Abstract
CTRP-1 is a novel member of the C1qTNF–related protein family containing family characteristic collagen and TNF-like domains and shows marked expression in vascular wall tissue. We observed that recombinant human CTRP-1 specifically bound to fibrillar collagen and blocked collagen-induced platelet aggregation. CTRP-1 completely or partially prevented VWF and GPVI-Fc4 binding to collagen, respectively. However, GPVI-Fc4 failed to compete for the binding of CTRP-1 to collagen. CTRP-1 had no effects on α2β1 integrin I–domain binding to collagen. Using whole human blood under flow at low and high shear rates, CTRP-1 prevented platelets from accumulating on a collagen-coated surface but had no effects on “platelet-rolling” on a surface coated with VWF. These data suggest that CTRP-1 prevents collagen-induced platelet aggregation by specific blockade of VWF binding to collagen. By using the Folts vascular injury model in nonhuman primates (Macaca fascicularis), we were able to demonstrate that CTRP-1 can prevent platelet thrombosis in vivo. This effect was achieved in the absence of changes in activated-clotting time (ACT) and template cut bleeding times, suggesting that CTRP-1 has promising antiplatelet thrombotic activity and most likely acts by pacifying the thrombogenic site of vascular injury.
Introduction
C1qTNF-related protein-1 (CTRP-1) belongs to a family of proteins characterized by a common TNF alpha–like globular domain.1-3 A second, less conserved structural element in this family is the N-terminal collagen-like region with typical glycine-X-Y repeats. The basic structure of proteins in the CTRP family appears to be a trimer that, in turn, can form higher order structures.4 While structurally related, members of this protein family are functionally diverse and include the plasma protein C1q, which is involved in immune functions and possibly platelet hemostasis,5 and adiponectin6-8 and the hibernation proteins 20, 25, and 27, which are thought to be regulators of metabolism9 ; other CTRP family members appear to have more structural or extracellular matrix–related functions (eg, collagen types VIII and X, and CTRP-5).10
Since C1q may play a role in collagen-induced platelet activation,11 we investigated whether CTRP-1 might exhibit similar properties. Initial studies indicated that CTRP-1 had affinity for collagen and blocked collagen-induced platelet activation. This raises the potential significance of CTRP-1 for human disease because of the central role collagen-induced platelet activation and thrombus formation play in acute cardiovascular and cerebrovascular events. Spontaneous rupture of atherosclerotic plaque or medical interventions can cause an injury to the arterial endothelial lining, thus exposing the subendothelial extracellular matrix to circulating blood. Collagens are a major component of the extracellular matrix and can directly and indirectly activate platelets.12-16 Three platelet receptors are thought to mediate these interactions. Integrin α2β1 is thought to have a major role in adhesion and platelet anchoring; GPVI, a member of the Ig superfamily, is responsible for collagen binding, signaling, and platelet activation; and GPIb-V-IX (GPIb), through its indirect binding to collagen-bound von Willebrand factor (VWF), enables the initial “rolling” of platelets on a collagen-containing surface under high shear conditions.12-18
In the present work, the mechanism by which CTRP-1 interferes with activation and aggregation of platelets by collagen was investigated, and the efficacy to prevent platelet thrombosis in vivo was tested.
Materials and methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (University of Washington Regional Primate Center).
Protein expression, purification, and characterization
Full-length recombinant human CTRP-1 was produced in CHO-DG44 cells transformed with a pZMP18-CTRP-1 expression vector. CTRP-1 was purified to homogeneity from the cell culture medium by sequential chromatographic steps and was characterized by various methods including amino acid analysis, N-terminal sequencing, and peptide mapping. CTRP-1 was dissolved in PBS and stored at –80°C.
