Abstract
The protozoan parasite Toxoplasma gondii enters hosts through the intestinal mucosa and colonizes distant tissues such as the brain, where its progeny persists for a lifetime. We investigated the role of CD11c- and CD11b-expressing leukocytes in T gondii transport during the early step of parasitism from the mouse small intestine and during subsequent parasite localization in the brain. Following intragastric inoculation of cyst-containing parasites in mice, CD11c+ dendritic cells from the intestinal lamina propria, the Peyer patches, and the mesenteric lymph nodes were parasitized while in the blood, parasites were associated with the CD11c- CD11b+ monocytes. Using adoptive transfer experiments, we demonstrated that these parasitized cells triggered a parasitic process in the brain of naive recipient mice. Ex vivo analysis of parasitized leukocytes showed that single tachyzoites remained at the cell periphery, often surrounded by the host cell plasma membrane, but did not divide. Using either a dye that labels circulating leukocytes or an antibody known to prevent CD11b+ circulating leukocytes from leaving the microvascular bed lumen, and chimeric mice in which the hematopoietic cells expressed the green fluorescent protein, we established that T gondii zoites hijacked CD11b+ leukocytes to reach the brain extravascular space.
Introduction
Many microorganisms initiate interactions with vertebrate hosts through digestive mucosa. Some will develop only in these peripheral host tissues, whereas others, including the protozoan parasite Toxoplasma gondii, disseminate throughout the host organism and colonize distant nonmucosal tissues. When hosts, including humans, ingest T gondii–containing cysts or oocysts, free T gondii zoites are released in the gut lumen. They subsequently enter enterocytes, where they multiply and initiate the parasitic process per se.1-3 Enterocytes loaded with zoites secrete chemokines such as monocyte chemotactic protein 1 (MCP-1/CCL-2), macrophage inflammatory protein 1α, and β (MIP-1α and β/CCL3 and CCL4), as well as MIP-2/CXCL2,4,5 which recruit leukocytes in the lamina propria (LP) extravascular space. Parasites then disseminate to several distant tissues, including the brain, a major site supporting T gondii progeny latency.6,7 Such features have important clinical implications since T gondii can remain, for a lifetime, cryptic as bradyzoite, a slowly replicative intracellular stage under the control of unique host-dependent immune signals. However, upon rupture of this equilibrium, such as the one occurring in HIV-loaded individuals or following tissue transplantation and cancer therapy, bradyzoites can differentiate into tachyzoites, which massively replicate. This process of parasite reactivation is assessed by pathology, especially severe to fatal cerebral toxoplasmosis.8,9 To date, whether they are acting at the cell lineage or tissue levels, the mechanisms underlying T gondii dissemination and entry into the brain remain largely unknown.
T gondii zoites display unique motile properties in vitro that enable them to efficiently glide onto a substrate.10 While T gondii multiplies intracellularly, the tachyzoites can survive in serum-containing medium for several hours. Therefore, in vivo, they might disseminate and cross endothelia as free parasites. Alternatively, they can exploit host cells that display basal or rapidly inducible motile properties to traffic from the gut to the brain. Among zoite carriers and motile candidates are leukocytes of the mononuclear phagocyte lineage, in particular dendritic cells (DCs, mostly CD11c+), which are known to survey tissue integrity and to transport virus, fungi, or bacteria from epithelia to draining lymph nodes.11-14 Previous observations reported that mouse secondary lymphoid organs are heavily infected after T gondii cyst per oral inoculation, indicating a spread of parasites by the lymphatic network during the initial phase of parasitism.15 Although known for their T-cell stimulatory properties, DCs might also transport T gondii: indeed, tachyzoites efficiently invade and survive within these cells in vitro.16,17 Among the other blood leukocyte lineages— the neutrophils, lymphocytes, and monocytes—that are permissive to parasite growth in vitro,17 the monocytes, especially the ones that could be recruited in inflamed LP, could also be hijacked by T gondii to disseminate throughout the host.
Here, we investigated the role of phagocytic leukocytes in T gondii trafficking during the early step of parasitism in and from the intestinal wall as well as during subsequent parasite localization in the brain. For this, a well-characterized mouse model was used that relies on intragastric delivery of parasites, thus mimicking the natural entry of T gondii in mammals.1,18,19 Additional strategies, including adoptive transfer of isolated DCs or monocytes/macrophages and on green fluorescent protein (GFP)–bone marrow chimeric mice, were also selected. In vivo and ex vivo approaches have allowed us to document, for the first time, that single T gondii tachyzoites subvert DCs and monocytes/macrophages as shuttle cells to disseminate to the brain of C57BL/6, BALB/c, or CBA/J mice. Such processes led to the first wave of T gondii multiplication and the subsequent persistence in the mouse brain.
Materials and methods
Mice
Female 8- to 10-week-old C57BL/6, BALB/c, and CBA/J mice were obtained from Charles River Laboratories (L'Arbresle, France). Transgenic mice (C57BL/6 genetic background) expressing the GFP20 were obtained from the breeding animal facility of Institut Pasteur (Paris, France).
Parasites and parasite titration
T gondii cysts from the strain 76K were prepared from the brains of CBA/J mice. Twenty cysts were inoculated intragastrically to mice. Parasite titration by real-time polymerase chain reaction (PCR) were performed with the LightCycler system (Roche Diagnostics, Basel, Switzerland) targeting the parasite B1 gene.21 The standard curve established over concentrations ranging from 106 to 10 per 4 μL showed linearity over a 6-log concentration range. At different time points after inoculation different mouse tissues were recovered and their DNA extracted with the DNeasy Tissue Kit (Qiagen, Hilden, Germany). For each sample, the parasite count was calculated by interpolation from the standard curve. In some experiments, the parasite numbers were normalized to the number of mouse cells using a real-time PCR targeting the mouse brain–derived neutrophic factor (Bdnf) gene.22,23 In B6 mice for which mesenteric lymph nodes (MLNs) were removed, 20 cysts of T gondii were inoculated intragastrically, 3 weeks after the surgery. At different times after inoculation, tissues or blood were recovered and the parasitic load was quantified by real-time PCR.
