Abstract
Transmembrane adaptor proteins (of which 7 have been identified so far) are involved in receptor signaling in immune cells. They have only a short extracellular region, with most of the molecule comprising a substantial intracytoplasmic region carrying multiple tyrosine residues that can be phosphorylated by Src- or Syk-family kinases. In this paper, we report an immunohistologic study of 6 of these molecules in normal and neoplastic human tissue sections and show that they are restricted to subpopulations of lymphoid cells, being present in either T cells (LAT, LIME, and TRIM), B cells (NTAL), or subsets of both cell types (PAG and SIT). Their expression in neoplastic lymphoid cells broadly reflects that of normal lymphoid tissue, including the positivity of plasma cells and myeloma/plasmacytoma for LIME, NTAL, PAG, and SIT. However, this study also revealed some reactions that may be of diagnostic/prognostic value. For example, lymphocytic lymphoma and mantle-cell lymphoma showed similar profiles but differed clearly from follicle-center lymphoma, whereas PAG tended to be selectively expressed in germinal center-derived subsets of diffuse large B-cell lymphoma. These molecules represent a potentially important addition to the panel of immunophenotypic markers detectable in routine biopsies that can be used in hematopathologic studies.
Introduction
Cytoplasmic lymphocyte-associated signaling molecules have been widely studied because of their importance in immune-cell function, but relatively little attention has been paid to their potential as immunocytochemical markers of lymphoid cells. We recently reported that 8 different signaling molecules (eg, BLNK, Fyn, Hck, Lyn, PLC-γ1 and 2, SLP-76, and Syk) are all detectable, using appropriate antibodies, in sections of paraffin-embedded lymphoid tissue. Furthermore, they show differential lineage- or maturation-related expression patterns comparable to the selective labeling profiles that have been well documented for many conventional CD surface markers.1 We have also reported that several B-cell-associated signaling molecules detected in this way can be informative in the immunohistologic study of lymphoma, including Hodgkin disease and mediastinal B-cell lymphoma.2,3
In the present study we report an immunohistologic analysis of another category of molecule involved in intracellular signaling in lymphoid cells, namely, transmembrane adaptor proteins.4 A total of 7 molecules of this type have been identified, namely, LAT, LAX, LIME, NTAL (alias LAB), PAG (alias Cbp), SIT, and TRIM, all of which comprise a short extracellular region followed by a single transmembrane sequence and a substantial cytoplasmic region containing up to 10 tyrosine residues. These residues undergo phosphorylation by Src- or Syk-family kinases after ligation of cell-surface receptors, thus creating docking sites for SH2-domain-containing proteins. In some cases, receptor stimulation may induce dephosphorylation of these molecules. Because transmembrane adaptor proteins are anchored within the cell-surface membrane, they probably serve to organize complexes of other molecules involved in the early stages of lymphoid-cell signaling. Four of them (LAT, LIME, NTAL, and PAG) are, due to their palmitoylation, incorporated into lipid rafts, membrane structures that are enriched in other signaling molecules such as Src-family kinases or G proteins.
In this study, we have investigated the expression of 6 of these transmembrane adaptor molecules (Table 1) in normal and neoplastic lymphoid tissue, using antibodies reactive with their targets in routine paraffin-embedded tissue. A seventh member of this family, LAX, was not studied for want of suitable antibodies. Our results indicate that transmembrane adaptor proteins represent a new category of markers that may be of relevance to the hematopathologist for the study of reactive and neoplastic lymphoid tissue.
Materials and methods
Tissue samples
Paraffin-embedded tissue samples were obtained from the routine diagnostic service of the authors' institutions. Lymphoma samples were studied in sections from either routinely processed biopsies or from tissue arrays containing 0.6- to 1-mm cores.20 One of the authors (G.R.) provided an array containing normal nonlymphoid tissues (adrenal gland, appendix, brain, breast, bronchus, colon, duodenum, fallopian tubes, gallbladder, hypophysis, kidney, larynx, liver, lung, muscle, ovary, pancreas, parathyroid, placenta, prostate, skin, small intestine, stomach, testicle, thyroid, trachea, urinary bladder, and uterus). In addition, we analyzed paraffin-embedded tissue sections of lymph node, spleen, and thymus of rat (kindly provided by Ms Jacqueline Cordell [LRF Immunodiagnostics Unit, John Radcliffe Hospital, Oxford, United Kingdom]).
