Abstract
Congenital dyserythropoietic anemia type I (CDA I) is a rare autosomal recessive disorder with ineffective erythropoiesis and iron overloading. More than 100 cases have been described, but with the exception of a report on a large Bedouin tribe, these reports include only small numbers of cases, and no data on the lifetime evolution of the disease are available. Since 1967, we have been able to follow 21 cases from 19 families for up to 37 years. Twenty-one patients with a confirmed diagnosis of CDA I exhibited chronic macrocytic anemia of variable severity, requiring regular red cell transfusions only in 2 individuals. Four developed gallstones before the age of 30 years. Fifteen of 16 cases alive at the time of analysis showed mutations of at least one allele from exons 6 to 28 within CDAN1. Iron overloading is to be expected in all patients. In 9 patients, iron depletion was started between the ages of 7 and 36 years. Splenectomy, which was performed in 7 patients, did not result in improvement of hemoglobin values. Five patients were treated with interferon α-2a, and all responded with a rise in hemoglobin concentration of between 25 and 35 g/L (2.5 and 3.5 g/dL) starting within 4 weeks.
Introduction
The congenital dyserythropoietic anemias (CDAs) comprise a group of rare hereditary disorders of erythropoiesis that is characterized by ineffective erythropoiesis as the predominant cause of anemia and by distinct morphologic abnormalities of the majority of erythroblasts in the bone marrow. The term was first used by Crookston et al1 for cases later classified as CDA II and by Wendt and Heimpel for cases later classified as CDA I.2 In addition to the index cases, a pair of nonidentical twins, we were able to find 2 unrelated cases with the CDA I phenotype, by reviewing material of patients with unclassified congenital anemias.3 Further families were described mainly from France and Great Britain.4,5 Seventy-seven cases from 68 families have been published as case reports, including 9 of 21 confirmed cases of CDA I collated in the German CDA registry. In addition, there are 70 more cases in the large Bedouin tribe described by Tamary et al6,7 and Shalev et al8 from Israel in 1996. CDA I is characterized by congenital anemia, ineffective erythropoiesis, and characteristic morphologic abnormalities on both light and electron microscopy.9,10 The severity of anemia varies considerably within and between families.4,5 Nonhematologic dysmorphologies may be present, particularly peripheral limb abnormalities.11,12 As in CDA II, the main complication is iron overloading independent of transfusions. In members of the Bedouin tribe, the CDAN1 gene was mapped to chromosome 15q15.1-3,6 and a group of scientists from Israel and Europe recently cloned the mutant gene and described different mutations in 9 families of Arabian, Polynesian, and European origin. The protein encoded by the CDAN1 gene was named codanin-1.13
Clinical characteristics, complications, and problems of therapy are contained in case reports only, except for studies on the founder population of 45 children or young adults from the 4 extended Bedouin tribes from Israel.8,14 Here we report epidemiologic data, clinical manifestations, and the course of the disease in 21 patients from 19 unrelated families followed up by one of the authors. Mutations of the CDAN1 gene were investigated in all 16 patients who were alive at the time of analysis.
Patients, materials, and methods
Patients
A total of 25 patients from 22 families originally classified as CDA I were collated in the German CDA Registry. Diagnosis of CDA I was based on evidence of congenital chronic anemia and/or hyperbilirubinemia, with normal or inadequately increased reticulocyte counts and distinct hyperplasia of erythropoietic precursors suggesting ineffective erythropoiesis. All cases were reviewed in detail at the time of analysis. In 21 patients from 19 families, morphologic abnormalities of red cell precursors (“Results”) were typical for CDA I (Table 1). The following analysis is based on data from these 21 patients.
Clinical data were obtained from the institutions and physicians responsible for the patients' management from 1967 to 2004 and collated in the German CDA Registry. The study was approved by the Ethics Committee of the University of Ulm, Germany. Informed consent was obtained, in accordance with the Declaration of Helsinki, for additional blood or bone marrow samples taken for research purposes. A unique patient number was assigned to each individual using a code identifying the family, the subjects, and their relatives. Family trees were constructed by Cyrillic 2.1.2 (Cherwell Scientific Publishing, Oxfordshire, United Kingdom). Cross-sectional data of 9 patients from 8 families were published previously.2,3,11,15-17
Clinical and laboratory data
Data used to analyze the course of disease were retrieved by both retrospective search for patients' charts and other sources and by prospective monitoring after the diagnosis had been made and the patient was reported to the registry. Observation times, defined as the interval between the first and the last set of laboratory tests, ranged from 6 to 46 years. At the time of analysis, 5 patients had died between the ages of 31 and 57 years, and living patients were between 6.5 and 55 years old. The number of days from which laboratory data were obtained varied between 4 and 44. For presentation of relevant parameters, means of all representative data after the age of 3 months and before splenectomy or treatment with interferon-α or phlebotomy were used, if not otherwise stated. Data measured after transfusion or at the time of aplastic crisis or severe, unrelated illness such as neoplasia were excluded.
