Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplantation (HSCT). Migration of donor-derived T cells into GVHD target organs plays a critical role in the development of GVHD and chemokines and their receptors are important molecules involved in this process. Here, we demonstrate in murine bone marrow transplantation models that the expression of the inflammatory CC chemokine receptor 2 (CCR2) on donor-derived CD8+ T cells is relevant for the control of CD8+ T-cell migration and development of GVHD. Recipients of CCR2-deficient (CCR2-/-) CD8+ T cells developed less damage of gut and liver than recipients of wild-type CD8+ T cells, which correlated with a reduction in overall GVHD morbidity and mortality. Assessment of donor CD8+ T-cell target organ infiltration revealed that CCR2-/- CD8+ T cells have an intrinsic migratory defect to the gut and liver. Other causes for the reduction in GVHD could be excluded, as alloreactive proliferation, activation, IFN-γ production and cytotoxicity of CCR2-/- CD8+ T cells were intact. Interestingly, the graft-versus-tumor effect mediated by CCR2-/- CD8+ T cells was preserved, which suggests that interference with T-cell migration by blockade of CCR2 signaling can separate GVHD from GVT activity.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a well-established therapy for a variety of malignant and nonmalignant disorders of the hematopoietic system and for certain solid tumors.1 A major complication limiting the success and wider application of allogeneic HSCT is the occurrence of acute graft-versus-host disease (GVHD), which is a rapidly progressive illness with immunosuppression, cachexia and specific target organ damage of liver, intestines, skin, and lung.2 Donor-derived alloreactive T cells play a major role in the pathogenesis of GVHD and depletion of T cells from the donor cell inoculum remains the most effective approach to prevent the development of disease.3 However, alloreactive donor T cells also display graft-versus-tumor (GVT) activity, which is increasingly being recognized as an important component of the overall antitumor effect of an allogeneic HSCT.4 Recent murine bone marrow transplantation studies suggest that specifically interfering with T-cell migration represents an attractive therapeutic approach toward the goal of amelioration of GVHD without reducing GVT activity.5,6 It is known that 3 families of migration molecules (selectins, chemokines, integrins, and their respective ligands and receptors) control T-cell migration in homeostasis and inflammation7 and members of all 3 families have been identified as important players during GVHD.5
CC chemokine receptor 2 (CCR2) and its main ligand chemokine ligand 2 (CCL2) are among the chemokine receptor-ligand pairs that control leukocyte migration during inflammatory processes.8,9 CCL2 (originally termed monocyte chemoattractant protein-1 [MCP-1]) belongs to the family of inflammatory CC chemokines and was one of the first identified chemoattractive proteins shown to be relevant for monocyte10 and T-cell chemoattraction.11 In GVHD, the expression of CCL2 in the gut, liver, skin, and lung coincides with the increase of the inflammatory cytokines IL-1, TNF-α, IFN-γ early after transplantation12-14 and the time course of CCL2 levels correlates with the severity of cellular infiltrates in the lung during GVHD-related idiopathic pneumonia syndrome (IPS).15 Blockade of CCL2 with a monoclonal antibody results in reduced severity of IPS after allogeneic HSCT.16
CCR2 belongs to the class of hepta-helical G-protein–coupled transmembrane CC chemokine receptors and is the predominant receptor for CCL2.17 CCR2 is expressed on many hematopoietic cell types such as monocytes, macrophages, dendritic cells, and effector, memory, and activated T cells.17,18 CCR2-deficient leukocytes show reduced migration toward CCL2 in vitro,19,20 reduced adhesion to the microvascular endothelium21 and reduced extravasation into areas of inflammation.20-22 CCR2-deficient mice develop normally and are not severely immunocompromised,20-22 but show a reduced susceptibility to a variety of inflammatory disorders,20-28 including T-cell–mediated disorders such as experimental autoimmune encephalomyelitis (EAE)29,30 and dextran sodium sulfate-induced colitis.31 Although a reduction of T-cell numbers within the inflammatory infiltrates of CCR2-/- mice has been described in these models, an intrinsic migratory defect of CCR2-/- T cells without possible confounding effects of CCR2-deficiency in other cell types (such as defective migration of CCR2-deficient dendritic cells [DCs]32,33 ) has not been shown so far.25,29 Therefore no studies to date have been able to demonstrate a clear role for CCR2 in in vivo migration of activated T cells.
We here sought to specifically define the role of CCR2 expression on donor CD8+ T cells in GVHD. We chose to focus in our initial studies on CD8+ T cells to avoid the confounding complexity of unselected or CD4+ T cells, which also contain CD4+CD25+ regulatory T cells and are deserving a separate study altogether. We found that recipients of CCR2-/- CD8+ T cells develop significantly less overall GVHD morbidity, mortality, and reduced target organ damage to gut and liver than recipients of wild-type (WT) CD8+ T cells and that this is due to an intrinsic migratory defect of CCR2-/- alloreactive CD8+ T cells. Importantly, the GVT activity of CCR2-/- CD8+ T cells was preserved, which suggests that interference with T-cell migration by blockade of CCL2-CCR2 signaling could separate GVHD from GVT activity.
Materials and methods
Cell lines
P815 (H-2d), a mastocytoma cell line of DBA/2 origin, was obtained from the American Type Culture Collection (Manassas, VA). A20 (H-2d), a BALB/c B-cell lymphoma, was kindly provided by A. Houghton (Memorial Sloan-Kettering Cancer Center, New York, NY). 32Dp210 (H-2k), a C3H/HeJ myeloid leukemia cell line, was kindly provided by J. Griffin (Dana Farber Cancer Institute, Boston, MA).34 Cells were maintained as previously described.35
Antibodies and flow cytometry
All of the following fluorochrome- or biotin-labeled antimurine antibodies were obtained from Pharmingen (San Diego, CA): CD16/CD32 FcR block (2.4G2), CD3 (145-2C11), CD4 (RM4-5, GK1.5), CD8 (53-6.7), CD62L (MEL-14), IFN-γ (XMG1.2), TNF-α (MP6-XT22), CD44 (IM7), Ly-9.1 (30C7), CD45.1 (A20), H-2Kk (36-7-5), rat IgG2a, κ (LOU, R35-95), rat IgG2b, κ (A95-1), and Streptavidin. FACS staining was performed as previously described.35 Cells were acquired on an LSR cytometer (Becton Dickinson, San Jose, CA) with CellQuest software (Becton Dickinson). Data were analyzed with FlowJo (Treestar, Ashland, OR).