Preparation of mouse leukocyte subsets
Isolation of LP cells from the small intestine was performed as previously described.24 Peyer patches (PPs) and lymph nodes (LNs) were recovered, cut into small pieces, and digested (15 minutes, 37°C) in Dulbecco modified Eagle medium (DMEM) (Gibco, Carlsbad, CA) containing 0.14 Wünsch units/mL of liberase blendzyme 3 (Roche Diagnostics) and 100 Kunitz of DNase I (Sigma-Aldrich, St Louis, MO). Collagenase activity was stopped by adding 10 mL of DMEM supplemented with 10% fetal calf serum (FCS) and containing 10 Kunitz of DNase I. Single cell suspensions were washed in phosphate-buffered saline (PBS), supplemented with 5% FCS and 5 mM EDTA. Brains were removed from mice, minced, passed through an 18-gauge needle, and digested (45 minutes, 37°C) in DMEM containing liberase and DNase I as described for PPs and LNs. Then cell suspensions were washed and fractionated on 30%/60% Percoll (Pharmacia, Uppsala, Sweden) gradients. Brain mononuclear cells were collected from the interface.25 Blood leukocytes were prepared using the Lympholyte-Mammal solution according to the manufacturer's instructions. For all experiments, cells were pooled from 3 to 9 mice per condition, and cell viability was assessed by trypan blue exclusion. For selection of CD11c+CD11b+/- and CD11c-CD11b+ cells from LP, PPs, MLNs, blood, and brain single-cell suspensions, the CD11c+ and CD11c- cells were fractionated using CD11c-coated magnetic beads (Miltenyi Biotec, Auburn, CA). Secondly, the CD11c- cells were incubated with CD11b-coated magnetic beads (Miltenyi Biotec). The purity of the populations was at least 95% as controlled by flow cytometry analysis, except for PPs (≥ 70% pure).
Immunolabeling and flow cytometry
Prior to staining, freshly isolated cells were incubated with an anti-FcγIII/II receptor mAb (2.4G2, BD-Pharmingen, San Diego, CA) in PBS containing 2% FCS, 0.01% NaN3 (20 minutes, 4°C). Cells were stained with the following mAbs (BD Pharmingen): anti-CD11c (HL3, biotinylated or phycoerythrin conjugated), anti-CD11b (M1/70, allophycocyanin conjugated), or/and anti-Ly6G/C (Gr-1) (RB6-8C5, phycoerythrin or fluorescein isothiocyanate (FITC) conjugated). Allophycocyanin or peridinin chlorophyll-a protein conjugated streptavidin was used as secondary reagent. The isotype controls used were rat IgG2a (R35-95), rat IgG2b (A95-1), and hamster IgG1 (G235-2356). For each sample, between 50 000 and 100 000 viable cells were analyzed using CELLQuest software and a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde and permeabilized or not with saponin. They were labeled with first, anti-SAG1 or anti-p36 mouse mAb and the Alexa-594 or -546 goat antimouse conjugate (Molecular Probes, Eugene, OR), and secondly with reagents from BD-Pharmingen: either biotinylated anti-CD11c HL3 (1/40), or anti-CD11b M1/70 (1/50), or anti–MHC class II molecules I-Ab, d, q and I-Ed, k (Bhattacharya et al26 ; 10 μg/mL) or anti-CD45 (1/40) or FITC-conjugated anti-CD19 1D3 (1/40) or NIMP-R14 supernatant (1/10; Lopez et al27 ). When necessary, Texas-red–conjugated streptavidin (10 μg/mL) or Alexa-488 goat antirat (Molecular Probes; 2 μg/mL) were used. DNA was stained with 4′, 6 diamino-2-phenylindole (DAPI), and cells were mounted in MOWIOL 4-88 (Calbiochemical, La Jolla, CA). Images were acquired under an Axiovert fluorescence microscope (Zeiss) or using a confocal microscope (Leica). They were analyzed using coolsnap HQ camera (Roper Scientific Photometrics) and MetaMorph software (Universal Imaging Corporation). About 100 parasites and 1000 mouse cells were counted for each sample.
In vivo injection of CD11b-binding monoclonal antibodies
At day 6 and day 7 after cyst intragastric inoculation, 0.3 mg of the anti–mouse CD11b (clone 5C6, IgG2b, Serotec, Raleigh, NC) or an irrelevant isotype (IgG2b negative control, Serotec) were intravenously injected in infected B6 mice (6 mice per group). At day 8 after inoculation, MLNs, blood, and brain cells were recovered and examined for their parasite DNA load.
Intravenous injection of 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE)
To trace the migration of blood leukocytes, the fluorescent dye (Molecular Probes) was intravenously injected (0.7 mg in 0.3 mL PBS-DMSO 7%) in the mouse tail vein. Three hours or 2 days after the intravenous dye injection, blood and brain cell suspensions were screened for the presence of CFSE+ cells by flow cytometry. Mice of the control group received 0.3 mL PBS-DMSO 7%.
Adoptive transfer of leukocyte subsets
The MNLs and blood of C57BL/6 mice were recovered on days 5 and 7 after inoculation, respectively, and were used as sources of donor cells: CD11c+ MLN cells and CD11b+ blood cells were purified and (5 × 104 MLN CD11c+ or 105 blood CD11b+ cells) were injected intravenously into a naive syngeneic recipient mouse. At different time points following the transfer, brain mononuclear cells were recovered to titrate parasites. Free 76 K tachyzoites were prepared from human foreskin fibroblasts (HFF) infected with cyst-containing bradyzoites. Free tachyzoites were recovered in the culture medium. Following PBS washes, 10 or 50 parasites were intravenously injected in naive B6 mice. One day and 5 days later, brain cells of the recipient mice were recovered and examined for their parasite DNA content.