Splenomegaly was defined as a palpable spleen or a size greater than reference values according to age and weight on ultrasound. Laboratory procedures depended on the time period in which they were performed. Estimation of red cell survival followed standard procedures.18 Electron microscopy was performed as described elsewhere.9
Analysis of the CDA I gene on genomic DNA
Genomic DNA was isolated by the QIAmp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Coding sequences and the exon/intron boundaries of the CDA I gene were amplified using the Taq polymerase system (QIAGEN). Polymerase chain reaction (PCR) products were sequenced directly using the BigDye Terminator v1.1 Cycle Sequencing Kit and an ABI 3100 Sequencer (Applied Biosystems, Weiterstadt, Germany). Primers used for PCR and sequencing and PCR conditions are available on request (klaus.schwarz@medizin.uni-ulm.de).
Statistics
Microsoft Access 2000 (Microsoft, Seattle, WA) was used as the data bank management system, and statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC). Event rates were calculated according to the method of Kaplan-Meier, and subgroups were compared by the log-rank test. If not otherwise stated, the Spearman rank correlation coefficient was used to correlate laboratory values and the Wilcoxon signed rank test, to compare paired data.
Results
Epidemiology and inheritance
Seventeen and 4 patients came from 15 and 4 families resident in Germany or Switzerland, respectively. One family was of Turkish and one of Yugoslavian origin. In 11 of the 17 families of German or Swiss origin, ancestry could be traced back to at least 3 (maximum 9) generations without showing origin from regions outside Central or Northern Europe. Consanguinity of parents of the probands was detected in 2 families. Basic laboratory data are available from 26 first-degree relatives; there was no unexplained chronic anemia or hyperbilirubinemia, except in 3 affected siblings of probands, which is compatible with the autosomal recessive heredity mode of inheritance of CDA I. Six female and 6 male patients had 8 and 10 healthy children, respectively.
Diagnosis and clinical presentation
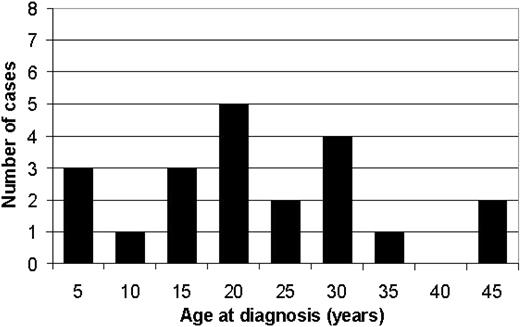
Anemia and/or jaundice were usually recognized in childhood or in young adults. The ages at first diagnosis of CDA I ranged from 0.1 to 45 years (median, 17.3 years) (Figure 1). In 2 patients, the diagnosis was made immediately after birth. Prior incorrect diagnoses included congenital hemolytic anemia (11), pernicious anemia (5), iron deficiency (4), or thalassemia (3). Seven patients had additional congenital malformations such as a sixth toe and syndactyly (3), a ventricular septal defect (1), short stature (1), double kidneys (1), or hip dysplasia (1). None of the patients was mentally retarded. At the time of diagnosis, the spleen was enlarged in 17 patients.
