Abstract
Most primary central nervous system lymphomas (PCNSLs) in immunocompetent patients are diffuse large B-cell lymphomas (DLBCLs), characterized by poor prognosis, compared with systemic forms. A germinal center B-cell–like (GCB) origin of PCNSL was hypothesized on the basis of BCL-6 expression and ongoing mutational activity. Our goal herein was to determine, for 83 PCNSLs, the percentages of GCB and activated B-cell–like (ABC) phenotypes and their prognostic significance. CD10, BCL-6, MUM1, BCL-2, and CD138 antigens were immunohistochemically labeled on paraffin-embedded sections; the first 4 were positive in 2.4%, 55.5%, 92.6%, and 55.5% of the tumors, respectively. None of the 56 tested samples expressed CD138. Among the 82 patients with complete information, 79 (96.3%) were classified as ABC; 42 (51.2%) expressed BCL-6+MUM1+, suggesting an “activated GCB” origin; 33 (40.2%) were exclusively MUM1+, and the remaining 4 (4.9%) were negative for all markers tested. These findings provide new insights into interpreting the poor PCNSL prognostic, which may, in part, be due to biologic aggressiveness associated with its activated B-cell–like pattern. We postulate assigning PCNSL a histogenetic “time-slot,” overlapping late GC and early post-GC, that could explain the predominant ABC phenotype observed.
Introduction
According to the World Health Organization (WHO) classification,1 most primary central nervous system lymphomas (PCNSLs) are diffuse large B-cell lymphomas (DLBCLs). Unlike human immunodeficiency virus (HIV)–associated PCNSLs, these lymphomas developing in immunocompetent patients are not associated with Epstein-Barr virus (EBV)2 and occur usually over the age of 50 years. While the incidence of HIV-associated PCNSL has decreased dramatically over the past few years with the use of highly active antiretroviral therapies, the PCNSL incidence in immunocompetent patients has continued to rise for unexplained reasons.3
Systemic DLBCLs, the most common non-Hodgkin lymphomas, are known to be clinically and morphologically heterogeneous.4 Using the unsupervised learning technique of hierarchic clustering on customized cDNA microarrays (lymphochip), Alizadeh et al5 identified 2 main subgroups: those with expression patterns similar to normal GC B cells (germinal center B-cell–like, or GCB), and those whose phenotypes resembled that of in vitro–activated peripheral blood cells (activated B-cell–like, or ABC). In their pioneering paper, Alizadeh et al5 showed that GCB immunoprofile was associated with a significantly better overall outcome than ABC. Rosenwald et al6 confirmed their results, showing in an extended population that the GCB subgroup had a significantly higher likelihood of survival after chemotherapy than the ABC phenotype and the newly identified type 3 subgroup. In contrast, Shipp et al did not find any such correlation distinguishing between cell-of-origin and outcome in their analysis of 6817 genes. However, comparisons between the 2 studies are difficult because different genes were measured on the array with different microarray technologies and different computational approaches.7
Several studies attempted to confirm the prognostic reliability of these proteins, using a panel of immunohistochemical markers8-10 : 2 for the GC, CD10, and BCL-6; and 2 for activation, MUM1 (multiple myeloma-1/interferon regulatory factor-4) and CD138. Two of those studies8,9 showed that immunohistochemical classification (GCB versus ABC) also carried prognostic significance.
Primary DLBCLs arising in the brain are morphologically similar to systemic forms, and the WHO classification does not consider them to be separate diseases.1 Concerning their histogenesis, PCNSLs are thought to be of GC origin, based on their BCL-6 expression11 and ongoing mutational activity.12,13 Although PCNSL prognosis has improved the most over the past decade, it remains poor, with a 5-year overall survival (OS) rate of approximately 30% for the patients benefiting from the best treatment protocols.14-17 It is still unclear whether the poor outcome is attributable to the specific cerebral location or reflects an exceptionally aggressive biologic behavior of tumor cells.
The goal of this study of 83 patients with PCNSL was to determine the percentages of tumors expressing the GCB or ABC phenotype and their respective prognostic significance.