Cell lines
The T-cell lymphoma-derived cell lines CCRF-CEM and HUT-78 were obtained from Prof E. Macintyre (Hôpital Necker-Enfants Malades, Paris, France). Another lymphoma-derived T-cell line Jurkat and the Raji (Burkitt-derived) B-cell line were obtained from the Sir William Dunn School of Pathology (Oxford, United Kingdom). The Hodgkin lymphoma-derived cell line, KM-H2, was provided by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) cell collection (Braunschweig, Germany). The Thiel myeloma cell line was provided by Dr Karen Pulford (LRF Immunodiagnostics Unit, John Radcliffe Hospital, Oxford, United Kingdom). Four diffuse large B-cell lymphoma-derived cell lines (OCI-LY-3, OCI-LY-10, SU-DHL-4, and SU-DHL-6) were obtained from Dr R. Eric Davis (Metabolism Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD). Cell pellets and cytospins were prepared from these cell lines as previously described.2
Antibodies
The following mouse monoclonal antibodies were used in this study, all of which had been produced in the laboratory of one of the authors (V.H.): LAT-01 (IgG1), reactive with an epitope in the intracellular domain of human LAT (amino acids 30-263); LIME-10 (IgG2a), reactive with an epitope in the intracellular domain of human LIME (amino acids 281-296); NAP-06 (IgG1), reactive with an epitope in the intracellular domain of human NTAL (amino acids 140-243); MEM-255 (IgG2a), reactive with an epitope in the intracellular domain of human PAG (amino acids 235-280); TRIM-10 (IgG2b), reactive with an epitope in the intracellular domain of human TRIM (amino acids 29-186); SIT-02 (IgG1), reactive with an unidentified epitope in the intracellular domain of human SIT. Each of these antibodies had been prepared and characterized (by testing their reactivity against relevant transfectants and recombinant proteins) in the laboratory of one of the authors (V.H.).
In addition, more than one monoclonal antibody against 4 of the mentioned molecules was evaluated (by immunohistologic staining on lymphoid tissue). Five anti-LIME antibodies (clones 1, 3, 5, 8, and 11) were compared and all showed similar reactivity to the reagent used in this study (LIME 10). Four anti-NTAL antibodies (clones 02, 03, 07, and 08) were evaluated: 2 of them (NTAL-07 and -08) gave the same reaction as the standard reagent (NTAL-06), but NTAL-03 labeled in addition epithelial cells, and NTAL-02 showed a different staining pattern, reacting with macrophages and endothelial cells. The reaction of the anti-PAG antibody used in this study (MEM-255) was identical to that of a second reagent (MEM-253). A monoclonal antibody to LAT (kindly provided by Prof G. Delsol, Laboratoire d'Anatomie Pathologique, INSERM U563, CHU-Purpan, Toulouse, France) was also evaluated and gave results indistinguishable from those observed with LAT-01. In the case of SIT and TRIM, only single clones were available, so that no comparison with other antibodies of the same putative specificity could be performed.
Immunocytochemistry
All antibodies were used as hybridoma supernatants. They were tested at a range of dilutions in phosphate-buffered saline (PBS) containing 10% human serum (to minimize possible nonspecific binding) and used at a concentration that gave background-free selective cellular labeling.
Tissue sections (3 μm) were cut from paraffin blocks, coated on electrically charged slides (Surgipath Europe, Peterborough, United Kingdom), and processed and immunostained using a conventional immunoperoxidase technique as described elsewhere.21,22 Cell pellets or cytospin preparations of cell lines were stained by the peroxidase-based EnVision method.21
Western blotting
Protein lysates from cell-pellet preparations of the mentioned cell lines and from normal human tonsil were subjected to Western blotting as previously described3 for LIME, NTAL, PAG, SIT, and TRIM.
Results
Immunostaining of tissue biopsies
The data obtained by immunostaining routine tissue biopsies of normal and neoplastic tissues for the 6 transmembrane adaptor molecules are summarized in Tables 2, 3 and illustrated in Figures 1, 2, 3, 4. Staining was present in the cytoplasm but there was often also stronger peripheral labeling in the region of the cell membrane.
Normal lymphohematopoietic tissue
T-cell-associated adaptor proteins: LAT, LIME, and TRIM. Each of these 3 molecules was expressed by T cells in normal lymphoid tissue (tonsil/lymph node, spleen, and thymus; Figure 1), being absent from B cells (including monocytoid B cells in a case of toxoplasmosis) and from nonhematopoietic cells (eg, epithelial and endothelial cells). LIME was also found in plasma cells in tonsil and bone marrow, and in the latter site the anti-TRIM antibody also labeled some mononuclear cells. Furthermore, LAT was found (as previously described24 ) in bone marrow megakaryocytes and in scattered mast cells. An interesting additional finding was that intraepithelial T lymphocytes in the small intestine expressed LAT and TRIM but not LIME (Figure 1).