Laboratory data
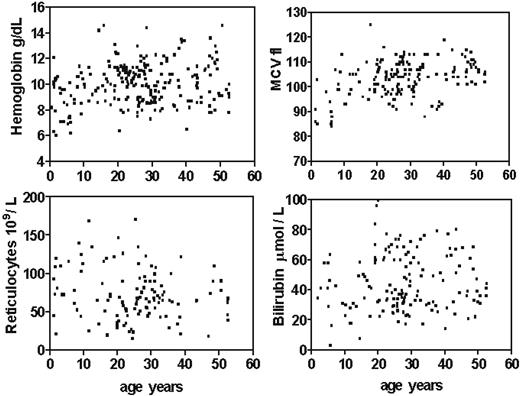
Key laboratory data of the 21 patients with confirmed CDA I are shown in Table 1. The degree of anemia varied, with mean hemoglobin concentrations between 64 and 132 g/L (6.4 and 13.2 g/dL). These values were almost stable throughout adult life, with the exception of single low values during severe infections or at pregnancy. Most but not all cases showed mild to distinct macrocytosis. The blood smear always showed anisopoikilocytosis as described elsewhere,10,19 with basophilic stippled erythrocytes in all and Cabot rings in 3 cases. Absolute reticulocyte counts were normal or moderately increased. Hemoglobin concentration and mean cellular volume (MCV) were lower, and relative and absolute reticulocyte counts were higher in children compared with adults (Figure 2). This could be shown by linear regression of all data (not shown). There were no significant differences between female and male patients. However, when values were compared only in individual patients with data available from an age of younger and older than 15 years the differences were not significant. Before splenectomy, white blood cell (WBC) and platelet counts were in the normal range throughout.
Red cell survival measured in 6 patients was moderately shortened, with apparent half-times of 16 to 25 days (normal, 25 to 35 days). Total serum bilirubin at the time of diagnosis was moderately increased in 90% of all cases. This was exclusively due to an increase of the indirectly reacting fraction. When follow-up data were included, all cases showed hyperbilirubinemia. Independent of the observed fluctuations, some patients had consistently higher levels than others. In all cases, serum haptoglobin was absent or below age-adjusted reference values.
Morphology
All patients had typical bone marrow findings as previously described.4,9,10,20 Bone marrow specimens obtained by aspiration and/or trephine biopsy invariably showed distinct hypercellularity due to erythroid hyperplasia. The ratio of erythropoietic to granulo-poietic cells (E/G ratio) varied between 3 and 8, compared with a normal range of 0.2 to 1. All 4 major abnormalities were seen: megaloblastoid aberrations of chromatin structure, large polyploid cells with an irregularly shaped nuclear mass often consisting of 2 segments, double- or triple-nucleated erythroblasts with nuclei of different sizes and structure, and pairs of predominantly mature cells connected by thin chromatin bridges in 1.1% to 3.7% of all erythroblasts. Thirty percent to 60% of all erythroblasts were considered abnormal.
Molecular genetics
Peripheral blood samples were available from 16 patients who were alive at the time of analysis (no samples were available from the 5 deceased patients). Fifteen patients with confirmed diagnosis of CDA I showed mutations of at least one allele from exons 6 to 28 within CDAN1 (Table 2) with all but one mutation being localized in exons 12 to 28. In all, 17 different disease-associated mutations were identified: 7 had been described previously,13,24 whereas 10 novel mutations were discovered (E 28 del -10 to +31 nts; del 1902-1904, 3259 insT; 3138 insTT; E 21 +2 nts insCCG; Gln1182Stop; Arg724Trp; Arg1064Gln; Gly749Cys; Phe359Leu). These novel mutations were not detected in 58 additional genomes (ie, 116 CDANI alleles), excluding polymorphism. Of the novel mutations, 2 were deletions, 3 were insertions, 1 mutation generated a premature stop codon, and 4 were missense mutations. Two of the novel mutations were predicted to affect the splice machinery. As expected, mutations were identical in 2 pairs of siblings (kindreds 026 and 409), and in the 2 alleles of case 447/01, the daughter of first-degree cousins of Turkish origin, both of whom were healthy heterozygotes (data not shown). Four additional parents of compound heterozygous patients analyzed exhibited CDANI heterozygosity without any signs of CDA I. In 6 patients, we were unable to describe more than one mutation, with the unidentified mutations most likely being located either in the 5′ or 3′ UTR, in the promoter, in enhancer regions, or in introns of CDANI. Results at the protein level are lacking due to the unavailability of an antibody. Quantification of codanin as well as search for its function are crucial points to be resolved in the future. In independent kindreds, mutations Pro671Leu and Pro1129Leu were present each on 3 alleles. Of interest, no homozygotes or compound heterozygotes for null-type mutations have been identified, supporting an earlier notion that codanin-1 may have a unique function and may be essential during development.