Patients, materials, and methods
Patients
Clinical data and tumor biopsy specimens from 83 PCNSLs were analyzed retrospectively. Thirty-eight (45.8%) patients had been included in 2 prospective multicenter clinical trials conducted by the GOELAMS (Groupe Ouest Est des Leucémies et Autres Maladies du Sang): SNC98 and LCP99. Forty-five (54.2%) patients enrolled in the Association des Neuro-Oncologues d'Expression Française (ANOCEF) protocol had been treated and followed at the Pitié-Salpêtrière Hospital, Neuro-oncology Department (Pr J. Y. Delattre), and tumor specimens were provided by Pr J. J. Hauw (Department of Neuropathology, Laboratoire R. Escourolle). All patients received chemotherapy regimens based on high-dose methotrexate. Approval for the present study was obtained from the GOELAMS scientific board. All clinical protocols conducted by either GOELAMS or ANOCEF groups were done with informed consent, according to the Declaration of Helsinki.
Tumor specimens
All specimens were obtained for diagnosis, before starting chemotherapy and/or radiotherapy. All diagnoses of DLBCL were confirmed by histologic review (S.C.-B., A. Martin, A. Moreau) and were subclassified, when possible, according to the Kiel classification as proposed by the WHO classification.1 The B-cell phenotype of all samples was confirmed by anti-CD20 and/or CD79a antibody labeling.
For this study, patients were selected on the basis of the availability of fixed and paraffin-embedded tumor tissues. For GOELAMS specimens, immunohistochemistry was done on conventional sections and recorded prospectively during histologic review. For the ANOCEF specimens, 2 tissue microarray (TMA) blocks were constructed using the tissue arrayer (Beecher Instruments, Silver Spring, MD), with 3 or 4 representative cores per patient, 0.6 mm in diameter. In situ hybridization and immunohistochemistry were done on 4-μm thick sections, obtained from either conventional or TMA paraffin-embedded blocks.
In situ hybridization for EBV
In situ hybridization was performed in a routine manner, as previously described, on paraffin-embedded tissue, using fluorescein isothiocyanate (FITC)–labeled oligonucleotide complementary to the small nuclear EBV-encoded RNA1 (EBER PNA probe/FITC, Y5200; Dako, Glostrup, Denmark).18 For detection, a monoclonal mouse anti-FITC antibody (clone DAK-FITC4, M0878; Dako), rabbit antimouse antibodies (Z0259; Dako), and an alkaline phosphatase–antialkaline phosphatase (APAAP) mouse system (D0651; Dako) were applied successively.
Immunohistochemical analysis
Sections were deparaffinized and antigen retrieval was carried out by microwaving. Sections were then incubated with the working dilution of each antibody raised against the following antigens: BCL-2 (clone 124; Dako; diluted 1:50); CD10 (clone NCL-CD10-270; Novocastra; New-castle, United Kingdom; diluted 1:50); BCL-6 (clone PG-B6p; Dako; diluted 1:50); MUM1 (goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:50); CD138 (clone B-B4; Diaclone, Stamford, CT; diluted 1:50).
CD10 and BCL-2 labelings were visualized using a commercially available streptavidin-biotin-peroxidase kit (Abcys Biospa, Milan, Italy), and MUM1 with an antigoat avidin-biotin-peroxidase kit (Vector Laboratories, Peterborough, United Kingdom). BCL-6 and CD138 were labeled in an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's instructions.
Mature plasma cells and reactive T lymphocytes were used as internal positive controls for CD138 and BCL-2, respectively. Reactive lymph node and 2 TMA slides including 45 systemic DLBCLs were used as external positive controls for CD10.
Two pathologists (SCB, AMa), with no knowledge of clinical data, interpreted immunohistochemical labelings independently. For CD10, BCL-6, MUM1, and CD138, the labeling was analyzed visually, with estimation of 10% increments, according to the method published by Hans et al.8 Tumors with more than 30% positively labeled nuclei were considered positive. BCL-2 labeling was interpreted according to the method previously published for systemic DLBCL,19 that is, the specimen was considered positive when at least 50% of the tumor cells expressed the BCL-2 protein.
Classification of tumors as GCB or ABC
Tumors were subclassified according to their expression of GCB and ABC markers, using the methods published by Hans et al8 and Chang et al.9
Using a decision tree, the Hans classification distinguishes 2 subgroups with the following 3 markers: CD10, BCL-6, and MUM1.8 Briefly, the GCB subgroup includes all CD10+ tumors and those with a CD10-BCL-6+MUM1- immunophenotype. Other patients are assigned to the second group, the so-called non-GC, which includes MUM1+ tumors, regardless of their BCL-6 status (CD10-BCL-6+MUM1+ or CD10-BCL-6-MUM1+). According to this method, tumors expressing none of the 3 markers are classified as non-GC.