Generation of tumor lines for in vivo bioluminescence imaging (BLI)
P815 and A20 cells were retrovirally transduced to express a triple fusion protein consisting of Herpes simplex virus thymidine kinase, enhanced green fluorescent protein (eGFP), and firefly luciferase (TGL; plasmid was kindly provided by V. Ponomarev, Memorial Sloan-Kettering Cancer Center, New York, NY).36 Transduced cells were expanded and individual clones with high eGFP expression were sorted into 96-well plates using a MoFlo Cell Sorter (DakoCytomation, Ft Collins, CO). Bioluminescent signal intensity of single clones was determined, and the brightest clones (termed P815 TGL and A20 TGL) were used for subsequent experiments.
Mice, bone marrow transplantation, and tumor induction
Female C57BL/6 (B6) (H-2b), C3FeB6F1 (H-2b/k), B6D2F1 (H-2b/d), BALB/c (H-2d), C57BL/6-Thy1.1+ (B6-Thy1.1) (H-2b), and C57BL/6-Ly5.1+ (B6-Ly5.1) (H-2b) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6.CCR2-KO (CCR2-/-) were kindly provided by W.A. Kuziel (University of Texas, Austin, TA).21 Mice used in bone marrow transplantation (BMT) experiments were between 8 and 12 weeks old. Bone marrow (BM) cells were isolated by flushing femurs and tibias. Donor BM was T-cell depleted (TCD) by incubation with anti–Thy-1.2 for 30 minutes at 4°C, followed by incubation with Low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, Canada) for 40 minutes at 37°C. The donor T-cell inoculum consisted of CD8+ T cells selected from WT or CCR2-/- splenic T cells with anti-CD8 microbeads (Miltenyi, Auburn, CA). Cells were transplanted by tail vein injection into lethally irradiated recipients (BALB/c: 900 cGy, C3FeB6F1, and B6D2F1: 1300 cGy total body irradiation from a 137Cs source as a split dose with a 3-hour interval between doses). In GVT experiments animals received tumor cells intravenously in a separate injection on day 0. BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Mice were housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated drinking water (pH 3.0).
Assessment of GVHD and GVT, BLI
Survival was monitored daily. A clinical GVHD score was generated weekly as previously described,37 and mice scoring 5 or greater were killed. The cause of death (tumor versus GVHD) was determined by necropsy and histopathology as previously described.38
For quantitative assessment of P815 TGL or A20 TGL tumor burden by BLI, mice received an intraperitoneal injection of 3 mg/mouse D-Luciferin (Xenogen, Alameda, CA). Fifteen minutes later, mice were anaesthetized and placed into the IVIS bioluminescence imaging system (Xenogen) and bioluminescent signal intensity was recorded. Pseudocolor images showing the whole body distribution of bioluminescent signal intensity were superimposed on conventional grayscale photographs and total flux (photons/sec) was determined for individual mice.
Histopathologic analysis, analysis of thymic cellularity, and complete blood counts (CBCs)
Mice were killed at days 21 and 55 after BMT for blinded histopathologic analysis of GVHD target organs (small and large bowel, liver, and skin). Organs were harvested, formalin-preserved, and subsequently paraffin-embedded, sectioned, and hematoxylin and eosin-stained by K. La Perle (Cornell University Medical College, New York, NY). Gut and liver samples were analyzed by C.L., and a semiquantitative score consisting of 19 to 22 different parameters associated with GVHD was calculated.39 Skin samples were examined by G.F.M., and the number of apoptotic cells per millimeter of epidermis was determined.40 To assess thymic damage, thymic cellularity and T-cell subset distribution was analyzed at day 21 after BMT. To assess damage to the bone marrow, CBCs were determined at 1, 3, 5, and 9 weeks after BMT on a Hemavet 950FS cell counter (Drew Scientific, Oxford, CT).
Assessment of donor CD8+ T-cell target-organ infiltration
For assessment of CD8+ T-cell target-organ infiltration, lethally irradiated C3FeB6F1 recipients were transplanted with TCD WT (B6-Ly5.1) BM cells and 2 × 106 WT (B6-Thy1.1) CD8+ T cells mixed with 2 × 106 CCR2-/- CD8+ T cells. Organs (liver, small bowel, spleen, mesenteric lymph nodes, peripheral lymph nodes, Peyer patches) were harvested at days 6, 14, and 28 after BMT. Spleen, liver, and lymph nodes were processed by homogenizing between frosted microslides and liver mononuclear cells were subsequently enriched by centrifugation over a Percoll gradient. The small bowel was dissected from the gastric-duodenal to the ileo-cecal junction, Peyer patches were removed, and the gut was cut longitudinally and washed. Subsequently, gut pieces were incubated on a shaker at 37°C in intraepithelial lymphocyte (IEL) separation media containing Hanks balanced salt solution (HBSS), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM EDTA (ethyleneediamine-tetraacetic acid), and 10% fetal bovine serum (FBS). After 20 minutes of incubation, gut pieces were vortexed, and IEL containing supernatant was transferred into a new tube. This procedure was repeated twice. For all organs, the percentage of infiltrating WT and CCR2-/- CD8+ donor T cells was determined by FACS analysis of CD8, Thy1.1, Ly5.1, and H-2Kk expression.