No mutations of the CDAN1 gene were detected in patient 303/01, who showed the definite phenotype of CDA I with 33 chromatin bridges in 1000 erythroblasts counted; of interest, iron loading was less than in other patients with 50% transferrin saturation and normal serum ferritin at the age of 40 years. For this case, pathogenic mutations remain to be discovered either in the CDA I gene or in a second disease locus.
Prognosis and course of disease
At the time of analysis, the age of living patients was between 6 and 55 years. Five patients had died between the ages of 31 and 56 years. All adults had completed at least 8 years of elementary and middle school education and 6 had graduated from college or acquired a similar professional qualification. Except for one patient who maintained hemoglobin concentrations of more than 110 g/L (11 g/dL), adolescents felt that their fitness was diminished compared with their classmates, and adults complained of moderate fatigue that became more marked during minor infections or social stress. Major health problems that can be regarded as dependent on the CDA are shown in Table 3. Patient 026/01 had a paravertebral bulk of extramedullary hematopoiesis that was removed without complications, and foci of hematopoiesis were detected in a liver biopsy in case 406/01. Leg ulcers, if present, were bilateral, overlaying, or proximal to the medial or lateral malleoli or both.
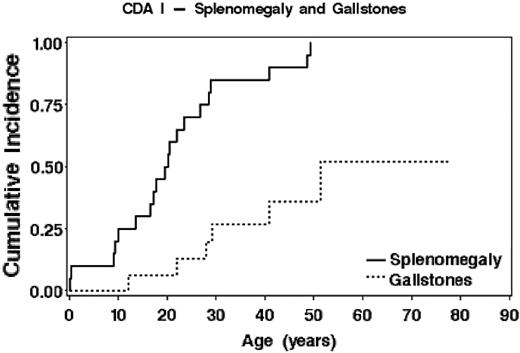
Moderate splenomegaly was present before or at diagnosis in 17 of 21 patients. In the majority of the other patients, splenomegaly became apparent in the first 3 decades of life. Gallstones were found in 4 patients before the age of 30 years and were sometimes detected in childhood or adolescence (Figure 3). Cholecystectomy was performed in 2 siblings at the ages of 44 and 51 years.
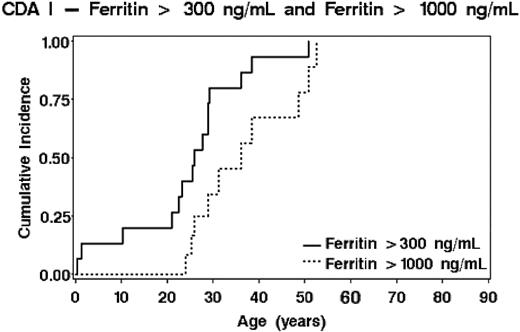
Twenty of 21 patients developed iron overloading, as ascertained by serum ferritin and transferrin saturation and/or invasive or noninvasive estimation of liver iron. Only data obtained before splenectomy or interferon therapy and before a first course of iron depletion were used. Probability of ferritin values of 300 μg/L or more or 1000 μg/L or more dependent on age is shown in Figure 4. There were large interindividual differences. Maximal increments were seen between the ages of 20 and 40 years, although only one adult patient received regular transfusions. No differences between males and females were seen (log-rank, P = .45). Concentrations of more than 2000 μg/L with maximal values up to 5000 μg/L were observed in 7 patients in whom the diagnosis was delayed (or unknown before 1967), or in patients not properly monitored or incompliant to iron depletion therapy.
At the time of analysis, 5 patients had died between the ages of 31 and 57 years, with heart and liver disease reported as the cause of death in 3 patients (027/01, 076/01, 417/01), squamous carcinoma of the ear in 1 patient (413/01), and septicemia in 1 splenectomized patient (028/01). Autopsy data available for 2 patients (027/01, 076/01) who died at the ages of 31 and 48 years revealed severe hemochromatosis.
Therapy
Red-cell transfusions were given to 12 patients. Five patients received 1 to 10 transfusions in the first 4 years of life, but not thereafter. Four females were transfused during pregnancy in order to ensure hemoglobin concentrations higher than 80 g/L (8 g/dL). With the exception of one male who received 28 transfusions, no adult patient was transfusion dependent.