Bone marrow chimeric C57BL/6 mice
Mice were irradiated with 5.4 Gy. Then, they received intravenously 107 fresh bone marrow cells recovered from femurs and tibias of C57BL/6 mice expressing GFP.20 Every 2 weeks, the presence of GFP+ leukocytes was monitored by flow cytometry in small blood samples from the reconstituted mice. Six weeks after the bone marrow cell injection, 20 cysts of T gondii 76K were inoculated intragastrically. At different time points after inoculation, brain mononuclear cells were examined for the presence of GFP+ cells. On days 7 and 9 after inoculation, GFP+ cells were selected by FACS (MoFlow, Becton Dickinson) to analyze their parasitic load and their phenotype using cytofluorometry. Brain cells from uninfected chimeric mice were similarly processed.
Results
T gondii delivered intragastrically reaches the blood and the brain: a process relying on trafficking through the intestinal lymphatic vessels?
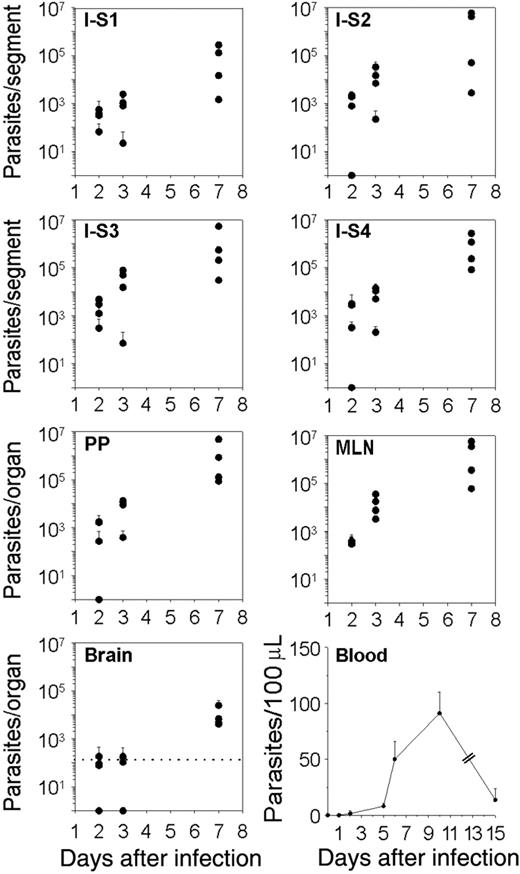
To address the question of how T gondii spreads from the intestinal wall to the brain, the number of parasites in various organs was quantified at different time points after the cyst intragastric delivery in different organs. Two days after cyst inoculation, parasites were detected in all the small intestine segments deprived of PPs, and their amount significantly increased between day 3 and day 7, suggesting a round of parasite multiplication within the intestine (Figure 1). Interestingly, parasites were first detected in the blood at day 5, then their amount increased up to 11.5-fold and was subsequently reduced by 6.7 times at day 15 after inoculation (Figure 1). Since genetically different mice vary with respect to the reactivity of the small intestine reached by cysts, especially with regard to their immune status, parasites were tracked in different tissues from BALB/c and of CBA mice, and similar data were obtained (data not shown). Of note, the limited inflammatory processes occurring in the small intestine of the BALB/c and CBA/J, which received 20 cysts, did not prevent the detection of parasites in the blood, and the kinetics of parasite DNA load in distant tissues were similar to the ones observed in B6 mice (data not shown). In the brain, the earliest detection of parasite DNA occurred at day 7 after inoculation, independently of the mouse genotype (Figure 1). The parasitic titer was significantly lower in the spleen (P ≤ .05) than in the gut-associated lymphoid organs at days 2, 3 and 7 after inoculation. Additionally, parasites were not found until day 7 in the LNs that do not drain the site of parasite entry, including the popliteal, inguinal, retro-maxillar, and cervical nodes (data not shown).
Altogether, these results suggest that once the bradyzoites released from the cysts have initiated the colonization of the small intestine wall, the downstream lymphatic system is the first to be subverted by T gondii before it colonizes the blood circulation and then the brain. However, in our mouse model, MLNs were not an obligatory relay for T gondii dissemination to the brain. Indeed, surgical removal of MLNs, 3 weeks before the intragastric delivery of cysts, induced a significant increase of parasite burden in the LP and in the PPs (P ≤ .05) at day 7 after inoculation, but parasites were still detected in the brain at the same time point as in sham-operated mice (data not shown).
Kinetics analysis of T gondii dissemination in C57BL/6 mice. Parasites were tracked using quantitative real-time PCR. Number of parasites per intestine, PP, MLN, and brain. The small intestine was cut into 4 equal-size segments (S), the S1 and S4 being proximal and distal to the stomach, respectively. The dot line represents the cut-off of sensitivity for the assay in the brain. Number of parasites per 100 μLof blood were estimated. Values represent mean number plus SD for 4 mice per group and 2 separate experiments.
Kinetics analysis of T gondii dissemination in C57BL/6 mice. Parasites were tracked using quantitative real-time PCR. Number of parasites per intestine, PP, MLN, and brain. The small intestine was cut into 4 equal-size segments (S), the S1 and S4 being proximal and distal to the stomach, respectively. The dot line represents the cut-off of sensitivity for the assay in the brain. Number of parasites per 100 μLof blood were estimated. Values represent mean number plus SD for 4 mice per group and 2 separate experiments.