Chang's classification9 was based on 2 GCB markers, CD10 and BCL-6, and 2 activation markers, MUM1 and CD138. Their GCB pattern (called pattern A) is characterized by CD10 and/or BCL-6 positivity but no activation markers. Unlike the Hans classification, Chang et al split the second group into 2 distinct subgroups: the activated GCB pattern (their pattern B) expressing at least 1 GCB marker and 1 activation marker, and the activated non-GCB pattern (their pattern C), expressing activation markers (MUM1 and/or CD138) but none of the GCB proteins.
Statistical analysis
Clinical-biologic correlations were tested using the chi-square test (or Fisher exact test, when required) with the following categoric clinical variables: age (< 60 versus ≥ 60 years), sex, number of lesions (1 versus ≥ 2), involvement of deep brain structures (absence versus presence), Eastern Cooperative Oncology Group (ECOG) performance status (0-1 versus > 1), cerebrospinal fluid (CSF) cellularity (absence versus presence of tumor cells), lactate dehydrogenase (LDH) concentration (normal versus elevated), and histologic subtype (immunoblastic versus others).
OS was calculated from the date of PCNSL diagnosis until death or last follow-up visit. Failure-free survival (FFS) was calculated from the date of PCNSL diagnosis until death, recurrence, progression, or last follow-up visit. Survival rates were derived from Kaplan-Meier20 estimates with their 95% confidence interval (95% CI). Survival distributions were compared for univariate analysis by the log-rank test.21
Our PCNSLs expressing an ABC immunophenotype were compared with the ABC and type 3 systemic DLBCLs published by Rosenwald et al.6 The data from that study are posted on the internet with corresponding clinical and outcome data (http://llmpp.nih.gov/lymphoma/). All analyses were performed with the S-Plus software package (Insightfull, Seattle, WA). Only P values less than or equal to .05 were considered significant.
Results
Patients, conventional histology, and in situ hybridization for EBV
Patients' clinical characteristics are detailed in Table 1. The male-female sex ratio was 0.73, and mean age was 60 years (range, 23 to 80 years). Fifty (60.2%) specimens were from stereotaxic biopsies. Forty-one (49.4%) patients had multiple brain lesions, and 45 (54.9%) patients had involvement of deep structures (periventricular regions, basal ganglia, corpus callosum, brainstem and/or cerebellum). Lymphomatous cells were found in the CSF of 2 (3.1%) of 65 patients. The LDH concentration was elevated in 14 (26.4%) of 53 patients. Histologic review was able to assign a histologic subtype to 72 PCNSL: 65 (90.3%) of 72 centroblastic, and 7 (9.7%) of 72 immunoblastic. All 70 PCNSLs tested by in situ hybridization were EBER-1–mRNA negative.
Immunohistochemical labeling patterns illustrating the 3 PCNSL immunoprofiles. (A) GCB: CD10+BCL-6+MUM1-CD138-. (B,C) The 2 predominant ABC patterns, respectively: activated GCB-cell CD10-BCL-6+MUM1+CD138- and non–GCB-cell CD10-BCL-6-MUM1+CD138-. Images were observed using a LEITZ DMR microscope (Leica, Wetzlar, Germany) with a 20 ×/0.70 numeric aperture objective, operating with a 3CCD color video camera (DXC.93OP; Sony, Tokyo, Japan). Image acquisition was performed using Analysis software (SIS Soft Imaging Software, Reutlingen, Germany).
Immunohistochemical labeling patterns illustrating the 3 PCNSL immunoprofiles. (A) GCB: CD10+BCL-6+MUM1-CD138-. (B,C) The 2 predominant ABC patterns, respectively: activated GCB-cell CD10-BCL-6+MUM1+CD138- and non–GCB-cell CD10-BCL-6-MUM1+CD138-. Images were observed using a LEITZ DMR microscope (Leica, Wetzlar, Germany) with a 20 ×/0.70 numeric aperture objective, operating with a 3CCD color video camera (DXC.93OP; Sony, Tokyo, Japan). Image acquisition was performed using Analysis software (SIS Soft Imaging Software, Reutlingen, Germany).
Immunohistochemical results
The 3 immunohistochemical profiles are shown in Figure 1. The comparisons of antigen expression rates in our PCNSLs and previously published DLBCLs8-10 are reported in Table 2.