Several patients had been treated with iron (7), a variety of vitamins (5), or with prednisone (1) without evidence of improvement. Five patients (026/01, 026/02, 177/01, 178/01, 406/01) were treated with interferon α-2a with a starting dose of 9 million units per week, and in one patient this was later switched to pegylated interferon α-2a 50 μg per week. They all responded with a rise of hemoglobin concentrations between 25 and 35 g/L (2.5 and 3.5 g/dL), the increase starting within 4 weeks after the first application. Hemoglobin concentrations fell to pretreatment levels in 2 patients who felt that side effects decreased their quality of life.
Splenectomy was performed on 7 patients ranging in age from 9 to 28 years. Data before and after splenectomy are available in 6 of those patients. Transient increase of hemoglobin concentration was seen in 2 cases, but was not different before and after splenectomy for the group as a whole (Wilcoxon signed rank test, P = .44). Improvement of quality of life was not documented. The sister of one patient (case not included in this series) died one month after splenectomy of “hemolytic anemia.” All splenectomized patients had long-standing thrombocytosis with maximum levels between 450 and 830 × 109 cells/L (median, 780 × 109 cells/L). Two had splanchnic or major deep venous thrombosis. Further increase of ferritin after splenectomy was documented in 4 of the splenectomized cases and all had to be treated with deferoxamine.
Altogether, 9 patients were treated with deferoxamine and/or deferiprone in doses recommended for treatment of secondary hemochromatosis in thalassemia major.25 Treatment was usually begun when plasma ferritin reached a serum concentration of 1000 μg/L (1000 μg/L). Age at initiation of deferoxamine therapy ranged from 7 to 35 years (median, 21 years). Reduction of elevated serum ferritin concentrations was achieved in all patients, while normal ferritin concentrations (< 300 μg/L) were reached in all patients with satisfactory compliance. Two patients underwent phlebotomies of 200 to 300 mL every 4 to 6 weeks, with normalization of ferritin and transferrin iron saturation.
Risk of iron overloading dependent on age. The probability of reaching concentrations of 300 μg/L and of 1000 μg/L serum ferritin is shown.
Risk of iron overloading dependent on age. The probability of reaching concentrations of 300 μg/L and of 1000 μg/L serum ferritin is shown.
Discussion
CDA I is a rare but well-defined clinical entity that has been observed in many regions in the world, most cases having been reported from the Central and Western European countries, North Africa, and the Near East.4,5,26 Descent from non-European populations could not be detected in pedigrees of 19 of 21 families residing in Germany or Switzerland (Table 1), and there is no evidence of a higher incidence in the large Mediterranean immigrant populations living in these countries than in families of Central European ethnicity. However, since the diagnosis requires microscopic assessment of bone marrow erythroblasts by an experienced expert, the ascertainment rate probably depends on the access of patients with chronic anemia to qualified hematologic diagnosis; therefore, many cases go undetected. Indirect evidence for this hypothesis is the large variation of the ages at which the diagnosis is made. The distribution of patients' age as shown in Figure 1, is similar to that of 50 of 70 cases identified from the literature (data not shown), excluding the patients of the highly inbred Bedouin tribes, in which all members were systematically investigated.7 This is also true for cases that were newly diagnosed after CDA I was recognized as an independent entity in the late 1960s.