B-cell-associated adaptor protein: NTAL. NTAL was present in B cells, including plasma cells, and was absent from T cells and other cell types (Figure 1). In thymus, NTAL was found in some cells in the medulla and in rare cells at the periphery of the cortex. In addition, NTAL was present in scattered mononuclear nonerythroid cells in the bone marrow; their lineage was not further defined, but their low frequency is in keeping with studies that have reported the presence of NTAL in natural killer (NK) cells, monocytes, and mast cells.7,11,12
Adaptor proteins present in subpopulations of both T and B cells: PAG and SIT. PAG was strongly expressed in germinal center (GC) B cells and also in T cells and plasma cells, but mantle zone B cells were negative (Figure 1). GC B cells were negative or weakly positive for SIT, whereas plasma cells were strongly positive (Figure 1). SIT was also found in T cells, and it was noted that cortical thymocytes were more strongly stained than T cells in the medulla and peripheral lymphoid tissue (Figure 1).
Lymphoma
All results are summarized in Table 3 and the immunostaining patterns of B-cell lymphomas for 3 of these markers are shown schematically in Figure 4A. Briefly, the T-cell-associated proteins LAT, LIME, and TRIM were expressed in many T-cell lymphomas (Figure 2). In contrast, B-cell neoplasms were negative, with the exception of 2 of 19 cases of mantle-cell lymphoma, in which the tumor cells showed very weak expression of LIME. In addition, cases of myeloma/plasmacytoma were positive for LIME (9 of 12 cases) and some expressed TRIM (3 of 12 cases), although none were positive for LAT (Figure 2). In 3 cases of enteropathy-type T-cell lymphoma, tumor cells were positive for LAT and moderately so for TRIM but negative for LIME (and for NTAL, PAG, and SIT).
The B-cell-associated protein NTAL was expressed in all categories of non-Hodgkin B-cell lymphoma, in keeping with its distribution in normal lymphoid tissue. However, among mantle-cell lymphomas NTAL was found in only 2 of 19 cases, and 5 of the 7 positive cases of small lymphocytic lymphoma (of 18 studied) gave only weak reactions. NTAL was also present in a minority of cases of classic Hodgkin lymphoma (Figure 2) and in most cases of lymphocyte predominant Hodgkin lymphoma (Table 3 and Figure 2). T-cell neoplasms were negative for NTAL, with the exception of 5 of 11 T-lymphoblastic tumors (Figure 2) and 2 of 10 anaplastic lymphoma kinase-positive (ALK+) lymphomas (Figure 2).
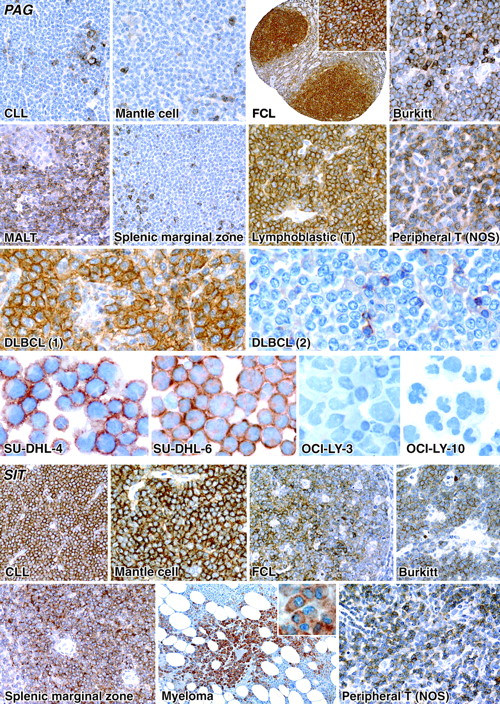
PAG and SIT were expressed in both T- and B-cell lymphomas. Among B-cell lymphomas, PAG tended to be negative in small-cell lymphomas (eg, chronic lymphocytic leukemia [CLL] and mantle-cell lymphoma; Figure 3) and myeloma/plasmacytoma, but it was expressed in mucosa-associated lymphoid tissue (MALT) lymphomas (Figure 3), and in some cases of nodal and splenic marginal zone lymphoma (Figure 3), and it was also commonly found in T-cell lymphomas (Figure 3). SIT contrasted with PAG in that it was found in small-cell lymphomas (eg, CLL and mantle-cell lymphoma; Figure 3) and myeloma/plasmacytoma (Figure 3).