CD11c+ DCs or CD11b+ leukocytes recovered from LP, PPs, MLNs, blood, and brain are parasitized
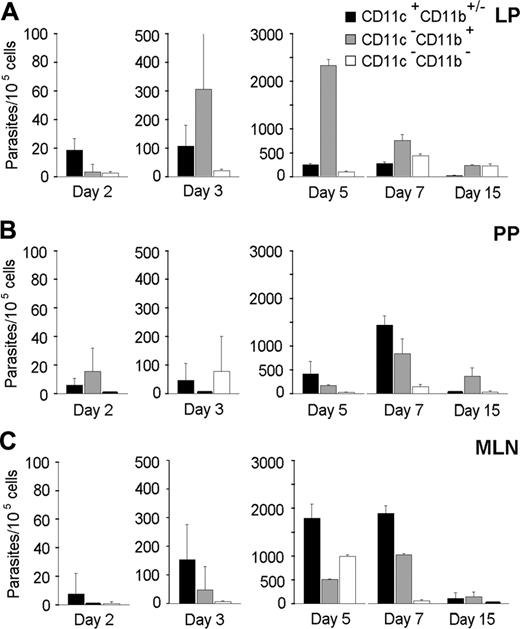
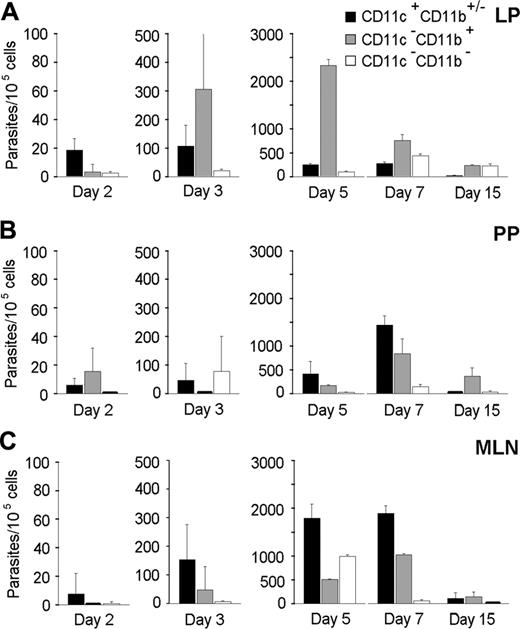
The frequencies and numbers of CD11c+ and CD11b+ cells in the LP, PPs, MLNs, blood, and brain were increased after T gondii cyst inoculation (data not shown). For example, the frequencies of CD11c+ cells increased about 2-fold by day 5 after inoculation in the MLN. In the brain, the increase in CD11c+ and CD11b+ cell numbers coincided with parasite detection at day 7 after inoculation. Therefore, the number of parasites associated with the CD11c+ and CD11b+ leukocyte subsets was quantified following their selective recovery from the LP, PPs, MLNs, blood, and brain. Parasites were preferentially associated with CD11c+ cells in the LP at day 2 and in the MLNs from days 3 to 7 (Figure 2A-C). Thus, the CD11c+CD11b+/- DCs may participate to the early dissemination of T gondii from the intestinal wall to MLNs. Interestingly, DCs recovered from PPs also contained parasites but not as many as DCs from the LP (Figure 2A,B). At day 15 after inoculation, the amount of parasites decreased in all the intestinal compartments examined, probably following T gondii clearance known to occur through T-lymphocyte activity.28
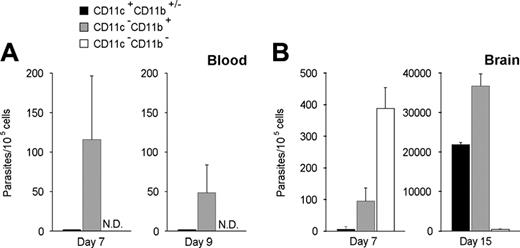
In the blood, at day 7 after inoculation, parasites were detected in the CD11b+ but not in the CD11c+ leukocytes (Figure 3A). Because CD11b+ cells can also be B lymphocytes or neutrophils, we checked whether parasites were associated with cells expressing either the B-cell marker CD19 or the neutrophil molecule NIMP-R14+. Parasites were not found in these leukocyte lineages (data not shown). Therefore, parasitized CD11b+ blood leukocytes were likely to be monocytes, and they were candidates to promote parasite colonization of the brain. In the brain, however, while the CD11b+ cell population contained parasites (Figure 3B), at day 7 after inoculation, the majority of parasites were found in CD11c -CD11b- but not CD45+ cells (∼ 400 parasites per 105 cells). Among these CD11c - CD11b- cells, we detected neutrophils, CD8+ and CD4+ lymphocytes, B lymphocytes, and MHC class II+ cells. Remarkably, at day 15 after inoculation—meanwhile the amount of parasites increased about 100-fold—these were no longer detected in the double-negative cells but in both the CD11c+CD11b+/- and CD11c-CD11b+ leukocyte populations (Figure 3B). Accordingly, parasites were also preferentially detected in the brain of CD11c+CD11b+/- and CD11c-CD11b+ cells recovered from BALB/c or CBA/J mice that had received 20 cysts 17 days before (data not shown).
Quantification of T gondii parasites associated to CD11c+CD11b+/- DCs and CD11b+ intestinal cells. Cell subsets were isolated from infected C57BL/6 mice, and associated parasites were numerated using real-time PCR. Number of parasites per 105 cells from LP (A), PPs (B), and MLN (C). Values represent mean number plus SD for 7 to 9 mice and 2 to 3 separate experiments.
Quantification of T gondii parasites associated to CD11c+CD11b+/- DCs and CD11b+ intestinal cells. Cell subsets were isolated from infected C57BL/6 mice, and associated parasites were numerated using real-time PCR. Number of parasites per 105 cells from LP (A), PPs (B), and MLN (C). Values represent mean number plus SD for 7 to 9 mice and 2 to 3 separate experiments.
The low parasitic load detected in the intestine at day 15 after inoculation, together with the restricted time frame within which parasites were found in blood leukocytes, argues for a massive parasite replication in the brain upon its invasion by T gondii.
MLN CD11c+ DCs and blood CD11b+ cells recovered from T gondii–parasitized mice initiate the parasitic process once intravenously transferred to naive syngeneic mice
To support T gondii dissemination, the shuttle cells must also enable parasite survival. Since both DCs and CD11c-CD11b+ leukocytes display direct and indirect microbicidal properties, the infectivity of T gondii associating with these cells was further examined.
