrane (C), or within the blood circulation (D) was determined 4 hours later. The data represent the mean ± SD of 2 independent experiments. **P < .01; *P < .05.
Macrophage migration from the peritoneum to the lymph nodes. A mixture of PKH67-labeled WT and PKH26-labeled Mac-1-/- macrophages (i) or PKH67-labeled WT and PKH26-labeled WT macrophages (ii) were separately injected intraperitoneally into the WT mice, followed by intraperitoneal injections of PBS or LPS. Four hours later, the number of the adoptively transferred WT and Mac-1-/- macrophages within the peritoneum was analyzed by dual-color FACS analysis (A), and their migration into the lymph nodes was visualized by fluorescence microscopy of the corresponding frozen sections (B). The data shown are representative of 2 independent experiments.
Macrophage migration from the peritoneum to the lymph nodes. A mixture of PKH67-labeled WT and PKH26-labeled Mac-1-/- macrophages (i) or PKH67-labeled WT and PKH26-labeled WT macrophages (ii) were separately injected intraperitoneally into the WT mice, followed by intraperitoneal injections of PBS or LPS. Four hours later, the number of the adoptively transferred WT and Mac-1-/- macrophages within the peritoneum was analyzed by dual-color FACS analysis (A), and their migration into the lymph nodes was visualized by fluorescence microscopy of the corresponding frozen sections (B). The data shown are representative of 2 independent experiments.
Discussion
Monocyte migration from the blood circulation to the inflammatory sites has been studied extensively in the past.2-4,6-10 However, the migration of macrophages, which have more distinct morphologies, gene expression patterns, and migratory behavior than their precursor monocytes, within the extravascular matrices, is poorly understood. Here, we report that genetic inactivation of Mac-1 does not compromise the accumulation of blood monocytes within the peritoneum. Instead, Mac-1 is involved in the accelerated migration of activated macrophages across the mesothelial layer into the lymphatics. Because inflammatory macrophages do not die locally via apoptosis, this Mac-1-mediated macrophage efflux may facilitate the removal of the proinflammatory macrophages from the site of injury, thus limiting the scope of local inflammation. In addition, macrophage migration to the lymph nodes may also facilitate antigen presentation and the development of adaptive immunity against the invading pathogens.
Persistent macrophage accumulation within the site of injury is a hallmark of many chronic inflammatory diseases, including atherosclerosis, restenosis, and rheumatoid arthritis.25 Past studies are largely focused on the recruitment of blood monocytes into the lesions and their role in disease progression. However, a recent study demonstrates that the failure of monocyte-derived cells to migrate out of the atherosclerotic plaques into the lymph nodes also represents a major cause of plaque progression.17 Consistent with this study and the study of Bellingan et al,13 we found that infiltrated macrophages did not undergo apoptosis locally with or without LPS stimulation (Figure 2), which contrasts sharply to the rapid apoptosis of peritoneal neutrophils immediately after their extravasation.10 Because WT macrophages, which express high levels of Mac-1 (Figure 7), did not die by apoptosis (Figure 2), the enhanced accumulation of Mac-1-/- monocytes/macrophages within the peritoneum (Figure 1) could not be caused by the defective apoptosis of the Mac-1-/- cells. Potential mechanisms that lead to enhanced monocyte/macrophage infiltration may include (1) changes in the levels of active chemokines within the peritoneum of Mac-1-/- mice and (2) changes in the sensitivity of Mac-1-/- mice toward TG stimulation. Further studies will be needed to clarify these mechanisms.
Another interesting feature of leukocyte migration is its dependence on the specific cell types, their activation status, and their corresponding extracellular environments.26 Thus, Mac-1 is required for migration of activated macrophages across the mesothelium (Figure 6) but is dispensable for neutrophil9,10 and monocyte (this study) accumulation within the inflamed peritoneum (Figure 1). Furthermore, the spontaneous macrophage efflux is relatively less efficient13 and is dependent on the β1 integrins.14 In contrast, the LPS-accelerated macrophage efflux observed in this study and in the study of Szaba et al18 is much more efficient and depends instead on Mac-1 (Figure 6). The mechanism that triggers the switch from a β1- to a Mac-1-dependent migration is currently unknown. Given that stimulation of macrophages by LPS potentially leads to blood coagulation and fibrin formation, and that fibrin is a physiologic ligand of Mac-1,19 we speculate that LPS stimulation may allow Mac-1 to engage fibrin and thereby enhance the efficiency of macrophage adhesion/migration across the peritoneal membrane. In support of this hypothesis, Szaba et al18 reported using a similar animal model that blockade of fibrin formation by a thrombin inhibitor (hirudin) or genetic inactivation of Fg in mice leads to defective macrophage adhesion/migration in response to antigen or LPS stimulation.
In summary, we showed in this study that the infiltrated macrophages do not undergo significant apoptosis and are not permanently trapped within the peritoneal membranes. Instead, a majority of them migrate across the mesothelium into the lymphatics, where they further move to the lymph nodes and to the blood circulation. Macrophage migration can be enhanced by cell activation, which is associated with a switch to the Mac-1-dependent mechanism. Acceleration of macrophage efflux by Mac-1 could potentially help the adequate removal of proinflammatory macrophages from the site of injury and thereby limit the scope of local inflammation. It may also facilitate antigen presentation to the lymphocytes and the initiation of an adaptive immune response.
Prepublished online as Blood First Edition Paper, July 7, 2005; DOI 10.1182/blood-2005-03-1288.
Supported in part by grants from the National Institute of Health (NHLBI R01 HL61589-01 and NHLBI 2P01 HL54710-06).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ballantyne of the Baylor College of Medicine for providing the Mac-1-deficient mice.