Most adult patients with CDA I have moderate macrocytic anemia with rather stable hemoglobin concentrations between 80 and 110 g/L. Median values in our patients correspond well with data reported by others.4,14,27 Occasionally, anemia may be severe enough to require blood transfusions in infancy and childhood but not thereafter, and exceptional patients remain transfusion dependent in later life.28 As a newborn, one female had severe anemia and neonatal sepsis requiring multiple red cell transfusions and intensive care, but was able to maintain stable hemoglobin concentrations after the age of 4 years. Similar observations have been made by others,29,30 and 17 of 31 patients of Bedouin tribe members were symptomatic in the newborn period.8 On the other hand, one patient (UPN 409/04) showed macrocytosis without anemia, whereas his sister had moderately low hemoglobin values between 100 and 120 g/L (10-12 g/dL) documented for 30 years. Only 3 cases with borderline anemia were described previously.21,31,32
Diagnosis of CDA I is based on evidence of an initially unspecified congenital dyserythropoietic anemia4,26,33 and typical morphology of bone marrow erythroblasts.4,9,10,20 Evidence of ineffective erythropoiesis was based on absence of adequate reticulocytosis in all our patients, contrasting with evidence of increased hemoglobin turnover as shown by greatly increased cellularity of bone marrow due to distinct hyperplasia of erythroblasts, indirect hyperbilirubinemia, and depletion of haptoglobin. In addition, ineffective erythropoiesis had been previously demonstrated by combined DNA—measurement and 3H-thymidin labeling in 234 and by ferrokinetic analysis in 3 of our patients3 as well as in cases reported in the literature.35,36 All 21 patients showed the characteristic morphologic changes of erythroblasts, confirmed in 9 cases by electron microscopy. Chromatin bridges, which are a hallmark of CDA I, may be seen in no less than 1% of erythroblasts, and at least 500 consecutive erythroblasts should be examined when searching for this abnormality.4 In contrast to CDA II,33 to date no features other than morphology have been available to establish the diagnosis. Dgany et al13 were the first to demonstrate that mutations of the condanin-1 gene first observed in the Bedouin families were also present in 6 unrelated families with CDA I from Europe and in one each from Arabia and Polynesia, as well as in 8 kindreds from France.24 The observation of mutations of the codanin-1 gene in 15 of 16 patients from Germany and Switzerland confirms these results. In accordance with similar clinical features as observed by the lifelong follow-up of our patients, these results suggest that CDA I is more than a phenotypic entity, and that the presence of codanin-1 mutations strongly supports the diagnosis of CDA I. DNA was available from an additional 4 of the 25 patients originally enrolled in the German CDA Registry but not confirmed by review, and no mutations could be detected. CDA I may be of variable severity, but this variability seems to reflect a spectrum rather than the presence of different subentities. In the small group of patients analyzed, no phenotype-genotype correlation could be established from our mutation analyses. Analysis of our cases according to the need for transfusions in childhood, degree of anemia or hyperbilirubinemia, or kinetics of iron loading failed to reveal correlations to the exon in which the mutation was detected. Since clinical severity was different in 2 pairs of siblings, factors determining “normal” variability of erythropoiesis are more likely to determine clinical expression than the codanin-1 mutation, per se.
Although many patients with CDA I are able to realize their social and professional goals, others may have relevant morbidity and if untreated may even die of sequels related to CDA I. As in CDA II,33 gallstones were detected more frequently and at younger age than expected in the healthy population. Bulky paravertebral erythropoiesis may occur, requiring thoracotomy in one of our patients. Iron overloading has been recognized as a potential consequence in most but not all patients described as case reports or cross-sectional studies of groups of cases,3,14,27,37,38 but our observations are the first to show that on long-term follow-up iron overloading with potential tissue damage is to be expected in almost all patients. Lifelong monitoring of serum ferritin is therefore mandatory, and iron depletion should be started if ferritin concentrations approach a level of 1000 μg/L, or if there is other evidence for organ damage by secondary hemochromatosis.
In contrast to CDA II, splenectomy is not recommended as a standard procedure even in patients with marked anemia, although in exceptionally severe cases it may lessen the need for transfusions.39 Our observations are consistent with the only report describing long-term follow-up in one patient.32 As in CDA II,33 splenectomy does not prevent further iron overloading. Interferon-alpha was effective in 5 of our patients and in all cases reported.5 According to a recently published follow-up of the first patient treated with interferon α-2a,40 this treatment, beyond normalizing the hemoglobin concentration, seems also to normalize the up-regulated enteral iron uptake, responsible for the iron overloading in CDA as well as in other states with chronic ineffective erythropoiesis.41
One successful allogeneic sibling bone marrow transplantation was reported.42 Like those with other rare anemias, patients with CDA I should be treated by hematologists in cooperation with specialized centers in order to recognize disease-specific complications and to avoid superfluous diagnostic procedures as well as potentially harmful therapies. Monitoring of iron status to prevent clinically relevant secondary hemochromatosis is essential. Collation of all cases in national or international registries should be attempted in order to establish evidence-based recommendations for the lifelong management of these patients and to define the indication for special therapeutic measures such as therapy with interferon-α or allogeneic stem cell transplantation in exceptionally severe cases.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-01-0421.
Supported by the Else Kröner-Fresenius-Foundation.
H.H. and K.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We should like to express our gratitude for excellent technical work to Tatjana Kersten and Christine Eggl, and to J. Forteza-Vila, R. Martin, and E. Schaefer for their help with EM. Data and blood samples of patients were obtained from many physicians in charge of the patients. This paper could not have been written without their interest and support.