Abstract
The Integruppo Italiano Linfomi (IIL) carried out a study to assess the outcomes of splenic marginal zone lymphoma and to identify prognostic factors in 309 patients. The 5-year cause-specific survival (CSS) rate was 76%. In univariate analysis, the parameters predictive of shorter CSS were hemoglobin levels below 12 g/dL (P < .001), albumin levels below 3.5 g/dL (P = .001), International Prognostic Index (IPI) scores of 2 to 3 (P < .001), lactate dehydrogenase (LDH) levels above normal (P < .001), age older than 60 years (P = .01), platelet counts below 100 000/μL (P = .04), HbsAg-positivity (P = .01), and no splenectomy at diagnosis (P = .006). Values that maintained a negative influence on CSS in multivariate analysis were hemoglobin level less than 12 g/dL, LDH level greater than normal, and albumin level less than 3.5 g/dL. Using these 3 variables, we grouped patients into 3 prognostic categories: low-risk group (41%) with no adverse factors, intermediate-risk group (34%) with one adverse factor, and high-risk group (25%) with 2 or 3 adverse factors. The 5-year CSS rate was 88% for the low-risk group, 73% for the intermediate-risk group, and 50% for the high-risk group. The cause-specific mortality rate (× 1000 person-years) was 20 for the low-risk group, 47 for the intermediate-risk group, and 174 for the high-risk group. This latter group accounted for 54% of all lymphoma-related deaths. In conclusion, with the use of readily available factors, this prognostic index may be an effective tool for evaluating the need for treatment and the intensity of therapy in an individual patient.
Introduction
Splenic marginal zone B-cell lymphoma is one of the marginal zone lymphomas (MZLs) recognized by the World Health Organization (WHO) classification.1,2 The presence of circulating villous lymphocytes defines splenic lymphoma with villous lymphocytes,3 which is considered the leukemic counterpart of splenic MZL.4 The relationship between splenic MZL and the other lymphomas of marginal zone origin and its modality of diffusion are still under debate.5-7 Diagnosis is traditionally based on spleen histology, presence of circulating villous lymphocytes, or both. Evaluation of the bone marrow infiltration pattern has been proposed as a reliable diagnostic criterion to replace histologic examination of the spleen.8 Most patients are asymptomatic at diagnosis and may not require treatment for years. The disease commonly pursues an indolent course9,10 ; median overall survival time exceeds 10 years.11 However, the disease follows an aggressive course in approximately one third of patients, who die within 4 years after diagnosis.12 Patients with an aggressive clinical course are more likely to have the 7q31 deletion, to lack IgVH somatic mutations, or both.13 Clinical and biologic prognostic factors reported thus far for splenic MZL are heterogeneous.14 The International Prognostic Index (IPI) score, initially proposed for aggressive lymphoma15 and validated for low-grade lymphoma,16 has no value in splenic MZL.17 It has been reported that survival time is significantly shorter in patients with a monoclonal component, high β2-microglobulin level, leukocyte count greater than 20 000/μL, and lymphocyte count greater than 9000/μL.17 In a series of patients with splenic MZL with villous lymphocytes, those who underwent splenectomy fared better than those who received chemotherapy.11 In fact, splenectomy is considered the treatment of choice for splenic MZL, especially if cytopenias are present.11,17
There is still no reliable clinical score system to provide an effective prognostic stratification of patients with splenic MZL. The Intergruppo Italiano Linfomi (IIL) carried out a multicenter study of clinical features and outcomes of patients with this uncommon lymphoma, the aim of which was to build a prognostic model for splenic MZL. Here we report the results of this study performed on 309 patients, and we describe a simple prognostic model for clinical use.