CD11c+ DCs were purified from the MLNs of 5-day parasitized mice and then transferred intravenously to naive mice. According to our quantification assays, at day 5 after inoculation, the total number of cells in an MLN was approximately 3 × 107, and 1% to 2% of them contained tachyzoites. Among the cells that were associated with parasites, 11.7% are CD11c+ cells. Therefore, 5 × 104 CD11c+ MLN DCs were injected intravenously to recipient naive mice, and 9 days after, these mice developed a parasitic process as assessed by the detection of parasites in their brain (78.1 ± 2 parasites per 106 cells).
Since parasites were associated with CD11b+ blood cells at day 7 after inoculation, 105 CD11b+ blood cells were transferred intravenously into naive mice. This number (105) represents approximately the total monocyte population circulating in a C57BL/6 mouse. As early as 1 day after the transfer of these CD11b+ leukocytes, T gondii were detected in the mononuclear cells recovered from the brain of the recipient mice (18.8 ± 3.7 parasites per 106 cells). These results demonstrate that live T gondii tachyzoites were associated with MLN DCs and CD11b+ blood leukocytes and contributed to the brain parasitism. Additionally, since a few free parasites were observed in the blood samples of parasitized mice, 10 or 50 free tachyzoites of the same parasite strain were injected into naive mice; no T gondii DNA was detected in the brain of the recipient mice 1 day or 5 days after their intravenous injection.
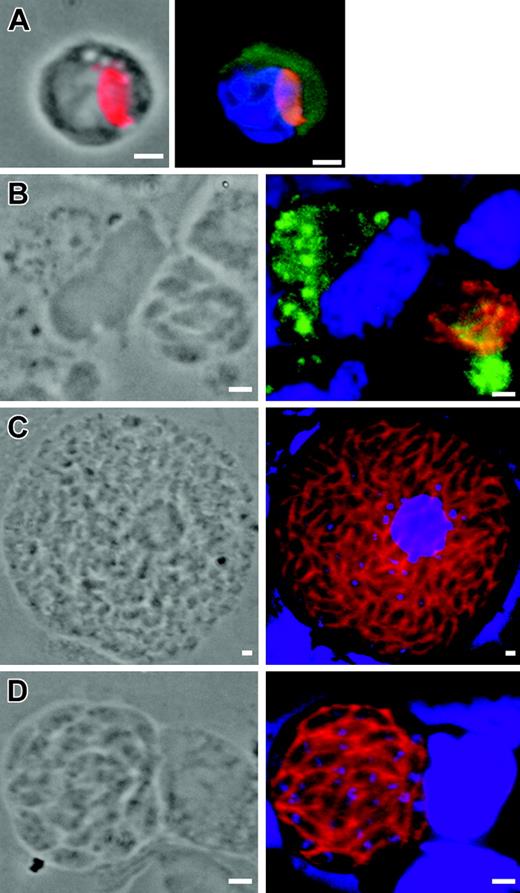
MLN CD11c+ DCs and blood CD11b+ cells harbor single tachyzoites
Staining the parasite membranes allowed detection of the typical shape of parasites, suggesting that they were not in the process of cell degradation (Figure 4). The parasites associated with CD11c+ DCs in MLN expressed SAG1, a tachyzoite-specific protein, at day 5 until at least day 9 after inoculation (Figure 4A). Remarkably, more than 90% of the parasite-positive cells carried a single tachyzoite, while very few cells contained a parasite-forming rosette (no. rosettes = 3 for > 105 MLN cells examined). In the blood, the detection of tachyzoites was a rare event, since we counted only 5 to 10 parasites out of about 105 cells. Again, the parasitized CD11b+ cells harbored a single tachyzoite (Figure 4F). The latter localized at the cell periphery; it could be bound to the surface, wrapped or not in plasma membrane projections, or it could be internalized but “arrested” underneath the plasma membrane. To discriminate between extracellular and intracellular parasites, we used selective detergent permeabilization immunostaining protocols and confocal microscopy. In MLN CD11c+ cells, 66% to 75% of T gondii tachyzoites were labeled only after cell permeabilization, arguing for their intracellular localization or for tight membranous folds surrounding the parasite. Additionally, analysis of a 0.4 μm section of MLN CD11b+ cells by confocal microscopy showed that most tachyzoites were indeed inside the host cells (Figure 4C,D). Confocal section of CD45-labeled host cell plasma membrane confirmed that these tachyzoites were surrounded by membrane folds (Figure 4E). In the blood leukocyte sample, tachyzoites were observed only after cell permeabilization, again consistent with intracellular localization (Figure 4F).
Quantification of T gondii parasites associated to CD11c+CD11b+/- and CD11b+ cells in the blood (A) and in the brain (B) of infected C57BL/6 mice using real-time PCR. Values represent mean number plus SD for 5 to 9 mice per group and 2 to 4 separate experiments.
Quantification of T gondii parasites associated to CD11c+CD11b+/- and CD11b+ cells in the blood (A) and in the brain (B) of infected C57BL/6 mice using real-time PCR. Values represent mean number plus SD for 5 to 9 mice per group and 2 to 4 separate experiments.
Immunolocalization of tachyzoite (anti-SAG1, red) associated to host cells. (A) MLN CD11c+ cell (green) at 5 days after inoculation. (B) MLN MHC class II+ (green) cell at 5 days after inoculation. Among the parasitized cells at day 5 after inoculation, approximately 59% express MHC class II molecules, which are constitutively expressed by DCs, 20.9% express CD11b and 11.7% express CD11c (n parasites > 100 for each subset). (C-E) Confocal microscopy (0.4 μm section) in MLN cells at day 5 after inoculation. Tachyzoites (red) are surrounded by CD11b+ host cell plasma membrane (green) (CD) or by CD45+ host cell plasma membrane (green). (F) Single tachyzoite (red) is associated with a blood CD11b+ host cell (green) at day 7 after inoculation. Nuclei were stained with DAPI (blue). Scale bar = 2 μm. Images were visualized with a 63×/1.25 Plan-neofluar objective lens (Zeiss, Le Pecq, France).