Intracellular cytokine staining
To measure intracellular cytokine production of in vivo activated donor T cells, splenocytes were harvested on day 7 from BMT recipients and subsequently restimulated with irradiated (2000 cGy) syngeneic (B6-Ly5.1) or allogeneic (C3FeB6F1) TCD stimulators in the presence of Brefeldin (Sigma, Saint Louis, MO). After 15 hours, cells were stained with (surface) fluorochrome-conjugated antibodies, fixed, and permeabilized with the Cytofix/Cytoperm Kit (Pharmingen). Cells were then stained with anti–IFN-γ or anti–TNF-α phycoerythrin (PE)–conjugated antibodies.
Enzyme-linked immunosorbent assay (ELISA)
Peripheral blood was obtained on day 7, 14, and 21 from mice in GVHD experiments. Serum was separated, and the concentration of IFN-γ was determined by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
CFSE staining
T cells were positively selected by magnetic cell sorting (MACS) from red blood cell (RBC)–lysed splenocytes and subsequently stained with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR). Transplanted into sublethally (900 cGy) irradiated allogeneic (C3FeB6F1) recipients were 10 to 20 × 106 stained cells. Recipient splenocytes were harvested 72 hours later and analyzed with FACS.
Mixed lymphocyte reaction (MLR)
CD8+ splenic T cells from WT and CCR2-/- mice were isolated by MACS, and 1 × 105 CD8+ T cells were incubated with 2 × 105 irradiated (2000 cGy) RBC-lysed allogeneic (C3FeB6F1) splenocytes for 5 days. One micro-Curie [0.037 MBq]/well of [3H]thymidine was added for the last 18 hours of stimulation. Cells were harvested with a Filtermate 196 harvester (Packard, Meridan, CT) and after addition of Microscint-20 scintillation fluid (Packard), counts per minute were measured with a Topcount NXT microplate scintillation counter (Packard). The basal proliferation rate of T cells without splenic stimulators was subtracted from specific proliferation against allogeneic stimulators.
Cytotoxicity assay
Effector splenocytes were harvested on day 7 from recipients of an allogeneic BMT. Target cells (32Dp210 and P815) were labeled for 2 hours with 100 μCi [3.7 MBq] 51Cr at 37°C. Labeled targets were plated at 5 × 103 cells/well in 96-well plates. Effector cells were added at various effector-target ratios, and plates were incubated for 4 hours at 37°C. Subsequently, 30 μL supernatant was removed from each well and counted in a Topcount NXT microplate scintillation counter (Packard). Spontaneous release was obtained from wells receiving target cells and medium only, and total release was obtained from wells receiving 10% Triton X-100 (Sigma). Percentage cytotoxicity was calculated by the following formula: % cytotoxicity = 100 × (experimental release - spontaneous release)/(total release - spontaneous release).
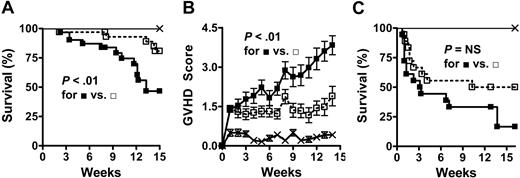
CCR2-/- CD8+ T cells induce less GVHD morbidity and mortality than WT CD8+ T cells. (A,B) Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells alone (×; n = 12) or in combination with 3 to 4.5 × 106 WT (▪; n = 31) or CCR2-/- (□; n = 33) CD8+ T cells. Data represent 3 combined experiments. (A) Kaplan-Meier survival curve. (B) Clinical GVHD score (mean ± SEM). (C) Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells alone (×;n = 9) or in combination with 2 × 106 WT (▪; n = 18) or CCR2-/- (□; n = 18) splenic T cells. Data represent 2 combined experiments. NS indicates not significant.
CCR2-/- CD8+ T cells induce less GVHD morbidity and mortality than WT CD8+ T cells. (A,B) Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells alone (×; n = 12) or in combination with 3 to 4.5 × 106 WT (▪; n = 31) or CCR2-/- (□; n = 33) CD8+ T cells. Data represent 3 combined experiments. (A) Kaplan-Meier survival curve. (B) Clinical GVHD score (mean ± SEM). (C) Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells alone (×;n = 9) or in combination with 2 × 106 WT (▪; n = 18) or CCR2-/- (□; n = 18) splenic T cells. Data represent 2 combined experiments. NS indicates not significant.
Statistics
All values shown in graphs represent the mean ± standard error of the mean (SEM). All groupwise comparisons except for the migration, survival, and GVHD score analysis were performed with the nonparametric unpaired Mann-Whitney U test. To assess differences between groups in the donor CD8+ T-cell target organ infiltration analysis, a permutation test based on the Wilcoxon rank sum statistic was used. Survival data were analyzed with the Mantel-Cox log-rank test. For GVHD scores the area under the curve (AUC) was used to summarize the GVHD trajectory of each mouse under study. Not all the mice were followed for the full length of the study (due to death or killing), and to eliminate this bias in the calculation of statistical significance, the AUCs were calculated up to the minimum follow-up time for each pairwise difference as described by Vardi et al.41 In the graphical presentation of GVHD scores, deceased mice were included with a score of 5. P < .05 was considered statistically significant. Only statistical significant differences are annotated in the graphs.
Results
CCR2-/- CD8+ T cells induce less GVHD morbidity and mortality than WT CD8+ T cells
To assess the role of CCR2 expression on allogeneic donor CD8+ T cells in the development of GVHD, we used a well-described major histocompatibility complex (MHC)–mismatched murine bone marrow transplantation model where lethally irradiated C3FeB6F1 hosts were transplanted with T-cell–depleted B6 WT bone marrow (BM) and purified CD8+ T cells from WT or CCR2-/- mice. This model allows the selective analysis of the effects of CCR2 deficiency on donor CD8+ T-cell migration and GVHD development without any confounding effects of CCR2 deficiency on host or donor BM-derived cells.