Patients and methods
We studied 309 patients with splenic B-cell marginal zone lymphoma, with or without villous lymphocytes, diagnosed between March 1989 and March 2004. Approval for this retrospective study, which was based on the use of archival data, was obtained from the Institutional Review Board of the Intergruppo Italiano Linfomi (IIL, Modena, Italy). Data management and analysis were performed in accordance with the ethical guidelines of the Modena Cancer Registry and the tenets of the Declaration of Helsinki of 1964, as revised in 2000. Spleen and bone marrow specimens were histologically reviewed by the panel of hematopathologists participating in the study and were classified according to WHO criteria.1,2 Histologic findings were combined with clinical, immunophenotypic, and laboratory data, including the following hallmarks: (1) detection of clonal, light chain–restricted, circulating CD19+/CD20+ B lymphocytes negative for CD5, CD10, CD23, and CD25; (2) greater than 10% typical villous lymphocytes in the peripheral blood; and (3) intrasinusoidal bone marrow infiltration by CD20+CD5–CD10– mature B lymphocytes. Patients without clinically palpable splenomegaly were included if they had typical bone marrow infiltration by CD20+CD5–CD10 mature B lymphocytes and peripheral blood involvement.5 A preliminary database of 372 patients was established, from which 63 patients were excluded for one or more of the following reasons: poor histomorphology, incomplete clinical data, diagnosis on the basis of cytology and flow cytometry only. Ninety-one patients from 2 previously published studies are included in the present series.9,10 Mucosa-associated lymphoid tissue (MALT) localization of lymphoma was not observed in any patient at diagnosis. Demographic characteristics, clinical symptoms, hematologic features, treatment, type of response, response duration, and cause of death were the data analyzed. Clinical and hematologic data were obtained before splenectomy.
Treatment
Eighty-one patients were observed without treatment. One hundred twenty-four underwent splenectomy (followed by no treatment, 75; chemotherapy, 47; interferon treatment, 2), 88 received chemotherapy only (alkylating agent, 40; anthracycline-containing regimen, 26; fludarabine-based regimen, 9; pentostatin, 8; cyclophosphamide, vincristine, prednisone [CVP], 5), 5 received immunochemotherapy, 3 received therapy with rituximab as a single agent, 4 received splenic radiotherapy, and 4 received interferon treatment alone.
Response criteria
Complete remission (CR) was defined as the complete disappearance of all detectable sites and symptoms of disease. Partial response (PR) was defined as a greater than 50% reduction in disease localizations. Progressive disease (PD) was defined as a greater than 25% increase in size of previously documented disease or the appearance of disease at any site. Shift to a more aggressive histologic pattern was also considered PD.
Statistical methods
Numeric variables are summarized by their medians and ranges. Categorical variables are described by counts and relative frequencies. Association between splenic MZL and the categorical variables was tested with the Fisher exact test (for 2 × 2 tables) or with χ2 approximation for larger tables. The Kaplan-Meier product-limit method was used to estimate survival curves, and the log-rank test was adopted to carry out comparisons between different groups of patients. For each patient, overall survival (OS) was calculated as the time between the date of diagnosis and the date of death or last follow-up for censored cases. Cause-specific survival (CSS) was calculated as the time between the date of diagnosis and the date of lymphoma-related death or last follow-up for censored cases. Event-free survival (EFS) was calculated as the time from the date of first-line therapy until the date of event (treatment after an initial watch-and-wait policy, relapse or progression of disease, death from any cause). Variables analyzed for influence on OS and CSS in the entire series were sex; age older than 60 years; lactate dehydrogenase (LDH) greater than normal value; largest spleen diameter greater than 20 cm; presence of bone marrow involvement; bone marrow involvement greater than 20% and greater than 30%, respectively; nodal disease; thoracic lymph nodes; superficial lymph nodes; hepatic involvement; extranodal sites (1 vs more than 1); performance status (ECOG 0-1 vs ECOG 2-3); Ann Arbor stage (I-II vs III-IV); IPI score (0-1 vs 2-3); hemoglobin level less than 12 g/dL; platelet count less than 100 000/μL; blood involvement; leukocyte count less than 3500/μL, greater than 20 000/μL, greater than 30 000/μL; villous lymphocytes (yes vs no); lymphocyte count less than 4000/μL, greater than 9000/μL, greater than 16 000/μL; β2-microglobulin level greater than 3 mg/mL; albumin level less than 3.5 g/dL; serum monoclonal component; HCV-serology (positive vs negative); HbsAg (positive vs negative); presence of B symptoms; splenectomy (yes vs no); chemotherapy (yes vs no); anthracycline-containing regimen (yes vs no); CR vs non-CR; autoimmune background; and history of other neoplasm. Factors independently associated with OS and CSS were identified in multivariate analysis by the Cox proportional hazard regression model. The multivariate model included all variables observed at diagnosis that significantly affected survival in univariate analysis. The limit of significance for all analyses was defined as P at or below .05. Variables associated with the highest hazard ratios (HRs) and lowest P values in multivariate Cox regression were then included in the computation of the prognostic model. Because HRs were of comparable magnitude, all factors were given the same weight; the presence of a risk factor contributed to the score with an increment of 1. Patients with 2 or 3 risk factors were grouped because of the small number of patients in the highest risk group. The prognostic value of the score was assessed by comparing survival in the different prognostic categories of the whole data set and within clinical groupings. All computations were performed using Statistica 7.1 (StatSoft, Tulsa, OK) and Excel 97 (Microsoft, Redmond, WA).