Immunolocalization of tachyzoite (anti-SAG1, red) associated to host cells. (A) MLN CD11c+ cell (green) at 5 days after inoculation. (B) MLN MHC class II+ (green) cell at 5 days after inoculation. Among the parasitized cells at day 5 after inoculation, approximately 59% express MHC class II molecules, which are constitutively expressed by DCs, 20.9% express CD11b and 11.7% express CD11c (n parasites > 100 for each subset). (C-E) Confocal microscopy (0.4 μm section) in MLN cells at day 5 after inoculation. Tachyzoites (red) are surrounded by CD11b+ host cell plasma membrane (green) (CD) or by CD45+ host cell plasma membrane (green). (F) Single tachyzoite (red) is associated with a blood CD11b+ host cell (green) at day 7 after inoculation. Nuclei were stained with DAPI (blue). Scale bar = 2 μm. Images were visualized with a 63×/1.25 Plan-neofluar objective lens (Zeiss, Le Pecq, France).
The blood CD11b+ monocytes deliver parasites into the brain
We investigated first whether the parasitized CD11b+ blood cells could migrate across the blood-brain barrier to promote parasite entry in the brain by using 2 approaches.
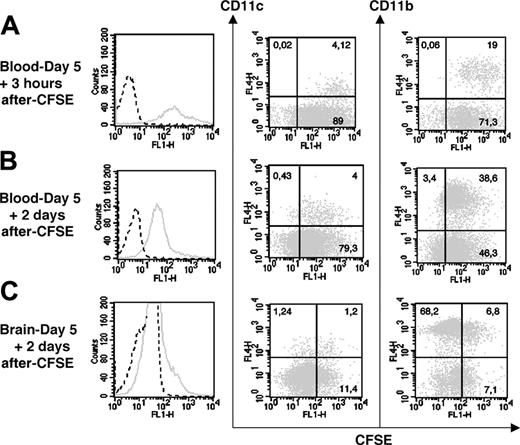
First, CFSE dye was injected intravenously into mice that were given 20 cysts 5 days before. CFSE was expected to label leukocytes in the blood and their late progenitors in the bone marrow, and thereby allow establishment as to whether CFSE+ leukocytes can reach the brain. Three hours after CFSE dye inoculation, 100% of CD11c+ and CD11b+ blood leukocytes were CFSE+ (Figure 5A). Two days after the dye injection (ie, day 7 after inoculation), the majority of CD11c+ and CD11b+ blood cells were still CFSE+, but their fluorescence intensity decreased 2-fold, suggesting that these cells had divided (Figure 5B). In the brain at day 7 after inoculation, we detected mostly CD11b+CFSE+ cells (48.9% of the CFSE+ cells) but also some CD11c+CFSE+ cells (9.5% of the CFSE+ cells). The CD11b+CFSE+ cells represented 9.1% of the CD11b+ cells, while the CD11c+CFSE+ cells represented 49.2% of the CD11c+ cells (Figure 5C). Remarkably, these 2 leukocyte subsets accounted for about 60% of the CFSE+ cells in the brain. These findings demonstrate that both leukocyte subsets trafficked from the blood compartment to the brain.
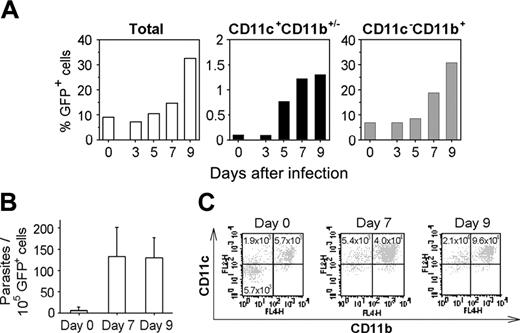
As a second approach, following sublethal irradiation, mice were reconstituted with actin-GFP+ bone marrow cells. Then, using cytofluorometry analysis, GFP+ leukocytes were tracked in the brain of parasitized chimeric mice. The accumulation of CD11c+GFP+ and CD11c-CD11b+GFP+ leukocytes was detected in the brain at day 7 after inoculation. The proportions of cerebral CD11c+GFP+ and CD11b+ GFP+ leukocytes in the brain increased by 13-fold and 2.8-fold, respectively, at day 7 (Figure 6A). Additionally, real-time PCR revealed that the GFP+ cells recovered from the brains of 7- or 9-day parasitized mice contained approximately 130 parasites per 105 cells (Figure 6B), whereas fewer than 30 parasites were quantified per 105 brain GFP- leukocytes (data not shown). Moreover, 90% of the GFP+ cells expressed the CD11c and/or CD11b integrins (Figure 6C). Importantly, in chimeric mice that were not inoculated with parasites, CD11c+ and CD11b+ GFP+ cells were detected in their brain, but they were 6-fold fewer than in the parasitized mouse brains (Figure 6C).
CFSE IV injection (0.72 mg/animal) in 5 day–infected C57BL/6 mice. CFSE fluorescence expressed by blood cells 3 hours (A) and 2 days (B) after dye injection. (C) CFSE fluorescence expressed by brain leukocytes 2 days after dye injection. Histograms represent the total of CFSE+ cells (solid line) and fluorescence measured after PBS-DMSO injection is shown as a dot line. Dot plots indicate the percentages of CD11c+CFSE+ and CD11b+CFSE+ cells present in the blood and the brain after the dye injection. Cells of 3 mice were pooled. The data shown are from a single experiment, representative of 2 separate experiments. Noninfected mice also received CFSE for control (data not shown).
CFSE IV injection (0.72 mg/animal) in 5 day–infected C57BL/6 mice. CFSE fluorescence expressed by blood cells 3 hours (A) and 2 days (B) after dye injection. (C) CFSE fluorescence expressed by brain leukocytes 2 days after dye injection. Histograms represent the total of CFSE+ cells (solid line) and fluorescence measured after PBS-DMSO injection is shown as a dot line. Dot plots indicate the percentages of CD11c+CFSE+ and CD11b+CFSE+ cells present in the blood and the brain after the dye injection. Cells of 3 mice were pooled. The data shown are from a single experiment, representative of 2 separate experiments. Noninfected mice also received CFSE for control (data not shown).