CD10 was positive in 2 (2.4%) of 82 PCNSLs, that is, 10 times lower than for systemic DLBCL (85 [26.4%] of 322) (P < .001). Our 2 CD10+ tumors had centroblastic morphology; both were BCL-6+, but 1 was MUM1+ and the other MUM1- (Figure 1A). PCNSL cell nuclear expression of BCL-6 was detected in 52 (64.2%) of 81 specimens, close to the cumulative values reported for systemic DLBCL (61.6%). Moreover, BCL-6+ tumor cells accounted for more than 30% of all tumor cells in 45 (55.5%) PCNSLs. More than 30% MUM1 positivity was found in 75 (92.6%) of 81 PCNSLs, a rate significantly higher than that reported for systemic DLBCL (161 [50.3%] of 320) (P < .001). Strong nuclear labeling in more than 50% of tumor cells was observed in 89.3% of MUM1+ PCNSL (67 of 75). BCL-6 and MUM1 were coexpressed in 42 (51.2%) PCNSLs (Figure 1B). Four cases were negative for all the markers tested. None of the 56 PCNSLs tested expressed CD138. BCL-2 expression was detected in 45 (55.6%) of 81 PCNSLs, comparable to the reported systemic DLBCL values (Table 2).
Classification in the GCB or ABC subgroup
For technical reasons (loss of few TMA cores), PCNSL assignment to the GCB or ABC subgroup could be assessed for 82 cases by immunohistochemical analysis. According to the Hans decision tree,8 3 (3.7%) of 82 were considered GCB and 79 (96.3%) of 82 were considered ABC (or non-GCB) (Table 3).
According to the Chang classification,9 77 PCNSLs could be classified: pattern A (GCB) was seen in 2 (2.6%) of 77, pattern B (activated GCB) in 43 (55.8%) of 77, and pattern C (activated non-GCB) in 32 (41.6%) of 77.
Clinical-biologic relationships
Because very few PCNSLs were CD10+, MUM1-, and CD138+, only the clinical-biologic correlations for BCL-6 and BCL-2 markers are given. BCL-6 expression was significantly associated with 2 clinical factors that are usually associated with poor prognosis: age older than 60 years (P = .01) and high ECOG performance status (P = .02). No significant relationship was found between BCL-6 expression and the other clinical or biologic parameters tested (sex, number of lesions, involvement of deep structures, tumor cells in CSF, LDH concentration, and histologic subtype) between BCL-6 and BCL-2 expression or between BCL-2 expression and all the clinical and biologic parameters tested.
Response to treatment
Of the 78 assessable patients with PCNSL (5 lost to follow-up), 49 (62.8%) achieved complete remissions, 12 (15.4%) partial responses, 1 patient's tumor remained unchanged (1.3%), 10 (12.8%) had progressive disease, and 6 (7.7%) died of toxic side effects.
Younger age at diagnosis (< 60 years) was associated with a higher complete remission rate (P = .05): 27 (75%) of 36 versus 22 (51%) of 43 of the older patients group (≥ 60 years). Complete remission was not significantly associated with the other clinical-biologic parameters tested. Also, CD10, BCL-6, MUM1, or BCL-2 expression did not significantly modify the probability of achieving complete remission. None of the clinical, biologic, or immunohistochemical parameters previously described were associated with the overall response rate, which included complete and partial responses to treatment.
Survival analysis
Overall survival. In our study, the median follow-up was 33 months. The median OS was 42 months (95% CI 18 to 66). The 2- and 4-year OS rates were 56% (95% CI 45 to 69) and 42% (95% CI 30 to 59), respectively. Univariate analysis showed that only younger age (< 60 years) was associated with a longer OS (P = .02); no prognostic impact on OS was found for the other clinical-biologic variables tested (see Table 1). Histologic subtype, PCNSL expression of CD10, BCL-6, MUM1, or BCL-2 had no significant impact on OS. Comparison of the survival durations of the 3 GCB patients, who were alive at 12, 32, and 63 months after diagnosis, to those of the ABC patients yielded no statistically significance difference (P = .15). Among the ABC cases, B (activated GCB) and C (activated non-GC) patterns had no impact on OS.
Failure-free survival. The median failure-free survival (FFS) for our PCNSLs was 33 months (95% CI 28 to 63). The 2- and 4-year FFS rates were 68% (95% CI 55 to 84) and 35% (95% CI 21 to 57), respectively. Univariate analysis showed that only younger age (< 60 years) was significantly associated with a longer FFS (P = .02). None of the other clinical-biologic factors (see Table 1), histologic subtype, or tumor CD10, BCL-6, MUM1, or BCL-2 expression had any impact on FFS.