Results
Clinical features
Clinical presenting features are summarized in Table 1. Of 309 patients (151 men, 158 women; median age, 64 years; range, 33-86 years), 296 received their diagnosis while in stage IV disease. Splenomegaly was detected at diagnosis in 290 patients (94%); 177 patients (59%) had peripheral blood involvement, and 38 patients (13%) had greater than 10% circulating villous lymphocytes in peripheral blood. Bone marrow was involved in 289 patients (94%). B symptoms were present in 61 patients (20%). Thirty patients (10%) had a poor performance status (ECOG score 2 or higher), 145 (48%) had hemoglobin levels below 12 g/dL, and 71 patients (24%) had platelet counts below 100 000/μL. Leukocyte counts were less than 3500/μL in 55 patients (18%), and lymphocyte counts were greater than 9000/μL in 77 patients (26%). LDH levels were above normal in 76 patients (28%). Twenty-five patients (8%) had a monoclonal component (19 IgM, 4 IgG, 1 IgG/IgM, and 1 IgA), with a median concentration of 1 g/dL. Autoimmune manifestations were detected in 28 patients (9%), as follows: autoimmune hemolytic anemia, 10 patients; immune thrombocytopenia, 2; vasculitis, 2; laboratory abnormalities without clinical signs of autoimmune disease, 14. IPI score was applicable to 270 patients; 60 (22%) ranked in the low-risk, 144 (54%) in the low-intermediate–risk, 60 (22%) in the intermediate-high–risk, and 6 (2%) in the high-risk groups. Twenty-four second cancers were registered (antecedent in 10, concomitant in 4, subsequent in 10). The overall response rate was 88% (30% CR, 58% PR). In particular, 25% of patients treated with chemotherapy entered CR and 51% entered PR. Among patients who underwent splenectomy alone, 20% entered CR and 80% entered PR. Among those who underwent splenectomy followed by chemotherapy, 60% entered CR and 37% entered PR.
Serologic testing for HCV was performed in 255 patients (83%), and results were positive in 49 patients (19%). Of 56 patients tested for HCV-RNA, 25 (45%) had positive findings. No patient had negative serologic and positive HCV-RNA. Genotype was available for 15 patients (1b in 10, 2b in 1, and 2a/2c in 4) Cryoglobulins were detected in 13 of 130 patients tested. HbsAg was positive in 13 (5%) of 246 patients tested. We compared the clinical features of HCV-positive and HCV-negative patients (Table 2). The 2 cohorts differed significantly regarding the male-female ratio, B symptoms, nodal disease, abdominal nonlocoregional lymph nodes, presence of villous lymphocytes, cryoglobulins, and monoclonal component. CR was achieved in 62% of HCV-negative patients and in 46% of HCV-positive patients (P = .04).