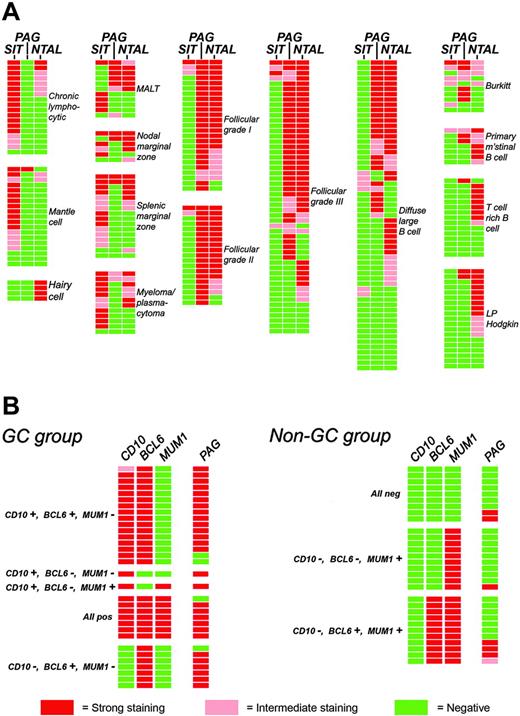
PAG was found in 46% of diffuse large B-cell lymphomas (DLBCLs). Immunostaining for PAG was reviewed in a group of 62 DLBCLs (comprising 39 cases from this study plus an additional 23 cases) that had been stained for CD10, BCL-6, and MUM1 (IRF4). This revealed (Figure 4B) that PAG expression was common among cases of GC origin (28 of 32), but was seen in only a minority (7 of 30) of non-GC cases.
Nonhematopoietic tissues
Normal nonhematopoietic biopsies were stained in a tissue array (see “Materials and methods”) for the 6 transmembrane adaptor molecules. All tissues were negative (apart from infiltrating white cells or plasma cells or both) with the exception of LAT that was present in pancreatic epithelium and PAG that was expressed in the stroma of chorionic villi and also weakly in the molecular layer of the cerebellar cortex (but not in Purkinje cells and granular cell layers).
Immunostaining of normal hematopoietic tissue and appendix for the T- and B-cell-associated transmembrane adaptor proteins. LAT, LIME, and TRIM: These molecules are all expressed in T-cell areas in tonsil (note unstained high endothelial venules, arrow) but are absent from both mantle zone (MZ) B cells and germinal center (GC) cells in B-cell secondary lymphoid follicles (the staining for LAT and LIME seen in GCs represents T cells). Plasma cells underlying the tonsil epithelium express LIME but not LAT or TRIM. LAT, LIME, and TRIM are also all expressed both in medullary (M) thymocytes surrounding Hassall corpuscles (HC) and in immature T cells in cortical (C) lobules. Tissue sections of the appendix show that intraepithelial T lymphocytes are positive for LAT and TRIM but negative for LIME. NTAL: B cells in the germinal centers (GC) and mantle zones (MZ) of secondary lymphoid follicles express NTAL, and this molecule is also found in plasma cells and monocytoid B cells (in a lymph node affected by toxoplasmosis). The thymus, in contrast, shows only scattered NTAL+ cells in the medulla (M) surrounding a Hassall corpuscle (HC) and no labeling in the thymic cortex (C). PAG: This molecule is absent from mantle zone (MZ) B cells but is found on germinal center (GC) B cells and monocytoid B cells. It is also expressed more weakly in T cells in tonsil and the thymic medulla. The immature T cells in the thymic cortex (C) are even more weakly labeled. SIT: This molecule is weakly expressed in the tonsil in GC B cells and in T cells (seen at higher magnification in the adjacent image). SIT is strongly expressed by plasma cells (arrow in the low-power view of the tonsil) and in immature T cells in the thymic cortex. (All staining performed on paraffin sections by the immunoperoxidase technique. Images were acquired using a Nikon Eclipse E800 microscope [Nikon, Tokyo, Japan] and a Zeiss Axiocam digital camera [Zeiss, Oberkochen, Germany], using a 10×/0.45 Plan Apo or a 20×/0.75, 40×/0.95, or 60×/1.4 Plan Fluor objective lens [Zeiss] Axiovision 3 image acquisition software [Zeiss] and Adobe Photoshop 7 image processing and manipulation software (Adobe, San Jose, CA) were also used.)