Expression and purification of soluble GPVI-Fc4 fusion proteins
Expression constructs encoding 2 versions of soluble human GPVI-Ig fusion proteins were generated by overlap polymerase chain reaction (PCR)19 and homologous recombination in yeast.20 The first construct encoding GPVI-wtγ1Fc was modeled after the version described.18 The second version encoding GPVI-Fc4 contained the same mouse κ signal sequence and GPVI extracellular domain sequences, but included a modified human IgG1 Fc containing amino acid substitutions that prevent FcγRI binding and complement fixation.21
Preparation of 125I-CTRP-1 and 125I-GPVI-Fc4
Labeling of CTRP-1 and GPVI-Fc4 with 125I was accomplished by a modified version of the indirect Iodogen labeling procedure as described by the manufacturer (Pierce Chemical, Rockford, IL). CTRP-1 and GPVI-Fc4 were labeled to specific activities of 4300 and 4900 cpm/ng, respectively. More than 95% of the cpm in the final preparations was precipitated in 10% TCA.
Synthesis and cross-linking of collagen-related peptide (CRP)
CRP was synthesized (GKPGPPGPPGPPGPPGPPGPPGPPGPPGPPGPPGKPGV) and cross-linked with glutaraldehyde as described.22 Valine was added to the sequence to improve recovery during resin cleavage; a molar ratio of CRP to glutaraldehyde of 1:290 was used. The majority of the cross-linked product was 30 kDa or larger.
Preparation of collagen-, BSA-, or CRP-coated plates
MaxiSorp microtiter plates (no. 473768; Nunc, Roskilde, Denmark) were coated overnight at 37°C with equine fibrillar collagen I (Chrono-Par no. 385; Chrono-Log, Havertown, PA) or bovine collagen VI (CC086; Chemicon, Temecula, CA) using collagen dissolved in 285 mM glucose, pH 2.7 to 2.9, to a final concentration of 0.01 mg/mL. Control plates were coated with an identical concentration of BSA dissolved in PBS. Dry, coated plates were stored at 4°C and were blocked for 1 hour at room temperature (0.05 M Na phosphate, 0.109 M NaCl, 0.1% BSA, pH 7.5) prior to use. Reacti-Bind Maleic Anhydride clear strip plates (no. 15100; Pierce Chemical) were coated with cross-linked CRP dissolved in PBS to a final concentration of 5 μg/mL. Prior to use, peptide-coated plates were blocked with Super Block (Pierce Chemical) in Tris-buffered saline (TBS) for 1 hour at room temperature.
Binding of 125I-CTRP-1 and 125I-GPVI-Fc4 to collagen- or CRP-coated plates
The indicated concentration of 125I-CTRP-1 or 125I-GPVI-Fc4 was incubated in the absence (total binding) and presence (nonspecific binding) of 250 μg/mL unlabeled CTRP-1 or GPVI-Fc4 in a final volume of 0.2 mL blocking buffer (0.05 M Na phosphate, 0.109 M NaCl, 0.1% BSA, pH 7.5). For competition of 125I-GPVI-Fc4 binding to cross-linked CRP, each well was incubated with 0.05 μg/mL 125I-GPVI-Fc4 in the absence (total binding) or presence (nonspecific binding) of the indicated fold excess of unlabeled GPVI-Fc4. Blocking buffer for CRP binding also contained 0.025% Tween. Plates were gently agitated for 3 hours at room temperature before being placed on ice. Media were removed, each well was washed 3 times with 0.2 mL ice-cold blocking buffer, and radioactivity was measured. Binding data were analyzed by nonlinear regression using Prism (GraphPad, San Diego, CA).
Competition for collagen binding under static conditions
Plates (96-well) were coated with 1 μg type I (no. 885-1; Sigma, St Louis, MO) or type III (no. C4407; Sigma) collagen dissolved in 0.1 mL TBS overnight at room temperature. Plates were subsequently blocked with 2% BSA in TBS for 1 hour at 37°C. A mixture of either purified A1 (0.75 μM) or A3 (0.75 μM) domains of VWF, full-length VWF (0.75 μM), or purified I-domain (0.75 μM) of α2β1 integrin was incubated in the presence and absence of CTRP-1 in a final volume of 0.2 mL TBS. After 1 hour at 37°C, incubation mixtures were removed and the wells were washed with TBS. Collagen-bound A1 and A3 were detected by the addition of CR-1 (0.3 μg/mL) and anti-His HRP (1/2500; Sigma) antibodies, respectively. The α2-I domain was detected by the addition of anti-His HRP antibody (1:2500), and VWF was detected by the addition of anti–VWF-HRP antibody (1:500; DAKO, Carpinteria, CA) and visualized using secondary anti–mouse HRP (1:2500; Pierce Chemical) and OPD reaction solution.