Outcome
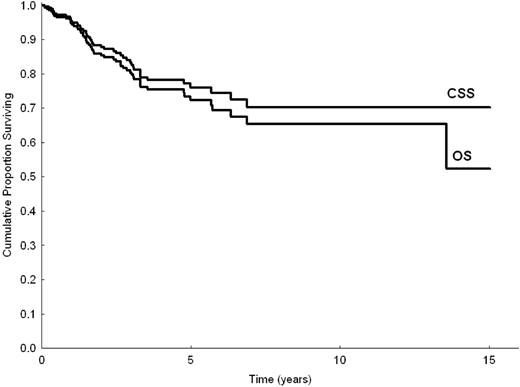
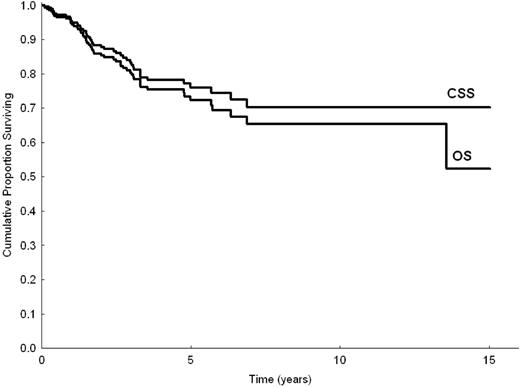
Mean follow-up time was 3.1 years (with 40% and 20% of patients followed up for longer than 3 and 5 years, respectively); 56 patients died (46 of disease-related causes, 4 of cardiovascular disease, 1 of solid cancer, and 5 of unknown causes). Five- and 10-year OS for the entire series were 72% (95% confidence interval [95% CI], 65%-79%) and 65% (95% CI, 56%-74%), respectively. Five- and 10-year cause-specific survival (CSS) were 76% (95% CI, 69%-82%) and 70% (95% CI, 61%-79%), respectively (Figure 1). The median survival time of patients who died was 1.6 years (range, 0.7-13.5 years). Of patients followed up with no treatment, 82% were expected to be alive at 5 years. Of those who received up-front therapy, 67% were expected to be alive at 5 years. Five- and 10-year EFS for all patients were 41% (95% CI, 33%-48%) and 19% (95% CI, 7%-31%), respectively. Five- and 10-year histologic shift rates were 5% (95% CI, 2%-10%) and 7% (95% CI, 2%-12%), respectively. Seven patients (2%) had disease relapse or disease progression to a MALT site during follow-up.
Univariate analysis
In univariate analysis, the following parameters were predictive of shorter OS and CSS (Table 3): hemoglobin level less than 12 g/dL, serum albumin level less than 3.5 g/dL, IPI score 2 to 3, LDH level higher than normal, age older than 60 years, platelet count less than 100 000/μL, HbsAg-positivity, and no splenectomy at diagnosis. The following parameters were predictive of shorter EFS: use of chemotherapy (P < .001), no splenectomy at diagnosis (P < .001), platelet count below 100 000/μL (P = .01), hemoglobin level below 12 g/dL (P < .001), IPI score of 2 to 3 (P = .004), LDH level above normal (P = .008), serum albumin level below 3.5 g/dL (P = .01), use of an anthracycline-containing regimen (P = .01), absence of CR (P = .01), nodal disease (P = .02), and B symptoms (P = .05).
Prognostic model
In multivariate analysis, the 3 parameters that maintained a negative prognostic influence on OS and CSS were hemoglobin level less than 12 g/dL, LDH level higher than normal, and albumin level less than 3.5 g/dL (Table 4). The 3 parameters maintained statistical significance in multivariate analysis after adjusting for HCV status, and the hazard ratio associated with HCV infection was not statistically different from 1. The same applies to the use of anthracyclines.
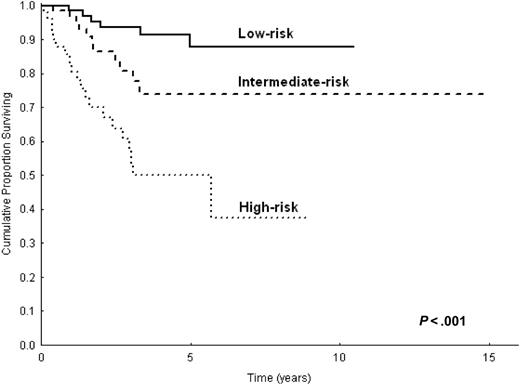
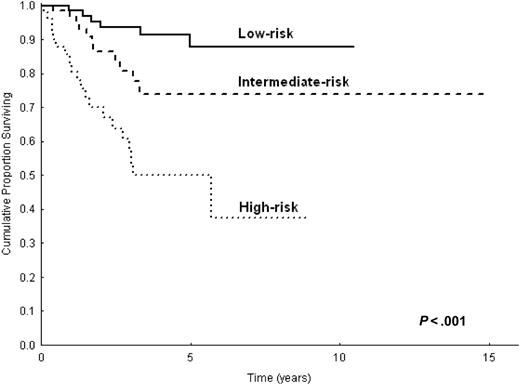
With the use of these 3 variables, we constructed a prognostic model in 232 patients. This model identified 3 prognostic groups with different OS and CSS (Figure 2; P < .001 for OS and CSS): group 1, low-risk (41%), with no adverse factors; group 2, intermediate-risk (34%), with one adverse factor; group 3, high-risk (25%), with 2 or more adverse factors. The 5-year CSS was 88% (95% CI, 77%-98%) for the low-risk group, 73% (95% CI, 58%-88%) for the intermediate-risk group, and 50% (95% CI, 33%-67%) for the high risk group. The cause-specific mortality rate (× 1000 person-years) was 20 for the low-risk group, 47 for the intermediate-risk group, and 174 for the high-risk group (Table 5). The high-risk group accounted for 54% (n = 25) of all lymphoma-related deaths, whereas the low- and intermediate-risk groups accounted for 16% (n = 7) and 30% (n = 14) of all lymphoma-related deaths, respectively. In addition, EFS curves for the 3 prognostic categories were significantly different (P = .01). OS and CSS of the 232 patients for whom all 3 parameters included in the prognostic model were available, and those of the remaining 77 patients in this study did not differ statistically (P = .1).