Immunostaining of normal hematopoietic tissue and appendix for the T- and B-cell-associated transmembrane adaptor proteins. LAT, LIME, and TRIM: These molecules are all expressed in T-cell areas in tonsil (note unstained high endothelial venules, arrow) but are absent from both mantle zone (MZ) B cells and germinal center (GC) cells in B-cell secondary lymphoid follicles (the staining for LAT and LIME seen in GCs represents T cells). Plasma cells underlying the tonsil epithelium express LIME but not LAT or TRIM. LAT, LIME, and TRIM are also all expressed both in medullary (M) thymocytes surrounding Hassall corpuscles (HC) and in immature T cells in cortical (C) lobules. Tissue sections of the appendix show that intraepithelial T lymphocytes are positive for LAT and TRIM but negative for LIME. NTAL: B cells in the germinal centers (GC) and mantle zones (MZ) of secondary lymphoid follicles express NTAL, and this molecule is also found in plasma cells and monocytoid B cells (in a lymph node affected by toxoplasmosis). The thymus, in contrast, shows only scattered NTAL+ cells in the medulla (M) surrounding a Hassall corpuscle (HC) and no labeling in the thymic cortex (C). PAG: This molecule is absent from mantle zone (MZ) B cells but is found on germinal center (GC) B cells and monocytoid B cells. It is also expressed more weakly in T cells in tonsil and the thymic medulla. The immature T cells in the thymic cortex (C) are even more weakly labeled. SIT: This molecule is weakly expressed in the tonsil in GC B cells and in T cells (seen at higher magnification in the adjacent image). SIT is strongly expressed by plasma cells (arrow in the low-power view of the tonsil) and in immature T cells in the thymic cortex. (All staining performed on paraffin sections by the immunoperoxidase technique. Images were acquired using a Nikon Eclipse E800 microscope [Nikon, Tokyo, Japan] and a Zeiss Axiocam digital camera [Zeiss, Oberkochen, Germany], using a 10×/0.45 Plan Apo or a 20×/0.75, 40×/0.95, or 60×/1.4 Plan Fluor objective lens [Zeiss] Axiovision 3 image acquisition software [Zeiss] and Adobe Photoshop 7 image processing and manipulation software (Adobe, San Jose, CA) were also used.)
Animal tissue
Antibodies to 3 of the adaptor proteins, namely, LAT, TRIM, and SIT, reacted strongly with tissue sections of lymph node, spleen, and thymus of rat origin, whereas LIME, NTAL, and PAG showed only faint positivity. The reactivity pattern was similar to that observed in human lymphoid organs.
Expression of transmembrane adaptor proteins in cell lines
The results of immunostaining and Western blotting performed on cell lines (with normal tonsil as a control) are summarized in Table 4 and Figure 5. In addition, 4 DLBCL cell lines were immunostained for PAG, and it was found that those of GC origin were positive (Figure 3), whereas no staining was seen in 2 DLBCL lines of “activated” type (Figure 3).
Discussion
The transmembrane adaptor molecules studied in this paper are believed to play important roles in immune-cell functions4 (Table 1). However, they have not been explored previously as immunohistologic markers of lymphoid tissues, with the exception of a paper describing the detection of LAT in biopsy samples using a polyclonal antibody.24 In the latter study, LAT was shown to be a selective marker within the lymphoid system for T cells (and it was also found in mast cells and megakaryocytes).
In the present paper we confirm these earlier data concerning LAT, using 2 monoclonal antibodies, and we also report that 2 other transmembrane adaptor proteins, LIME and TRIM, are selectively expressed by T cells. In contrast, NTAL (alias LAB) proved to be a selective marker of B cells, in keeping with previous data on its expression in cell lines and peripheral-blood cells.4,8,25 Our findings therefore indicate that 4 new lineage-associated molecules (LAT, LIME, NTAL, and TRIM) can be added to the traditional panel of pan-T and pan-B markers (eg, CD3, CD20) detectable with monoclonal antibodies in routine biopsy samples.
The other 2 adaptor proteins studied, PAG and SIT, are not restricted to the T or B lineage, but their expression patterns were nevertheless of interest. The presence of PAG in T cells is in keeping with previous studies that described the role of PAG in T-cell-receptor signaling.13,26-28 However, we also noted that PAG was differentially expressed in B cells in secondary lymphoid follicles, being present in GC cells but not in resting small B cells in mantle zones. PAG has not been extensively studied in B cells, although cross-linking of B-cell receptor (BCR) in B cells is known to induce an increase in PAG phosphorylation.29 Furthermore, transformation and lymphoproliferation in Theileria-transformed B cells correlates with decreased PAG expression and the loss of the PAG-associated kinase Csk.30 It may therefore be of interest to study in more detail the physiologic role of PAG in GC B cells. The sixth adaptor protein studied, SIT, has also not been widely investigated, but our finding of its expression in T cells (including cortical thymocytes, whose expression of SIT seemed higher than that of peripheral T cells) is in keeping with the first report on this molecule, in which a role in T-cell activation was described.18
Representative examples of lymphoma subtypes immunostained for T-cell-associated transmembrane adaptor proteins LAT, LIME, and TRIM and the B-cell-associated molecule NTAL. Note cases of T-lymphoblastic lymphoma expressing these markers and cases of ALK+ anaplastic large-cell lymphoma (ALCL) that are negative for LIME and TRIM. The reactivity of cases of myeloma for LIME and TRIM is also shown. In contrast, LAT is not expressed in this tumor (note positive megakaryocyte). NTAL is expressed in B-cell lymphomas, and examples are also shown of 2 NTAL+ T-cell neoplasms (lymphoblastic and ALK+). In Hodgkin disease, NTAL expression is shown in a case of lymphocyte-predominant Hodgkin disease (LPHD) and in the classic subtype (arrows indicate lymphocytic and histiocytic cells and Reed-Sternberg cells). Image acquisition was performed as described for Figure 1.