We found that recipients of CCR2-/- CD8+ T cells had a significant survival advantage over recipients of WT CD8+ T cells (Figure 1A) and that this correlated with a significant decrease in clinical GVHD scores (Figure 1B). When BMT experiments with unselected splenic T cells (ie, containing both CD4+ and CD8+ T cells) were performed, only a less prominent, nonsignificant survival advantage of CCR2-/- T-cell recipients was observed (Figure 1C). These results demonstrate that CCR2 is an important molecule for the function of CD8+ donor T cells during GVHD, while an additional role of CCR2 for CD4+ T cells appears unlikely.
CCR2-/- CD8+ T cells cause less intestinal and hepatic GVHD than WT CD8+ T cells, while no difference is observed for cutaneous and thymic GVHD and GVHD-associated pancytopenia
Histopathologic analysis of skin, liver, small and large bowel, flow cytometric analysis of the thymus, and complete peripheral blood counts were performed to determine if the reduced morbidity and mortality of CCR2-/- CD8+ T-cell recipients could be attributed to reduced damage to specific GVHD target organs and could therefore point toward any possible organ specifity of CCR2 in GVHD. Organs were harvested at indicated time points (Figure 2) from lethally irradiated C3FeB6F1 mice that received TCD WT BM together with WT or CCR2-/- CD8+ T cells. Intestinal and hepatic GVHD was assessed using a semiquantitative score consisting of 19 to 22 different parameters associated with GVHD,39 and cutaneous GVHD by determining the number of apoptotic keratinocytes per millimeter epidermis40 as previously described.
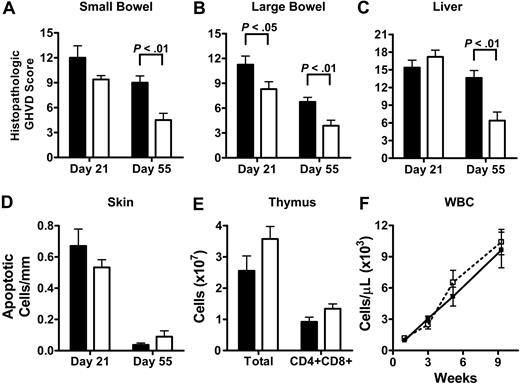
We found that recipients of CCR2-/- CD8+ T cells developed significantly less histopathologic damage of the large bowel at day 21 and of the small bowel, large bowel, and liver at day 55 (Figure 2A-C). However, when skin GVHD was analyzed, no significant differences for recipients of WT and CCR2-/- CD8+ T cell could be detected (Figure 2D). In order to assess thymic damage, thymic cellularity was analyzed by flow cytometry on day 21. GHVD is associated with a decrease in overall thymic cellularity as well as a decrease in the percentage of CD4/CD8 double-positive thymocytes (CD4+/CD8+).42 Again, no significant differences could be observed between recipients of WT and CCR2-/- CD8+ T cells (Figure 2E). GVHD is associated with pancytopenia, and to assess the effects of CCR2 deficiency of donor CD8+ T cells on the recovery of white blood cells, red blood cells, and platelets, CBCs were determined 1, 3, 5, and 9 weeks after BMT. No significant differences were observed for total peripheral blood white blood cell (WBC) counts and all other CBC parameters (Figure 2F and data not shown).
These results show that CCR2-/- CD8+ T cells induce less intestinal and hepatic GVHD, while cutaneous and thymic GVHD and GVHD-associated pancytopenia are not reduced. These data suggest that alloreactive CCR2-/- CD8+ T cells indeed have organ-specific functional defects during the development of GVHD.
CCR2-/- CD8+ T cells cause less gut and liver damage than WT CD8+ T cells while no difference is observed for skin, thymus, and bone marrow. Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells in combination with 3 to 4.5 × 106 WT (▪) or CCR2-/- (□) CD8+ T cells. (A-C) Small bowel, large bowel, and liver were taken on day 21 and day 55. Hematoxylin and eosin–stained slides were analyzed and scored for histopathologic damage. Shown is the mean ± SEM for 8 to 10 mice per group per time point. (D) Skin GHVD was determined on day 21 and day 55 by the number of apoptotic keratinocytes/mm epidermis. Shown is the mean ± SEM for 8 to 10 mice per group per time point. (E) Thymic cellularity (total cell counts and CD4+CD8+ thymocytes) was determined by FACS on day 21. Shown is the mean ± SEM for 7 mice per group. (F) Peripheral blood was obtained at weeks 1, 3, 5, and 9 after BMT and CBCs were determined. Shown is the mean ± SEM for WBC counts for 7 to 10 mice per group per time point.
CCR2-/- CD8+ T cells cause less gut and liver damage than WT CD8+ T cells while no difference is observed for skin, thymus, and bone marrow. Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT BM cells in combination with 3 to 4.5 × 106 WT (▪) or CCR2-/- (□) CD8+ T cells. (A-C) Small bowel, large bowel, and liver were taken on day 21 and day 55. Hematoxylin and eosin–stained slides were analyzed and scored for histopathologic damage. Shown is the mean ± SEM for 8 to 10 mice per group per time point. (D) Skin GHVD was determined on day 21 and day 55 by the number of apoptotic keratinocytes/mm epidermis. Shown is the mean ± SEM for 8 to 10 mice per group per time point. (E) Thymic cellularity (total cell counts and CD4+CD8+ thymocytes) was determined by FACS on day 21. Shown is the mean ± SEM for 7 mice per group. (F) Peripheral blood was obtained at weeks 1, 3, 5, and 9 after BMT and CBCs were determined. Shown is the mean ± SEM for WBC counts for 7 to 10 mice per group per time point.