The 3 GCB patients were alive and disease-free at 12, 32, and 63 months. Despite this small number of GCB PCNSL, the difference, compared to ABC patients, was at the limit of significance (P = .06). FFS did not differ significantly between B and C patterns.
Comparison of OS for patients with PCNSL or systemic DLBCL expressing the ABC phenotype
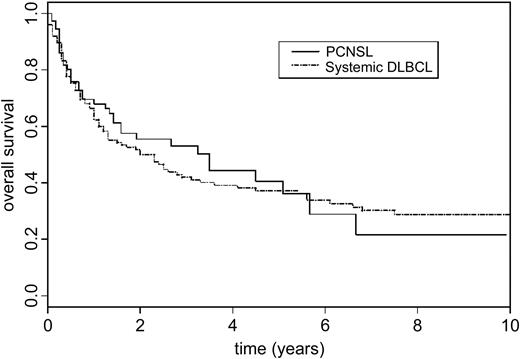
Rosenwald et al6 provided immunoprofile and clinical outcome information for 240 DLBCL patients classified as follows: 115 GCB, 73 ABC, and 52 type 3. The median OS durations were: 24 months (95% CI 12.0 to 39.6) for ABC patients and 30 months (95% CI 15.6 to 132.0) for type 3 patients. As stated in the original paper, ABC and type 3 did not differ in terms of OS (P = .46), and therefore we considered them as a single group for our analysis. For our 79 patients whose PCNSL expressed the ABC phenotype, the median OS was 42 months (95% CI 19.0 to 80.0). According to our univariate analysis, OS for these PCNSLs and systemic DLBCLs (ABC and type 3) did not differ significantly (P = .7) (Figure 2).
Comparison of overall survival rates for patients with PCNSL or systemic DLBCL expressing ABC phenotypes. DLBCLs were classified by cDNA microarray in the study published by Rosenwald et al.6
Comparison of overall survival rates for patients with PCNSL or systemic DLBCL expressing ABC phenotypes. DLBCLs were classified by cDNA microarray in the study published by Rosenwald et al.6
Discussion
This is the largest study to have investigated the GCB or ABC immunoprofile and its potential prognostic impact on PCNSL. We found that, unlike systemic DLBCLs, almost all PCNSLs express an activated immunophenotype.
We included only patients with primary brain localization of DLBCL. In accordance with previous series,2,22,23 centroblastic subtype predominated (78.3%), and immunoblastic subtype was much less frequent (8.4%). The CD10-BCL-2+ immunoprofile expressed by most of our PCNSLs argues against the hypothesis these lymphomas are Burkitt lymphomas, for which the percentage has varied widely in the literature.22 The CD138 negativity of the 56 PCNSLs tested is also in agreement with the uniform centroblastic morphology lacking late plasmacytic features. Our PCNSL samples included a mixture of tissues evaluated with TMA or conventional sections; the 2 techniques have been shown to give similar results.24 Moreover, the majority of PCNSL specimens were stereotaxic biopsies containing very small parts of the tumors, even when tested on conventional sections.
Most PCNSLs express an ABC phenotype
In PCNSL specimens, CD10 was rarely expressed and thus only a few were assigned to the GCB subgroup. The different threshold applied to define positivity may, in part, explain the discrepancy between our results and those published by Braaten et al.23 Those authors defined positivity as more than 10% labeled tumor cells and reported a higher percentage of CD10+ tumors (19%). We controlled the quality of our labeling by using reactive lymph nodes and 2 TMA from known systemic DLBCLs, which yielded one-third positive labeling, similar to previously reported observations (data not shown).8-10 Moreover, our low percentage of CD10+ PCNSLs was consistent with the high percentage of MUM1+ tumors, representing 92.6% versus 50.3% of systemic DLBCLs (Table 2). This CD10-MUM1+ immunophenotype classified the great majority of our PCNSLs in the ABC subgroup. Although the 3 GCB patients with PCNSL were alive and disease-free at their last follow-up visit, no definitive conclusion can be drawn in terms of the prognostic value of the immunoprofile for patients with PCNSL. Analysis of an even larger cohort is needed to reach sufficient statistical power.