Representative examples of lymphoma subtypes immunostained for T-cell-associated transmembrane adaptor proteins LAT, LIME, and TRIM and the B-cell-associated molecule NTAL. Note cases of T-lymphoblastic lymphoma expressing these markers and cases of ALK+ anaplastic large-cell lymphoma (ALCL) that are negative for LIME and TRIM. The reactivity of cases of myeloma for LIME and TRIM is also shown. In contrast, LAT is not expressed in this tumor (note positive megakaryocyte). NTAL is expressed in B-cell lymphomas, and examples are also shown of 2 NTAL+ T-cell neoplasms (lymphoblastic and ALK+). In Hodgkin disease, NTAL expression is shown in a case of lymphocyte-predominant Hodgkin disease (LPHD) and in the classic subtype (arrows indicate lymphocytic and histiocytic cells and Reed-Sternberg cells). Image acquisition was performed as described for Figure 1.
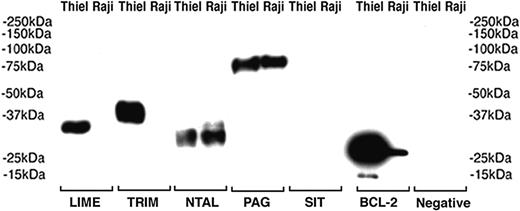
Of particular interest in this study was the observation that LIME, NTAL, PAG, and SIT were all present in plasma cells (despite the fact that LIME was otherwise restricted to T cells); this finding was confirmed also by Western blotting of a plasmacytoma cell line (Figure 5). Plasma cells are traditionally thought of as “end-stage cells” that are unresponsive to signals from the outside environment because they lack surface molecules such as CD20 and BCR and have also lost a number of signaling molecules associated with earlier stages of B-cell maturation.1 It would be of interest to determine the tyrosine phosphorylation status of LIME, NTAL, PAG, and SIT in plasma cells and to identify the proteins with which these molecules are physically associated, because this may hint at their role in these cells. In this context it may be noted that the plasma cell line Thiel expresses 4 of these 5 molecules (Table 4) and could therefore be used as a surrogate for fresh plasma cells in biochemical studies. A seventh transmembrane adaptor molecule, LAX, was not studied at the protein level for want of an antibody, but we have demonstrated its expression in the Thiel plasma cell line by reverse transcriptase-polymerase chain reaction (data not shown). However, it may be added that the expression of transmembrane adaptor proteins in plasma cells is not an absolute rule because LAT and TRIM were not found in these cells.
Immunostaining of PAG and SIT in neoplastic lymphoid cells. PAG is present in cases of both B- and T-cell neoplasia. Note the 2 cases of DLBCL, one positive and one negative. Two DLBCL-derived cell lines of GC type express PAG, whereas the other 2 (of “activated” subtype) are negative. SIT expression is shown in a variety of B-cell neoplasms, including a case of myeloma. Image acquisition was performed as described for Figure 1.
Immunostaining of PAG and SIT in neoplastic lymphoid cells. PAG is present in cases of both B- and T-cell neoplasia. Note the 2 cases of DLBCL, one positive and one negative. Two DLBCL-derived cell lines of GC type express PAG, whereas the other 2 (of “activated” subtype) are negative. SIT expression is shown in a variety of B-cell neoplasms, including a case of myeloma. Image acquisition was performed as described for Figure 1.