Migration of CCR2-/- CD8+ T cells into gut and liver during GVHD is reduced
We hypothesized that the decreased GVHD morbidity and mortality and the decreased intestinal and hepatic GVHD in recipients of CCR2-/- CD8+ T cells was due to a migration defect of CCR2-/- CD8+ T cells into these organs. Therefore, we assessed donor CD8+ T-cell target organ infiltration with an assay where lethally irradiated C3FeB6F1 mice were transplanted with TCD WT (B6-Ly5.1) BM and a mix of equal numbers of WT (B6-Thy1.1) and CCR2-/- CD8+ (B6-Thy1.2) T cells. Organs (liver, small bowel intraepithelial lymphocytes [IEL], mesenteric lymph nodes [MLNs], peripheral lymph nodes [PLNs], Peyer patches [PP]) were harvested at 6, 14, and 28 days after BMT, and the percentage of infiltrating WT and CCR2-/- CD8+ donor T cells (excluding BM-derived CD8+ T cells) was determined by FACS analysis of CD8, Thy1.1, Ly5.1, and H-2Kk expression.
We found that CCR2-/- CD8+ T cells showed intact or slightly increased accumulation in the secondary lymphoid organs on day 6 after transplant, whereas the infiltration of CCR2-/- CD8+ T cells into the liver was slightly reduced at this time point (Figure 3A-F). A more prominent reduction of infiltrating CCR2-/- CD8+ T cells in the liver was seen on days 14 and 28 and in the small bowel on day 14 (Figure 3A,B). Although a small decrease in migration of CCR2-/- CD8+ T cells to spleen and PLN was observed on day 14, the infiltration patterns for the spleen, PLN, MLN, and PP showed a significant accumulation of CCR2-/- CD8+ T cells on day 28, which might be due to selective trapping in these organs (Figure 3A-F). These results indicate that CCR2 is important for CD8+ T-cell migration to the gut and liver during GVHD.
Alloreactive CCR2-/- CD8+ T cells have intact proliferation, activation, IFN-γ production, and cytolytic activity
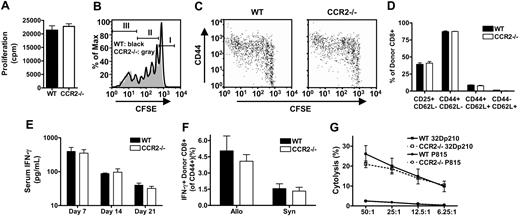
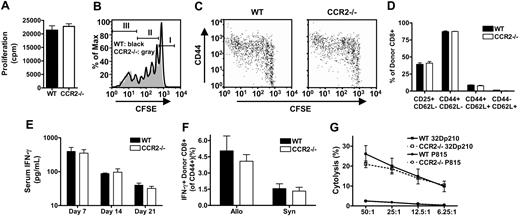
To analyze if alloreactive CCR2-/- CD8+ T cells have any other intrinsic defects besides reduced migration to the gut and liver during GVHD, we determined if proliferation, activation, cytokine production, or cytotoxicity of CCR2-/- CD8+ T cells were intact. In an MLR against irradiated allogeneic C3FeB6F1 splenocytes CCR2-/- CD8+ T cells did not have any defect in proliferation against alloantigens in vitro (Figure 4A). Intact expansion of alloreactive CD8+ T cells in vivo also was demonstrated by infusing CFSE-labeled WT or CCR2-/- splenic T cells into sublethally irradiated allogeneic C3FeB6F1 hosts. Proliferation kinetics were evaluated 72 hours after adoptive transfer (Figure 4B), showing similar proportions of nonproliferative cells (CFSEhigh, Figure 4B, population I), slow-proliferative cells undergoing homeostatic expansion (CFSEint, Figure 4B, population II), and fast-proliferative allo-activated CD8+ T cells (CFSElow, Figure 4B, population III).43 Also, activation of alloreactive CCR2-/- CD8+ T cells as determined by CD44 up-regulation on the fast-proliferative allo-activated population was comparable to WT CD8+ T-cell activation (Figure 4C). These data were confirmed by examining the percentage of activated (CD44hiCD62Llow) donor CD8+ T cells in the spleen, small bowel, liver, MLN, and PP of BMT recipients with GVHD (Figure 4D and data not shown). The analysis of serum samples obtained from lethally irradiated C3FeB6F1 recipients of TCD WT BM plus WT or CCR2-/- CD8+ T cells showed similar IFN-γ levels at 3 different time points (Figure 4E). Also, intracellular IFN-γ production of alloreactive T cells in the spleen of allogeneic HSCT recipients one week after BMT was comparable for recipients of WT or CCR2-/- CD8+ T cells (Figure 4F). Intracellular TNF-α was also similar for WT and CCR2-/- CD8+ T cells, but overall expression levels were low (data not shown). Finally, cytotoxic activity of alloreactive CCR2-/- CD8+ T cells was intact as measured in a 51Cr release assay of in vivo–activated CCR2-/- CD8+ T cells (versus WT CD8+ T cells) against a syngeneic tumor cell line and a third party control (Figure 4G).
Migration of CCR2-/- CD8+ T cells into gut and liver during GVHD is reduced. Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT (B6-Ly5.1) BM cells with a mix of 2 × 106 WT (B6-Thy1.1) and 2 × 106 CCR2-/- CD8+ T cells. Organs were harvested at days 6, 14, and 28 after BMT and analyzed with FACS. (A) Small bowel IEL. (B) Liver. (C) Spleen. (D) PLN. (E) MLN. (F) PP. Data represent the mean ± SEM of the percentage of infiltrating WT (▪) or CCR2-/- (□) CD8+ T cells of all donor CD8+ T cells excluding BM-derived CD8+ T cells. Eight mice were analyzed per time point.
Migration of CCR2-/- CD8+ T cells into gut and liver during GVHD is reduced. Lethally irradiated (1300 cGy) C3FeB6F1 mice received 5 × 106 TCD WT (B6-Ly5.1) BM cells with a mix of 2 × 106 WT (B6-Thy1.1) and 2 × 106 CCR2-/- CD8+ T cells. Organs were harvested at days 6, 14, and 28 after BMT and analyzed with FACS. (A) Small bowel IEL. (B) Liver. (C) Spleen. (D) PLN. (E) MLN. (F) PP. Data represent the mean ± SEM of the percentage of infiltrating WT (▪) or CCR2-/- (□) CD8+ T cells of all donor CD8+ T cells excluding BM-derived CD8+ T cells. Eight mice were analyzed per time point.