Altogether, these data strongly suggest that CD11b+ monocytes, exiting the bone marrow or the parasite-loaded extravascular tissue where they enter transiently and circulating in the blood, deliver T gondii into the brain extravascular space.
We then tested whether the CD11b integrin function in CD11b+ leucocytes could be involved in parasite dissemination to the brain by injecting CD11b-binding antibodies in T gondii–parasitized mice. Following relevant antibody injection at day 6 and day 7 after inoculation, the parasite burden decreased by approximately 2-fold in the MLN, blood, and the brain cells at day 8 after inoculation (Table 1).
CD11c+CD11b+/- cells deliver T gondii into the brain. (A) Accumulation of CD11c+CD11b+/- and CD11c-CD11b+ cells in the brain of infected C57BL/6 mice previously engrafted with GFP+ bone marrow cells. (B) Number of parasites harbored by GFP+ brain cells from naive or infected chimeric mice. Values represent mean number plus SD for 3 to 5 mice per group. (C) Dot plots of CD11c+ and CD11b+ GFP+ cells naive or infected chimeric mouse brains. Brain GFP+ cells were isolated by FACS and stained. Numbers are the total CD11c+ or CD11b+ GFP+ cells per brain. Data are from a single experiment, representative of 2 separate experiments.
CD11c+CD11b+/- cells deliver T gondii into the brain. (A) Accumulation of CD11c+CD11b+/- and CD11c-CD11b+ cells in the brain of infected C57BL/6 mice previously engrafted with GFP+ bone marrow cells. (B) Number of parasites harbored by GFP+ brain cells from naive or infected chimeric mice. Values represent mean number plus SD for 3 to 5 mice per group. (C) Dot plots of CD11c+ and CD11b+ GFP+ cells naive or infected chimeric mouse brains. Brain GFP+ cells were isolated by FACS and stained. Numbers are the total CD11c+ or CD11b+ GFP+ cells per brain. Data are from a single experiment, representative of 2 separate experiments.
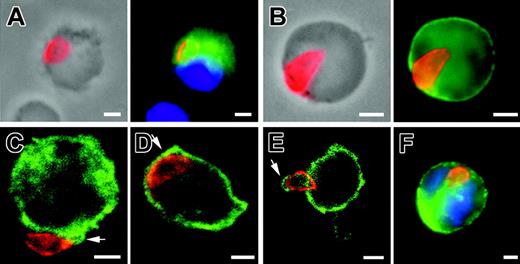
The last issue concerned the maintenance of parasites in the brain. Using immunostaining, both tachyzoites and bradyzoites were observed in CD11b+ brain cells between day 7 and 9 after inoculation. At day 7, the majority of parasitized cells harbored a single parasite in the periphery, few cells contained small rosettes of intracellular dividing parasites (no. parasites/cell = 2-4). In contrast, as early as day 9, most of the parasitized cells contained larger rosettes (Figure 7A-D). These results argue for an early phase of parasite replication in the brain, a process that has not been reported yet. In addition, consistent with the quantification of parasites in mouse cell subsets at day 7 (Figure 3B), we found that most of the rosettes were detected within CD11b- (Figure 7C,D). These cells were CD45+ MHC class II + CD19-NIMP-R14.
Discussion
In the natural parasitic process, T gondii zoites enter the gut epithelium and disseminate toward distant tissues including the muscles and the brain. Using laboratory mice as experimental hosts, we document that T gondii subverts CD11c+- or CD11b+-expressing phagocytic leukocytes to disseminate from the intestine to the brain.
Enterocytes from the small intestine are described as the first host cells encountered and subverted by the parasitic zoites,1,2 and this interaction is likely to elicit an early immune response.29-31 In the vicinity of enterocytes, in addition to resident and rapidly renewed DCs, other leukocytes are recruited from the blood, and these were proposed to contribute to the initial dissemination of T gondii via the lymphatic route.15,32
We observed that both DCs from the LP along the intestinal epithelium and DCs from the gut-associated lymphoid PPs and MLNs were host cells of T gondii. Such routes of entry have been described for the enteroinvasive bacteria. While most bacteria are internalized by M cells in PPs and DCs neighboring M cells,12,33 recent data suggest that bacteria can also be taken up from the lumen by resident DCs in the LP.34 Indeed, Salmonella can use CD18-expressing phagocytes to disseminate from the gastrointestinal tract to the bloodstream.35 Here, we report that CD11c+ DCs are subverted by tachyzoites as shuttle cells to reach the MLNs. Then, in the blood the tachyzoites were associated with CD11c-CD11b+ monocytes (NIMP-R14-, CD19-).
Clearly, the 76K tachyzoites associated with lymph node DCs or blood monocytes were invasive since both populations initiate a parasitic process in recipient syngeneic naive mice, whereas free 76K tachyzoites inoculated intravenously were unable to do so. These results indicate that T gondii–carrying monocytes are shuttling parasites from the blood to the brain. The parasite-free leukocyte migration across the endothelium—in steady state tissues or in tissues where inflammatory signals are transiently generated—is a complex and active process for both the leukocytes and the endothelial cells, the paracellular route being the route most studied over the transcellular one.36 The intravenous administration of a CD11b-binding antibody—screened on its blocking activity37 —in mice at days 6 and 7 after T gondii intragastric inoculation resulted in a 2-fold decrease in the blood and the brain parasite burden one day later. These data strongly support the function of CD11b integrin and CD11b+ cells in parasite dissemination to the brain. The CD11b+ Ly6Chigh monocytes have been shown to play a role during the onset of inflammatory process in the Listeria-loaded brain.38 More recently, CD31pos:Ly-6Cpos:CD11bpos:LY-6Glow leukocytes present in the bone marrow as early as 24 hours after the intravenous inoculation of Listeria monocytogenes and loaded with live bacteria have been shown to interact with ICAM-1 in brain endothelial cells, a process coupled to the delivery of the bacteria in the brain extravascular space.39 Here, we reported that blood T gondii–carrying monocytes delivered zoites to the brain within a narrow time frame, regardless of the host mouse strain and whether there is an inflammatory microenvironment or not (third paragraph of “Results”). Remarkably, this time frame slightly preceded the earlier detection of parasites in the brain. One cannot rule out that a flux of parasite-loaded leukocytes from the blood to the brain persists at a very low level, thereby undetectable by PCR.