The reactions of the markers evaluated were generally in keeping with their expression in normal lymphoid tissue. For example, the B-cell-associated adaptor molecule NTAL was expressed by many B-cell lymphomas (although it is of interest that it was negative in almost all cases of CLL and mantle-cell lymphoma) and generally absent from T-cell neoplasms. NTAL was present in hairy-cell leukemia, a neoplasm that was constantly negative for PAG and SIT. The presence of the B-cell-associated adaptor protein NTAL in 5 of 11 cases of T lymphoblastic leukemia/lymphoma tested is of interest. It is possible that the rare NTAL+ cells we observed in the thymic cortex represent the precursor cells of this lymphoma; alternatively, its expression in T lymphoblasts may occur only following neoplastic transformation, resembling the aberrant expression of the B-cell marker CD79 in a minority of T-lymphoblastic tumors.31
The 3 markers of normal T cells (LAT, LIME, and TRIM) also showed wide expression in neoplasms of this lineage. However, it is of interest that LIME was absent in the 3 cases of enteropathy-type T-cell lymphoma tested, in keeping with its absence from their cell of origin (intraepithelial T lymphocytes). The expression of LIME (and SIT) in normal plasma cells was matched by their presence in cases of myeloma/plasmacytoma. At present only 2 relatively selective markers of plasma cells (CD38 and CD138), together with a less specific marker p63 (detected by antibody VS38), can be detected in paraffin-embedded tissue samples, so that the introduction of new plasma-cell markers is to be welcomed.
The expression pattern of PAG in lymphomas (commonly present in follicle-center cell and Burkitt lymphomas but absent from CLL and, with one exception, from mantle-cell lymphoma) also mirrored its expression in normal lymphoid follicles (present in GCs, absent from mantle zones). It is of interest that PAG was also expressed in only a proportion of the DLBCLs studied. Our preliminary analysis (Figure 4B) revealed that PAG expression was most common in the “GC” category of DLBCLs (originally defined by gene-expression profiling studies).32-35 It may also be added that PAG was present in 2 DLBCL cell lines (SU-DHL-4 and SU-DHL-6) considered to be of GC origin, but absent in 2 (OCI-LY-3 and OCI-LY-10) of “activated” type. There are at present few immunohistologic markers for this prognostically favorable subtype of DLBCLs,23,36,37 and PAG may therefore have a clinical application in the assessment of these neoplasms by the hematologist.
Schematic representation of the expression of transmembrane adaptor proteins in B-cell lymphomas. Each horizontal row represents an individual patient. (A) The expression of 3 molecules in the spectrum of B-cell lymphomas is illustrated. The majority of the cases summarized in Table 3 are included, with the exception of a small number of samples for which it was not possible (for technical reasons) to evaluate all of these markers. (B) Expression of PAG in DLBCL according to cellular origin (GC derived or non-GC). Cases were assigned to these 2 categories on the basis of immunostaining for CD10, BCL-6, and MUM1 (IRF4) following the algorithm reported by Hans et al.23
Schematic representation of the expression of transmembrane adaptor proteins in B-cell lymphomas. Each horizontal row represents an individual patient. (A) The expression of 3 molecules in the spectrum of B-cell lymphomas is illustrated. The majority of the cases summarized in Table 3 are included, with the exception of a small number of samples for which it was not possible (for technical reasons) to evaluate all of these markers. (B) Expression of PAG in DLBCL according to cellular origin (GC derived or non-GC). Cases were assigned to these 2 categories on the basis of immunostaining for CD10, BCL-6, and MUM1 (IRF4) following the algorithm reported by Hans et al.23
The negative reactions of CLL and mantle-cell lymphoma for PAG are also potentially of practical interest, because a third category of small B-cell lymphoma (MALT lymphoma, believed to arise from marginal zone B cells) showed PAG positivity in more than 50% of the cases. In contrast, the expression of SIT in small B-cell neoplasms was the converse of the PAG pattern (ie, SIT was usually positive in CLL and mantle-cell lymphoma and was sometimes negative in MALT lymphoma). It may be possible to exploit these complementary patterns of marker expression in the differential diagnosis of small B-cell neoplasms.
Western blotting of transmembrane adaptor proteins in cell lysates from Burkitt (Raji) and myeloma (Thiel) cell lines. The proteins detected with antibodies to LIME, TRIM, NTAL, and PAG have the expected molecular weights. Anti-BCL-2 is used as positive control and primary antibody was omitted in the negative control.
Western blotting of transmembrane adaptor proteins in cell lysates from Burkitt (Raji) and myeloma (Thiel) cell lines. The proteins detected with antibodies to LIME, TRIM, NTAL, and PAG have the expected molecular weights. Anti-BCL-2 is used as positive control and primary antibody was omitted in the negative control.
In conclusion, our findings reveal patterns of expression of transmembrane adaptor molecules that may throw new light on their physiologic roles. Furthermore, these 6 markers can be added to the list of lineage/maturation-associated lymphoid signaling molecules detectable with monoclonal antibodies in routinely processed biopsy samples.1-3,38,39 Work from other groups,40-45 and a number of recent examples from our own laboratory (unpublished data, January 2005), provide further confirmation that signaling molecules represent a new category of marker of value to pathologists in the diagnosis and assessment of human lymphomas. Furthermore, there are several examples of malignancy-associated genetic abnormalities that alter the pattern of expression of intracellular molecules.39,46,47 In consequence, systematic screening for aberrant expression of signaling molecules (such as the ones we describe in this paper) in neoplastic white cells may identify new underlying genetic alterations of causal importance.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-06-2273.