Alloreactive CCR2-/- CD8+ T cells have intact proliferation, activation, IFN-γ production, and cytolytic activity. (A) MLR with WT and CCR2-/- CD8+ T-cell effectors and irradiated C3FeB6F1 stimulators. Bars represent mean ± SEM for specific proliferation of 12 replicate wells from one representative experiment out of 3; cpm indicates counts per minute. (B,C) Sublethally irradiated (900 cGy) C3FeB6F1 mice received CFSE-labeled WT or CCR2-/- T cells. Recipient spleens were harvested after 72 hours for FACS analysis. Data shown are from one representative mouse out of 4 mice from 2 experiments. (B) Histogram overlays for CFSE-labeled WT (black line) and CCR2-/- (gray area) donor CD8+ T cells. Markers indicate nonproliferative cells (I), slow-proliferative cells (II), and fast-proliferative T cells (III). (C) Dot plots of CFSE and CD44 expression on WT and CCR2-/- donor CD8+ T cells. (D-G) Lethally irradiated (1300 cGy) C3FeB6F1 received 5 × 106 TCD WT BM cells in combination with 3 × 106 WT (▪) or CCR2-/- (□) CD8+ T cells. (D) Recipient spleens were harvested at day 7 for FACS analysis of CD25, CD44, and CD62L expression. Shown is the mean ± SEM for 4 mice per group. (E) Serum cytokine levels were measured by ELISA at indicated time points. Shown is the mean ± SEM for 8 to 12 mice per group per time point. (F) Recipient spleens were harvested at day 7 and restimulated for 12 hours with irradiated TCD C3FeB6F1 splenocytes. Cells were subsequently stained for intracellular IFN-γ. Shown is the mean ± SEM for 4 mice per group. (G) Mice were killed on day 7, and splenocytes were used as effectors in a 51Cr cytotoxicity assay. Targets were allogeneic 32Dp210 and third party P815. Shown is the mean specific lysis ± SEM for 4 mice per group.
Alloreactive CCR2-/- CD8+ T cells have intact proliferation, activation, IFN-γ production, and cytolytic activity. (A) MLR with WT and CCR2-/- CD8+ T-cell effectors and irradiated C3FeB6F1 stimulators. Bars represent mean ± SEM for specific proliferation of 12 replicate wells from one representative experiment out of 3; cpm indicates counts per minute. (B,C) Sublethally irradiated (900 cGy) C3FeB6F1 mice received CFSE-labeled WT or CCR2-/- T cells. Recipient spleens were harvested after 72 hours for FACS analysis. Data shown are from one representative mouse out of 4 mice from 2 experiments. (B) Histogram overlays for CFSE-labeled WT (black line) and CCR2-/- (gray area) donor CD8+ T cells. Markers indicate nonproliferative cells (I), slow-proliferative cells (II), and fast-proliferative T cells (III). (C) Dot plots of CFSE and CD44 expression on WT and CCR2-/- donor CD8+ T cells. (D-G) Lethally irradiated (1300 cGy) C3FeB6F1 received 5 × 106 TCD WT BM cells in combination with 3 × 106 WT (▪) or CCR2-/- (□) CD8+ T cells. (D) Recipient spleens were harvested at day 7 for FACS analysis of CD25, CD44, and CD62L expression. Shown is the mean ± SEM for 4 mice per group. (E) Serum cytokine levels were measured by ELISA at indicated time points. Shown is the mean ± SEM for 8 to 12 mice per group per time point. (F) Recipient spleens were harvested at day 7 and restimulated for 12 hours with irradiated TCD C3FeB6F1 splenocytes. Cells were subsequently stained for intracellular IFN-γ. Shown is the mean ± SEM for 4 mice per group. (G) Mice were killed on day 7, and splenocytes were used as effectors in a 51Cr cytotoxicity assay. Targets were allogeneic 32Dp210 and third party P815. Shown is the mean specific lysis ± SEM for 4 mice per group.
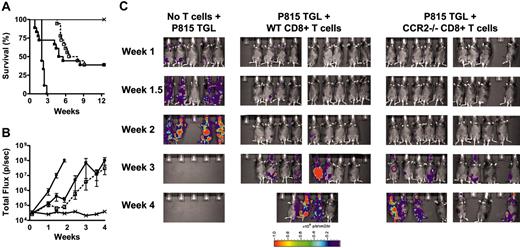
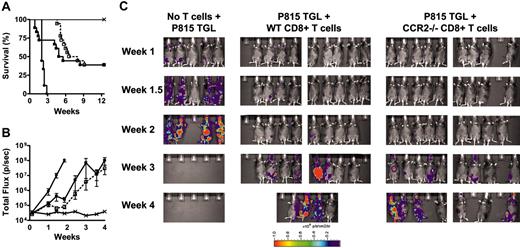
Graft-versus-tumor effect of CCR2-/- CD8+ T cells is intact. (A) B6D2F1 mice were lethally irradiated and subsequently received 5 × 106 TCD WT BM cells alone (×; n = 9), TCD WT BM and 1 × 103 P815 mastocytoma cells (•; n = 9), or TCD WT BM, 1 × 103 P815 mastocytoma cells and 3 × 106 WT (▪; n = 18) or CCR2-/- (□; n = 18) CD8+ T cells. Survival was monitored daily; see Table 1 for causes of death. Data represent 2 combined experiments. (B,C) B6D2F1 mice were lethally irradiated and subsequently received 5 × 106 TCD WT BM cells alone (×; n = 5), TCD WT BM and 5 × 103 P815 TGL mastocytoma cells (•; n = 5), or TCD WT BM, 5 × 103 P815 TGL mastocytoma cells and 3 × 106 WT (▪; n = 10), or CCR2-/- (□; n = 10) CD8+ T cells. Whole-body bioluminescent signal intensity was determined twice weekly, and mean ± SEM of total flux is shown.