The distribution of patients treated up front (either by splenectomy or chemotherapy) among the 3 prognostic categories was statistically different (P < .001) from the distribution of patients in whom treatment was deferred.
Among patients treated up front, 32% were in the low-risk group, 38% in the intermediate-risk group, and 30% in the high-risk group. Among patients who received no therapy, the percentages were 65%, 23%, and 12%, respectively. The percentage of patients who received up-front treatment increased from 56% in the low-risk group, to 81% in the intermediate-risk group, to 85% in the high-risk group. We also compared the use of chemotherapy across the 3 prognostic categories: 56% of patients in the low- and intermediate-risk groups received chemotherapy compared with 73% in the high-risk group (P = .05). The use of splenectomy was more frequent in the low- and intermediate-risk categories (57%) than in the high-risk group (33%) (P = .006).
Split sample analysis
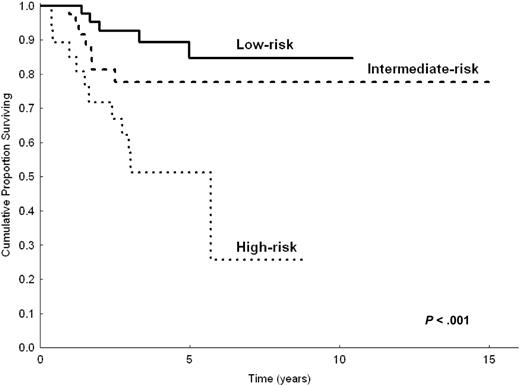
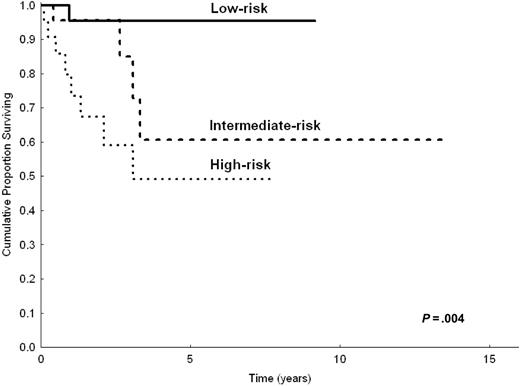
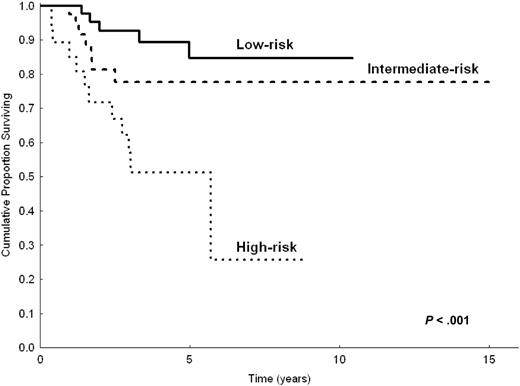
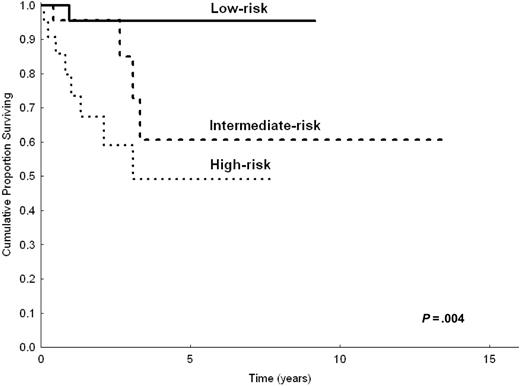
Even though the number of patients appeared relatively small to build a priori the prognostic model with a 2-step procedure (testing sample and validation sample), to demonstrate the robustness of our model we made the prognostic analysis after splitting the study population in 2 parts (two thirds and one third). Based on univariate analysis in two thirds of patients (n = 206) randomly chosen, the following parameters were predictive of shorter CSS: LDH level greater than normal (P < .001), hemoglobin level less than 12 g/dL (P = .002), serum albumin level less than 3.5 g/dL (P = .007), B symptoms (P = .03), platelet count less than 100 000/μL(P = .02), and nodal disease (P = .01). In multivariate analysis, the highest hazard ratios were reached by anemia (HR = 2.8), high LDH level (HR = 3.1), and hypoalbuminemia (HR = 2). However, because of the reduced number of patients, only anemia and high LDH level had significant P values (P = .03 and P = .01, respectively), whereas hypoalbuminemia did not (P = .1). The prognostic model, obtained by selecting the 3 variables with the highest HRs, still identified 3 prognostic groups (0 factors, 1 factor, 2 or more factors) with statistically different CSS (P < .001). This was applicable to the 154 patients in the testing sample for whom all 3 parameters were available (Figure 3). When applied to the remaining one third of patients (total, 103 patients; 78 had all 3 available parameters), the prognostic model maintained statistical significance (P = .004; Figure 4).
Discussion