Supported by research funding from the Leukaemia Research Fund (grants 9970 and 0382; J.C.P., H.R., D.Y.M., T.M.), Julian Starmer-Smith Lymphoma Fund (D.Y.M.), Center of Molecular and Cellular Immunology (project 1M6837805001; V.H., P.A.), and the Academy of Sciences of the Czech Republic (project AV0Z50520514; V.H., P.A.).
S.T. and J.C.P. were responsible for the majority of immunostaining; M.-L.H., Y.N., T.R., M.Q.D., G.R., L.S., N.M. and S.P. provided the tissue material; P.A. was responsible for the production of the antibodies; H.R. performed the Western blotting analysis; M.P. performed some immunostaining; R.B. performed the RT-PCR; M.-L.H., D.Y.M., and T.M. reviewed the immunostaining, controlled and analyzed the data and, together with V.H., wrote the manuscript.
S.T. and J.C.P. contributed equally to this study.
D.Y.M., T.M., and V.H. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors express their gratitude to Mr Ralf Liebertz and Mrs Bridget Watson for their excellent technical contribution and assistance, and José Francisco Garcia Verdes-Montenegro for providing sections of tissue array containing normal nonlymphoid biopsies.

![Figure 1. Immunostaining of normal hematopoietic tissue and appendix for the T- and B-cell-associated transmembrane adaptor proteins. LAT, LIME, and TRIM: These molecules are all expressed in T-cell areas in tonsil (note unstained high endothelial venules, arrow) but are absent from both mantle zone (MZ) B cells and germinal center (GC) cells in B-cell secondary lymphoid follicles (the staining for LAT and LIME seen in GCs represents T cells). Plasma cells underlying the tonsil epithelium express LIME but not LAT or TRIM. LAT, LIME, and TRIM are also all expressed both in medullary (M) thymocytes surrounding Hassall corpuscles (HC) and in immature T cells in cortical (C) lobules. Tissue sections of the appendix show that intraepithelial T lymphocytes are positive for LAT and TRIM but negative for LIME. NTAL: B cells in the germinal centers (GC) and mantle zones (MZ) of secondary lymphoid follicles express NTAL, and this molecule is also found in plasma cells and monocytoid B cells (in a lymph node affected by toxoplasmosis). The thymus, in contrast, shows only scattered NTAL+ cells in the medulla (M) surrounding a Hassall corpuscle (HC) and no labeling in the thymic cortex (C). PAG: This molecule is absent from mantle zone (MZ) B cells but is found on germinal center (GC) B cells and monocytoid B cells. It is also expressed more weakly in T cells in tonsil and the thymic medulla. The immature T cells in the thymic cortex (C) are even more weakly labeled. SIT: This molecule is weakly expressed in the tonsil in GC B cells and in T cells (seen at higher magnification in the adjacent image). SIT is strongly expressed by plasma cells (arrow in the low-power view of the tonsil) and in immature T cells in the thymic cortex. (All staining performed on paraffin sections by the immunoperoxidase technique. Images were acquired using a Nikon Eclipse E800 microscope [Nikon, Tokyo, Japan] and a Zeiss Axiocam digital camera [Zeiss, Oberkochen, Germany], using a 10×/0.45 Plan Apo or a 20×/0.75, 40×/0.95, or 60×/1.4 Plan Fluor objective lens [Zeiss] Axiovision 3 image acquisition software [Zeiss] and Adobe Photoshop 7 image processing and manipulation software (Adobe, San Jose, CA) were also used.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-06-2273/4/m_zh80010688660001.jpeg?Expires=1765976751&Signature=A~QViM~BwVj0xP5uxG4hl5LgtqN4D-3ri1rYz47mxCzlWbAMzfV7jj10037DEKTejP1EBcduNfNjG5dIPrG11hDt16W3SQf3eVWCmipLMaI8tFmfsvI4fQZnHZn2~tuTHMxETEDrYG2N3LNZ8IJrAalUp2FFCvcDZFwQAaZXhNhVBnc3dq7qLryAtMu9X9SFv7sdp54V7np-lH108cSVx0e72GVsNFMdUm8s0~HeBVCrhg6Z4amNWFW0QOuJtA7RGDMRJpjwYGav~VKtodowccilyfKCkqrUtY3pbqQzlIRQ2PhMz7d3usit~bGy3CrcLWX7f~MCSQlI7KCt-oTOlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)