Like other recent PCNSL protocols,14-17 35% of our patients were alive and disease-free 4 years after diagnosis. Concerning OS, we found no difference between PCNSL and systemic DLBCL expressing the ABC immunoprofile when comparing our data with those published by Rosenwald et al.6 Therefore, we hypothesize that the classic poor prognosis of PCNSL may, in part, be explained by the high percentage expressing the ABC phenotype. However, this immunoprofile was relatively uniform in our PCNSLs, and neither the GCB nor ABC pattern can explain why certain patients had relatively good prognoses. It would be highly relevant to have additional biologic prognostic markers able to identify high- or low-risk patients, who might benefit from different therapeutic protocols.
Histogenesis of PCNSL
Each B-cell lymphoma can be considered as arising from B cells arrested at a specific differentiation stage when malignant transformation took place. The WHO classification is based on the resemblance between neoplastic cells and their normal counter-parts.1 Our finding of a predominant ABC phenotype for PCNSL seems paradoxical, in comparison to earlier studies,11-13,23 whose authors postulated a GCB origin of PCNSL. That hypothetical GCB origin was based, in part, on BCL-6 expression by the majority of PCNSLs. Indeed, BCL-6+ tumor cells accounted for more than one-third of PCNSL cells in 9 (64.3%) of 14 immunocompetent patients, according to Larocca et al11 and 24 (72.7%) of 33 such patients studied Braaten et al.23 The second main argument supporting the GCB origin is the presence of ongoing mutational activity in some PCNSLs, demonstrated in 1 of the 3 PCNSL tested by Montesinos-Rongen et al12 and in 3 of the 5 tumors examined by Thompsett et al.13
In our series, 42 (51.2%) were characterized by the co-expression of a GC (BCL-6) and an activation (MUM1) marker. This frequency was higher for PCNSL than the 15% to 30% found in systemic DLBCL.8-10 Interpretation of this co-expression is difficult because it does not exist in reactive GC, where BCL-6 and MUM1 are mutually exclusive.25 Moreover, Carbone et al25 showed that MUM1, usually considered a post-GCB marker, is not restricted to interfollicular cells in reactive lymph nodes as it is also expressed in a subset of cells located in the GC and preferentially in the light zone (late GCB). The term “activated GC” pattern, proposed by Chang et al,9 seems to reflect more accurately the histogenesis of the BCL-6+ or MUM1+ lymphomas. The ABC pattern, corresponding to BCL-6+MUM1+ co-expression in 51.2% of our PCNSLs may explain previous findings, such as active hypermutation, which is concordant with an activated GC origin.
The second main group, comprising 40.2% of our patients with PCNSL, was characterized by its exclusive MUM1 positivity and can be classified as “activated non-GCB-cell” pattern. CD138 negativity corresponds to an early post-GC stage.
Among the ABC cases, distinguishing between GCB-cell and non–GCB-cell patterns had no prognostic impact in terms of OS or FFS. Those observations are similar to those obtained for systemic DLBCL patients9 and are in accordance with the classification of both in the ABC subgroup.
Is PCNSL a separate entity?
Although not considered in the WHO classification,1 some biologic differences between PCNSL and systemic DLBCL raise the question of whether PCNSL is a separate entity. For example, PCNSLs carry an extremely high load of somatic mutations of immunoglobulin genes and other proto-oncogenes (aberrant ongoing hypermutation), exceeding that of other lymphoma types.13,26 In contrast, comparative genomic hybridization analysis of chromosomal imbalances showed similar abnormalities in PCNSL and systemic DLBCL,27,28 arguing against that hypothesis. Our results suggest that, in contrast to systemic DLBCL, which represents a very heterogeneous entity,4 PCNSLs express a relatively homogeneous activated immunophenotype. Considering together the genetic similarities and differences in the immunophenotypes of PCNSL and systemic DLBCL, we hypothesize that PCNSL is quite similar to a DLBCL subset.
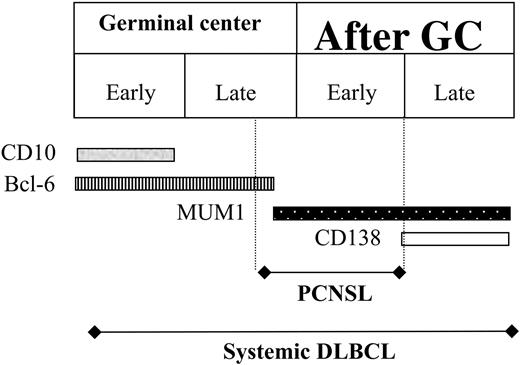
Figure 3 is a schematic representation of this hypothesis, based on the model of B-cell differentiation proposed by Carbone et al.25 While DLBCL can arise at any time—from early GC to late post-GC—PCNSL antigens correspond to those mainly expressed from late GC to early post-GC. The latter precise time span could explain, successively, for our PCNSLs, the very low CD10-expression rate, the predominant BCL-6+MUM1+ phenotype, and the CD138 negativity.