Graft-versus-tumor effect of CCR2-/- CD8+ T cells is intact. (A) B6D2F1 mice were lethally irradiated and subsequently received 5 × 106 TCD WT BM cells alone (×; n = 9), TCD WT BM and 1 × 103 P815 mastocytoma cells (•; n = 9), or TCD WT BM, 1 × 103 P815 mastocytoma cells and 3 × 106 WT (▪; n = 18) or CCR2-/- (□; n = 18) CD8+ T cells. Survival was monitored daily; see Table 1 for causes of death. Data represent 2 combined experiments. (B,C) B6D2F1 mice were lethally irradiated and subsequently received 5 × 106 TCD WT BM cells alone (×; n = 5), TCD WT BM and 5 × 103 P815 TGL mastocytoma cells (•; n = 5), or TCD WT BM, 5 × 103 P815 TGL mastocytoma cells and 3 × 106 WT (▪; n = 10), or CCR2-/- (□; n = 10) CD8+ T cells. Whole-body bioluminescent signal intensity was determined twice weekly, and mean ± SEM of total flux is shown.
These results indicate that the observed differences in morbidity, mortality, histopathology, and organ infiltration are due only to a migratory defect of alloreactive CCR2-/- CD8+ T cells and not due to defects in proliferation, activation, cytokine production, or cytotoxicity.
Graft-versus-tumor effect of CCR2-/- CD8+ T cells is intact
To test whether CCR2 deficiency of donor CD8+ T cells reduces GVT activity, we used a well-characterized GVHD/GVT model (B6→B6D2F1 with P815 mastocytoma). Lethally irradiated B6D2F1 mice received allogeneic TCD BM alone or in combination with WT or CCR2-/- CD8+ T cells. Mice also received a dose of 1 × 103 P815 mastocytoma cells in a separate injection and were monitored daily for survival. Cause of death (tumor versus GVHD) was determined by necropsy and histopathology as previously described.38 The administration of P815 mastocytoma cells was lethal within 20 days for all mice that received TCD, BM, and no T cells. However, overall mortality was reduced to about 60% if WT or CCR2-/- CD8+ T cells were added, suggesting an intact GVT effect of CCR2-/- CD8+ T cells (Figure 5A). Of note, recipients of WT CD8+ T cells showed significant mortality due to GVHD early after transplant (Table 1), whereas recipients of CCR2-/- CD8+ T cells did not die from GVHD until week 6, again demonstrating the reduced ability of CCR2-/- CD8+ T cells to induce lethal GVHD.
To better determine the effects of CCR2-/- versus WT CD8+ T cells on tumor growth, we performed GVT experiments with the newly established P815 TGL cell line, which expresses firefly luciferase under the viral LTR promoter and allows quantification of tumor burden by BLI.36 Figure 5B shows the time course of bioluminescent signal intensity, and Figure 5C shows pseudocolor images of bioluminescent signal intensity overlaid on conventional photographs. Both figures demonstrate that the GVT effect of CCR2-/- CD8+ T cells is preserved. Similar results were obtained in a B6→BALB/c model with A20 TGL tumor cells (data not shown). These results show that the GVT activity of CCR2-/-CD8+ T cells is intact.
Discussion
The important role of alloreactive donor CD8+ T cells in the development of GVHD and GVT is well described.2,4,44,45 In this study, we demonstrate that alloreactive CD8+ T cells deficient for the inflammatory chemokine receptor CCR2 induce less GVHD morbidity and mortality than WT CD8+ T cells, while their GVT activity is preserved. This decrease in GVHD activity is associated with an intrinsic migratory defect of CCR2-/- CD8+ T cells to the gut and liver and reduction in histopathologic damage to these organs.
It has previously been shown that chemokine receptors may specifically control the migration of alloreactive donor CD8+ T cells into different target organs during experimental GVHD. For example, it was demonstrated that CCR5 and its main ligand CCL3 are expressed at high levels in the liver during GVHD and that blockade of CCR5 with a monoclonal antibody leads to reduced liver infiltration by CD8+ T cells.46 CCR5 is also critical for the migration of donor CD8+ T cells into Peyer patches and for the subsequent induction of GVHD.47 Another study described CXCR3 as an important molecule for donor CD8+ T-cell infiltration into the small bowel, the development of histopathologic damage in liver and gut, and overall morbidity and mortality.48 However, the incomplete inhibition of GVHD by interference with CCR5 and CXCR3 and the redundancy of the chemokine system49 suggest that CD8+ T-cell migration into GVHD target organs is under the influence of more than one molecule.
In solid organ transplantation models, it was shown that CCL2 and CCR2 are up-regulated in allografts and that transplantation into CCR2-/- mice reduces leukocyte infiltration and prolongs graft survival.50-52 In murine allogeneic BMT models it was shown that CCL2 is expressed in the liver,13 skin,14 lung,15 and gut (M.R.M.v.d.B., unpublished observations, May 2001) early after transplantation and that CCL2 levels correlate with the severity of cellular infiltrates in the lung.15 A recently published clinical study on chemokine receptor gene expression after allogeneic HSCT found that CCR2 was up-regulated in the peripheral blood prior to the development of GVHD and might therefore be a valuable molecular marker to diagnose or monitor the disease.53 Studies in a sublethal graft-versus-host reaction (but not GVHD) model concluded that CCR2 expression was relevant for the function of a donor non–T-cell population with regulatory properties.54 In a study that analyzed the role of CCR2 expression on donor cells in murine idiopathic pneumonia syndrome and GVHD, it was shown that irradiated mice reconstituted with CCR2-/- BM and CCR2-/- T cells developed less IPS, but that hepatic and intestinal damage and overall mortality were not reduced.16 This study also described a significant reduction of CD8+ T-cell but not CD4+ T-cell numbers in the bronchoalveolar lavage when CCR2-/- T cells were administered. Together with data from a recent study that found that WT and CCR2-/-CD4+ T cells were equally potent to induce GVHD,54 this suggests a more prominent role of CCR2 on CD8+ donor T cells than on CD4+ donor T cells. In agreement with this hypothesis, we found that the administration of enriched CCR2-/- CD8+ T cells lead to an amelioration of GVHD, whereas the administration of CCR2-/- unselected T cells (ie, CD4+ and CD8+ T cells) did not lead to a significant reduction in overall morbidity and mortality. However, it also is possible that CCR2 is required for the function of CD4+CD25+ regulatory T cells (as recently suggested in a murine arthritis model55 ), and this might account for the observed CD8 bias. In this context it would be interesting to compare the potency of WT and CCR2-/- donor CD4+CD25+ regulatory T cells in modulating the immune response after allogeneic BMT.