Splenic MZL is a well-defined neoplasm that commonly follows an indolent course. The outcome of the disease, however, is heterogeneous. Most patients display a stable clinical course and do not require treatment for years, but in approximately one third of patients the disease follows an aggressive course and eventually causes death within 4 years.12 We studied clinical features and outcomes of a large series of patients with splenic MZL. The aim was to build a prognostic model to stratify patients at diagnosis into different risk categories.
This study provides a prognostic score for splenic MZL that combines 3 simple laboratory parameters—hemoglobin, LDH, and albumin levels. With the use of this model, 3 prognostic categories were identified with significantly different OS and CSS (Figure 2). The low-risk group was composed of 41% of the patients, all of whom were asymptomatic and whose lymphoma followed a true indolent course; disease-specific survival rate at 5 years was 88%. Conversely, the high-risk group was composed of 25% of the patients, 54% of whom eventually died of lymphoma. Identifying a subgroup with a worse prognosis seems particularly important for an indolent disorder such as splenic MZL, in which the indication for treatment is often uncertain. This model confirms the importance of anemia in the prognosis of lymphoproliferative disorders. The same degree of anemia (less than 12 g/dL) is one of the adverse factors included in the IPI score for aggressive lymphoma15 and in the Follicular Lymphoma IPI (FLIPI) score for follicular lymphoma.18 Anemia proved to negatively influence OS in a smaller series of patients with splenic MZL.10 Low serum albumin concentration, another adverse factor in this prognostic model, proved to be of prognostic value in other scores for malignant lymphomas, such as Hodgkin lymphoma.19
CSS of 233 patients with splenic MZL according to the 3 categories of the prognostic model.
CSS of 233 patients with splenic MZL according to the 3 categories of the prognostic model.
Recently, new prognostic markers have been proposed for splenic MZL on the basis of tissue and cDNA microarray studies.20 Shorter survival was associated with CD38 expression, naive IgVH genes, and NF-κB pathway gene expression (TRAF5, REL, PKCA). The use of these complex techniques confirms the existence of a subset of MZL patients with more aggressive disease.
A possible limitation of our analysis is represented by the relatively short follow-up, although 40% and 20% of patients were followed up for longer than 3 and 5 years, respectively. We excluded patients with dated diagnoses and with inadequate or incomplete data. A qualifying aspect of this study was the accuracy of diagnosis; in fact, we included only patients for whom good quality clinical, histologic, and immunophenotypic data were available at diagnosis. The long median survival time of patients with splenic MZL is well known.11 Our analysis focused on detecting the subset of patients who, though affected by a condition whose course is commonly considered indolent, have unfavorable outcomes. Identifying this subset might allow the application of more intensive treatment programs.