Immunolocalization of T gondii associated to host cells in the brain. (A) Single tachyzoite (anti-SAG1, red) associated with a CD11b+ cell (green) at 7 days after inoculation. (B) Bradyzoites (anti-P36, red) multiplying within a CD11b+ cell (green) at 9 days after inoculation. (C) Tachyzoites and (D) bradyzoites dividing within a CD11c- -CD11b- cell at day 9 after inoculation. Nuclei were stained with DAPI (blue). Scale bar = 2 μm. Images were visualized with a 63×/1.25 Plan-neofluar objective lens (Zeiss).
Immunolocalization of T gondii associated to host cells in the brain. (A) Single tachyzoite (anti-SAG1, red) associated with a CD11b+ cell (green) at 7 days after inoculation. (B) Bradyzoites (anti-P36, red) multiplying within a CD11b+ cell (green) at 9 days after inoculation. (C) Tachyzoites and (D) bradyzoites dividing within a CD11c- -CD11b- cell at day 9 after inoculation. Nuclei were stained with DAPI (blue). Scale bar = 2 μm. Images were visualized with a 63×/1.25 Plan-neofluar objective lens (Zeiss).
To favor dissemination, the parasite-host cell interaction may not only allow the survival of both cells, but also may alter the behavior of the zoite-loaded leukocyte trafficking properties to target selective organs. The blood brain barrier (BBB) protects the brain parenchyma from most steady-state disruptive signals or microorganisms. This barrier is composed of brain endothelial cells that are bound together by intercellular tight junctions but are also sheathed by cytoplasmic processes of the astrocytes as well as by pericytes.40 To successfully cross such an endothelium, leukocytes are expected to express molecules that support tethering and rolling on vascular ligands as well as adhesion molecules and others promoting diapedesis.40,41 We provided the first evidence that T gondii exploits the route(s) that has been identified for leukocyte migration across the BBB. First, parasitized CD11c+CD11b+ GFP+ cells were found concomitantly with the earliest parasite detection in brain of chimeric mice (ie, day 7 after inoculation), indicating that this population trafficked to the brain. Secondly, under our experimental conditions, in T gondii–parasitized mice, the leukocyte extravasation is 6-fold amplified compared to nonparasitized mice in which such process did occur under steady-state conditions. It will be of interest to further analyze if the expression of unique molecules could be triggered on the surface of parasite-loaded leukocytes by the interaction with T gondii and thereby promote crossing of the brain endothelium as well as other barriers.42
How T gondii tachyzoites efficiently hijack circulating leukocytes remains a central question. Part of the answer might be given by the peculiar interaction we observed between single tachyzoites and these cells. Indeed, tachyzoites are usually characterized by their replicative properties within an intracellular parasitophorous vacuole.43,44 In parallel, leukocytes such as monocytes display well-known microbicibial properties when opsonized tachyzoites are entering through phagocytosis.45 However, if parasites have evolved a way to enter the leukocytes through a nonphagocytic process and interact with leukocytes without proliferating and to escape from the host cell microbicidal machinery, such strategy is likely to promote survival of both cells. In this study, relying on confocal microscopy analysis, we established that almost all parasites associated as single cells with DCs or monocytes but remained at the cell periphery, surrounded by plasma membrane. In the future, the identity of parasite ligands and host cell receptors involved in this interaction will shed light on the underlying mechanisms that impede the classic modes of parasite entry into host cells and that modify parasite fate.
Altogether, our data support that these single parasite–associated CD11b-positive leukocytes entered the brain. Within days in the brain, the proportion of single parasite–associated cells decreased, and T gondii multiplication and/or differentiation were/was observed. We can, however, speculate that shuttle leukocytes together with their parasites were taken up by different populations of parenchymal macrophages into which parasites subsequently multiply (CD11c- microglia/brain perivascular macrophages). In favor of this are cumulative data reporting that at least in vitro46,47,48 and very likely in vivo,49,50,51 several populations of nonleukocytic brain lineages were differentially permissive to T gondii entry, multiplication, and differentiation. The CD11b+ and CD11c+ cell population size increase in the brain in mice where the 76K T gondii reach the persisting phase,52,53 and we documented for the first time that these CD11c+ and C11b+ cells are parasitized (day 15 after inoculation of B6, BALB/c, or CBA/J mice).
In conclusion, once T gondii are present in the CD11b or CD11c leukocytes, the basal host cell trafficking properties of these leukocytes are modified, as assessed by their amplified entry in the brain extravascular space. The molecular basis of this interaction is suggestive of an intimate crosstalk between T gondii zoites and CD11b+ or CD11c+ leukocytes, as well as their bone marrow precursors and/or other unique bone marrow cells that we aim to further explore.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-02-0666.
Supported by the CNRS (Action thématique et incitative sur programme [ATIP] grant, I.T.), the Institut Pasteur (G.M., D.B.-G.), and the National Institute of Health (grants AI19316 and AI30000, D.B.-G.). N.C. was supported by Ensemble Contre le Sida.
D.B.-G. and I.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We deeply thank Dr R. Ménard (Institut Pasteur, France) for reading of the manuscript. We also thank Dr J.-C. Antoine (Institut Pasteur, France), Dr J. F. Dubremetz (UMR CNRS 5539, France) and Dr L. Renia (Institut Cochin, France) for the gifts of immunologic reagents. We acknowledge S. Celli (Institut Pasteur, France) for her help in the surgical removing of the mouse mesenteric lymph nodes.