We found that CCR2-/- CD8+ T cells exhibited reduced infiltration of the gut and liver and caused less histopathologic damage in these organs, which finally lead to reduced overall GVHD morbidity and mortality. The fact that we did not observe a significant reduction of histopathologic damage to the skin in recipients of CCR2-/- CD8+ T cells despite expression of CCL2 might be due to the large number of chemokines up-regulated in this organ early after transplantation (including CXCL-1, -2, -9, -10, -11; CCL-2, -3, -5, -6, -7, -8, -9, -11, -19, -20),14,56 which might lead to a major redundancy in chemokine-triggered T-cell migration. In this context, a central role of CCL20-CCR6 signaling for T-cell migration into the skin after allogeneic BMT recently has been discovered.56 We excluded that the reduction of CCR2-/- CD8+ T-cell infiltrates in the gut and liver was due to a defective alloresponse of CCR2-/- CD8+ T cells early after transplantation by showing intact accumulation of CCR2-/- CD8+ T cells in secondary lymphoid organs as well as intact activation and clonal expansion in response to alloantigen. We also excluded that the observed differences for organ damage and GVHD severity were due to defective effector functions of CCR2-/- CD8+ T cells by showing intact cytokine production and cytotoxicity. Earlier studies had found defects in T-cell differentiation and Th1 cytokine production in CCR2-/- mice during some inflammatory conditions,20,57,58 but other reports support our data by demonstrating that the observed defects were not intrinsic to CCR2-/- T cells but rather secondary to DC recruitment deficiencies.16,32,33
To our knowledge, this is the first report showing a role for CCR2 in in vivo T-cell migration in a preclinical disease model. Other studies were either confounded by effects of CCR2 deficiency on other cell types or did not demonstrate a significant difference.25,29,32,33 For example, CCR2-/- mice have an increased susceptibility to M. tuberculosis, and this is accompanied by a reduced infiltration of macrophages, DCs, and T cells.24 However, the reduced T-cell numbers were most likely secondary to CCR2-dependent macrophage and DC recruitment deficiencies and not caused by a primary T-cell defect.25 Similarly, in a model of experimental autoimmune encephalomyelitis (EAE), CCR2-/- mice developed less cellular infiltrates in the spinal cord and developed less disease, but sorted antigen-restimulated T cells taken from CCR2-/- mice were still able to induce disease and form infiltrates in WT mice.29
However, our data are in agreement with in vitro assays that clearly demonstrate that activated T cells express high levels of CCR2,59,60 that these cells migrate toward CCL2,11,59 and that this migration is abrogated in T cells from CCR2-/- mice.19
A major goal for GVHD prevention and therapy is the specific blockade of T-cell functions that mediate GVHD while maintaining the beneficial effects of T-cell–mediated GVT activity. We found that absence of CCR2 did not reduce the GVT effect of CD8+ T cells against P815 mastocytoma and A20 B-cell lymphoma cells as demonstrated by analysis of survival and bioluminescence imaging. P815 and A20 tumors tend to form infiltrates in lymphohematopoietic organs including liver, spleen, lymph nodes, and bone marrow,61-63 and we speculate that the intact GVT activity of CCR2-/- CD8+ T cells is due to intact or even increased (as seen for the secondary lymphoid organs on day 28 of our studies) accumulation at most of these sites. The fact that the reduced potential of CCR2-/- CD8+ T cells to infiltrate the liver did not have a major impact on overall GVT activity suggests that the remaining cells were potent enough to target liver infiltrating tumor cells. Trapping of lymphocytes in secondary lymphoid organs, reduced organ infiltration, and subsequent amelioration of GVHD with intact GVT was previously described.6,38 Whether CCL2-CCR2 signaling is required for the trafficking of leukocytes into solid tumors and tumor rejection is still debated,64 and it would be interesting to examine the GVT effect against solid tumors.
In summary, we found that CCR2 is important for migration of alloreactive CD8+ T cells to the gut and liver and induction of GVHD while other T-cell functions and GVT are not affected. This establishes the rationale for the use of CCR2 antagonists possibly in combination with other chemokine receptor antagonists as novel therapeutic tools in GVHD.9,65-67
Prepublished online as Blood First Edition Paper, July 21, 2005; DOI 10.1182/blood-2005-05-1860.
Supported by grants HL69929, HL72412, and CA107096 from the National Institutes of Health and awards from the Emerald Foundation and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center, funded by William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research (M.R.M.v.d.B.); the Deutscher Akademischer Austauschdienst and Boehringer Ingelheim Fonds (T.H.T.); a Mildred Scheel-Stipendium from the Deutsche Krebshilfe (T.D.K.); and the Dr Werner Jackstaedt-Stiftung (J.L.Z.).
T.H.T. designed and performed research, contributed new reagents, analyzed data, and wrote the paper; T.D.K. performed research and wrote the paper; A.A.K, V.M.H., S.L., J.L.Z., T.R.-M., J.M.E., and S.J.M. performed research and analyzed data; G.H., G.F.M., and C.L. analyzed data, T.B.-A. designed research and contributed new reagents; O.A. designed and performed research; and M.R.M.v.d.B. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the staff of the Research Animal Resource Center for excellent animal care.