A 2-step procedure is usually required for building a prognostic index (1 for building and 1 for validation). In the field of lymphoma, this procedure has been used to build and to validate prognostic scores for common subtypes (eg, the FLIPI score for follicular lymphoma).18 Although the present series is the largest ever reported (309 patients), the number of study patients remains relatively small; as a consequence, partitioning the patients into 2 subgroups could have weakened the study's statistical power. However, we performed the analysis on a two thirds/one third partition of the study population, with the aim of demonstrating the robustness of the model. Multivariate analysis of two thirds of the patients showed high hazard ratios for the same 3 parameters that were valid for the entire population; in this subgroup, we were able to define 3 groups with statistically different CSS. The model was also effective in the remaining one third of patients.
CSS of 154 patients with splenic MZL, according to the 3 categories of the prognostic model. Calculation on two thirds (n = 206) of the study population.
CSS of 154 patients with splenic MZL, according to the 3 categories of the prognostic model. Calculation on two thirds (n = 206) of the study population.
CSS of 78 patients with splenic MZL according to the 3 categories of the prognostic model. Calculation on one third (n = 103) of the study population.
CSS of 78 patients with splenic MZL according to the 3 categories of the prognostic model. Calculation on one third (n = 103) of the study population.
In splenic MZL, splenectomy plays a dual role: diagnostic and therapeutic. Regarding diagnosis, surgical removal of the spleen is not essential because the integration of clinical data with bone marrow histology and flow cytometry enables a diagnosis without the need for surgery. Splenectomy, however, is considered the treatment of choice for patients with peripheral cytopenia or abdominal symptoms caused by an enlarged spleen.11,17,21 It has been shown that the extent of bone marrow infiltration by lymphoma may increase after splenectomy.22 As in previous reports, this study highlights that splenectomy is related to better OS and CSS as determined with univariate analysis. In multivariate analysis, however, splenectomy did not retain an independent prognostic value. Patients in low- and intermediate-risk prognostic categories more frequently underwent splenectomy. It is arguable that a significant proportion of these splenectomies were performed mainly for diagnostic purposes.
In this study, the HCV seroprevalence in patients with splenic MZL was 19%; this percentage was considerable despite the high seroprevalence of HCV infection in the Italian population.23 However, the association of HCV infection with splenic MZL has not been found in other series.17 A new clinical entity characterized by concurrent splenic MZL with villous lymphocytes, type II cryoglobulinemia, and HCV infection has been proposed.24 The reported antilymphoma activity of interferon-α in patients with HCV-positive splenic MZL24,25 and with other types of MZL26,27 supports a role for HCV infection in the lymphomagenesis of marginal zone lymphomas.
In conclusion, this study of a large population of patients shows that splenic MZL has a heterogeneous clinical behavior. Using readily available clinical factors, we designed a simple prognostic model that identified 3 categories with significantly different survival. Ongoing research on features closely related to the biology of the disease, such as karyotypic abnormalities, IgVH gene mutational status, and gene expression profile, might shed light on the biologic heterogeneity of this type of lymphoma. For clinical use, this prognostic model, based on simple variables, may be a useful tool to stratify patients in different risk groups, to establish the need for therapy, and to compare different treatment modalities.