Schematic representation of our hypothesis, developed to explain the histogenesis of PCNSL, taking into consideration the time of B-cell arrest and the corresponding antigen expression. This hypothesis expands upon the model proposed by Carbone et al,25 which was founded on the physiologic stages of B-cell maturation.
Schematic representation of our hypothesis, developed to explain the histogenesis of PCNSL, taking into consideration the time of B-cell arrest and the corresponding antigen expression. This hypothesis expands upon the model proposed by Carbone et al,25 which was founded on the physiologic stages of B-cell maturation.
Prognostic impact of immunohistochemical markers expressed by PCNSL
BCL-6 is required for GC formation.29 In B-cell lymphomas, its deregulated expression caused by either chromosomal translocation or somatic hypermutation at its 5′-regulatory region may contribute to lymphomagenesis by increasing B-cell resistance to apoptosis and/or suppressing B-cell differentiation. In systemic DLBCL, BCL-6 expression is associated with a favorable prognosis in most studies30,31 and is one of the key proteins allowing classification into the GCB subgroup.6 In PCNSL, the prognostic impact of BCL-6 expression was assessed in 2 studies that yielded opposite results. Braaten et al23 showed that, as for systemic DLBCL, BCL-6 expression in PCNSL was associated with a favorable prognosis. In contrast, Chang et al32 concluded, for their 14 patients, a trend toward a poor prognosis associated with BCL-6 expression. To the best of our knowledge, our PCNSL population is the largest to have been analyzed for immunohistochemical marker expression. We found no significant impact of BCL-6 expression on outcome. However, we cannot exclude bias due to the relationship between BCL-6 expression and old age (> 60 years), as the latter is a strong indicator of poor prognosis. It is worth noting that the BCL-6–age relationship cannot be explained by a systematic error because the slides were examined without any knowledge of clinical data.
Most of the studies of systemic DLBCL considered BCL-2 an independent marker of poor prognosis.19,33,34 No such prognostic impact has been reported for PCNSL,2,23,32 but the relatively limited numbers of patients, compared to systemic DLBCL studies, raises the problem of the lack of statistical power. Using the same method as that previously described,19 we have found no prognostic impact of BCL-2 expression in PCNSL.
In conclusion, PCNSLs are more homogeneous than systemic DLBCLs and predominantly express an ABC phenotype, either activated GCB or activated non–GCB cell. This finding provides new insights into interpreting the poor prognosis of PCNSL, which may be attributable, at least in part, to biologic aggressiveness, but the specific impact of brain localization remains to be clarified.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-03-1024.
Supported by a grant from GOELAMS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors sincerely thank Pr J. Diebold and Pr J. Audouin for stimulating discussions; Pr J. J. Hauw and Pr J. Y. Delattre for providing tissue samples and clinical information from the Pitié-Salpêtrière ANOCEF patients; B. Marmey and B. Lejeune for their invaluable technical support; and J. Jacobson for reviewing the manuscript. They also acknowledge the contributions of the following GOELAMS investigators: M. P. Algros, R. Angonin, O. Bay, A. M. Bergemer-Fouquet, C. Berthou, A. Brion, S. Caulet-Maugendre, P. Cuillere, P. Cumin, B. Desablens, M. Escoffre, C. Foussard, C. Himberlin, M. Gardembas, J. L. Kemeny, T. Lamy, E. Legouffe, P. Levillain, C. Le Maignan, F. Maitre, S. Michalak-Provost, S. Letortorec, C. Linassier, E. Menet, B. Le Mevel, M. Patey, B. Quesnel, I. Quintin-Roué, P. Quittet, M. P. Ramée, N. Rioux Leclercq, M. C. Rousselet, T. Rousset, M. M. Ruchoux, C. Sagan, P. Solal-Céligny, and B. Turlin. The authors are indebted to Rosenwald et al for providing free access to their survival data for their DLBCL patients.