Appendix
Participating institutions and principal investigators of the Intergruppo Italiano Linfomi on splenic marginal zone lymphoma are as follows:
Division of Hematology, IRCCS Policlinico San Matteo, University of Pavia, Pavia (Luca Arcaini, Nora Colombo, Sara Burcheri, Francesco Passamonti, Cristiana Pascutto, Mario Lazzarino); Division of Pathology, IRCCS Policlinico San Matteo, University of Pavia, Pavia (Marco Paulli, Emanuela Boveri, Marco Lucioni, Umberto Magrini); Division of Hematology, University of Palermo (Viviana Minardi, Emilio Iannitto); Division of Pathology, University of Palermo (Claudio Tripodo, Vito Franco); Division of Hematology, Ospedale Niguarda Ca' Granda, Milano (Livio Gargantini, Giovanna D'Avanzo, Enrica Morra); Division of Pathology, Ospedale Niguarda Ca' Granda, Milano (Marcello Gambacorta); Division of Hematology, Ospedali Riuniti Bergamo (Andrea Rossi, Sergio Cortelazzo); Division of Hematology, Ospedali Civili, Brescia (Alessandra Tucci, Giuseppe Rossi); Division of Pathology, Ospedali Civili, Brescia (Marco Ungari); Department of Clinical and Experimental Medicine, University of Verona, Verona (Achille Ambrosetti, Maura Colosio, Giovanni Pizzolo); Department of Pathology, University of Verona, Verona (Fabio Menestrina); Division of Hematology, Azienda Ospedaliera S. Giovanni Battista, Torino (Lorella Orsucci, Umberto Vitolo, Eugenio Gallo); Division of Pathology, Azienda Ospedaliera S. Giovanni Battista, Torino (Domenico Novero); Department of Hematology, University “La Sapienza” University, Roma (Alessandro Pulsoni, Natalia Frattarelli, Robin Foa); Division of Hematology, Ospedale S. Bortolo, Vicenza (Maurizio Frezzato, Francesco Rodeghiero); Division of Pathology, Ospedale S. Bortolo, Vicenza (Emanuela Bonoldi); Division of Hematology, Amedeo Avogadro University of Eastern Piedmont, Novara (Davide Rossi, Gianluca Gaidano); Division of Pathology, Ospedale Maggiore, Novara (Antonio Ramponi); Bone Marrow Transplant Unit, Azienda Ospedaliera Bianchi-Melacrino-Morelli, Reggio Calabria (Caterina Stelitano, Vincenzo Callea); Department of Hematology, IRCCS Ospedale Maggiore, University of Milano, Milano (Luca Baldini, Maria Goldaniga, Giorgio Lambertenghi Deliliers); Division of Medicine and Hematology, Ospedale G. da Saliceto, Piacenza (Daniele Vallisa, Patrizia Bernuzzi, Luigi Cavanna); Division of Hematology, Ospedale S. Maria Nuova, Reggio Emilia (Francesco Merli, Luigi Gugliotta); Department of Oncology and Hematology, University of Modena and Reggio Emilia, Modena (Stefano Luminari, Monica Bellei, Massimo Federico); Division of Hematology, University of Parma (Monica Crugnola, Vittorio Rizzoli); Division of Hematology, Ospedale S. Paolo, Milano (Lilj Uziel); Division of Pathology, Ospedale S. Paolo, Milano (Umberto Gianelli); Division of Medical Oncology A, National Cancer Institute, Aviano (Michele Spina, Umberto Tirelli); Division of Hematology, Ospedale S. Croce, Cuneo (Roberta Calvi, Silvia Tavera, Andrea Gallamini); Division of Hematology, Policlinico S. Maria alle Scotte, University of Siena (Alberto Fabbri, Francesco Lauria); Division of Hematology, Polytechnic University of Ancona, Ancona (Mauro Montanari, Pietro Leoni); Department of Hematology, Catholic University Medical School, Roma (Annalaura Di Febo, Maria Teresa Voso); Department of Hematology, Ospedale S. Maurizio, Bolzano (Atto Billio, Paolo Coser); Division of Hematology, University “Federico II,” Napoli (Amalia De Renzo, Bruno Rotoli); Division of Hematology, Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro (Renato Cantaffa); Division of Hematology, University of Padova (Giampietro Semenzato); Division of Hematology, Cà Foncello Hospital (Filippo Gherlinzoni); and Division of Hematology, Ospedale di Circolo, Rho (Alessandro Vismara), Italy.
Prepublished online as Blood First Edition Paper, February 21, 2006; DOI 10.1182/blood-2005-11-4659.
A complete list of the members of the Intergruppo Italiano Linfomi appears in the “Appendix.”
L.A., E.I., M.L., and M.P. wrote the manuscript. L.A., M.L., E.I., E.M., S.C., L.B., N.C., S.B., A.T., A.A., L.O., A.P., M. Frezzato, G.G., M. Federico, D.V., F. Merli, V.C., and V.M. served as clinical investigators. M.P., V.F., E.B., M.G., M.U., F. Menestrina, D.N., and C.T. formed the pathology panel. N.C. was responsible for data management.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Cristiana Pascutto for performing statistical analysis and Ziggy Kennell for linguistic review of the manuscript.