Collagen-induced platelet aggregation
Collagen-induced platelet aggregation was performed using human platelet-rich plasma (PRP). CTRP-1 was mixed with PRP by gentle rocking in a 96-well flat-bottom plate. Collagen I was added at a final concentration of 1.25 μg/mL, and the plate was agitated at 37°C on the microplate reader; turbidity was monitored as percent light transmitted at 632 nm after 21.5 minutes as described previously.23 Platelet aggregation was determined in an aggregometer, and platelet activation was determined by measuring ATP release using a Chrono-Lum luciferase reagent (Chrono-Log) following the manufacturer's instructions.
Platelet aggregation under flow conditions
The parallel plate flow chamber24 was composed of a polycarbonate slab, a silicon gasket, and a glass coverslip coated with type I collagen (Helena Labs, Beaumont, TX). Coverslips were incubated with a solution of 100 μg collagen or VWF/mL for 1 hour at 37°C. Citrated whole blood alone or with 20 μg CTRP-1/mL added was incubated for 5 minutes at room temperature, the platelets were labeled by incubating the blood sample with 0.2 M mepacrine for 10 minutes in the dark, and the blood was perfused over the slide containing the immobilized collagen. A syringe pump was connected to the outlet port that drew blood through the chamber at defined flow rates (13 and 50 dyne/cm2) to generate a wall shear. The flow chamber was mounted onto a Nikon Eclipse TE300 inverted stage microscope (Nikon, Garden City, NY) equipped with a Quantix high-speed digital camera (Photometrics, Tucson, AZ). Magnification was 400 × (40 ×/0.65 numeric aperture objective, 10 × eyepiece). Acquired images were analyzed offline using MetaMorph Imaging System (Universal Images, West Chester, PA).
Folts vascular injury and bleeding models
The antithrombotic efficacy of CTRP-1 was tested in nonhuman primates (Macaca fascicularis) using the Folts model of vascular injury.25 The right carotid artery was surgically exposed by blunt dissection, and a flow probe (Transonic Systems, Ithaca, NY) was placed around the artery. After determining baseline blood flow, a partial stenosis (85%-90% flow) was established by placing a nylon ring around the vessel proximal to the flow probe. A 2- to 4-mm–wide crush-type injury was performed on the exposed artery, a stenotic ring was then positioned over the crush area, and blood flow was monitored. Each time blood flow approached zero (< 0.7 mL/min), the vessel was lightly tapped to dislodge occluding thrombi, thereby creating cyclic flow variations (CFVs). Once CFVs were confirmed, animals were treated with the indicated amount of protein. Tapping the occluding blood vessel was ceased 30 minutes following treatment. Bleeding effects were examined by measuring activated clotting time (ACT) using the SCA2000 Coagulation Analyzer (Synbiotics, San Diego, CA). In addition, template bleeding cuts were made on the forearm and time to hemostasis was determined (Surgicutt; ITC, Edison, NJ).
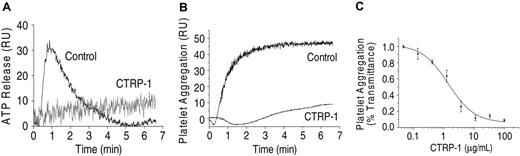
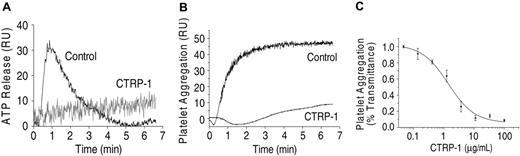
CTRP-1 prevents collagen-induced platelet activation and aggregation. Collagen-induced platelet activation (5 μg/mL collagen I) measured by ATP release (A) and platelet aggregation (B) are inhibited by CTRP-1 (5 μg/mL). The inhibition of platelet aggregation by CTRP-1 is dose dependent (C). For this experiment, PRP was incubated with 1.25 μg/mL collagen I. RU indicates relative units.
CTRP-1 prevents collagen-induced platelet activation and aggregation. Collagen-induced platelet activation (5 μg/mL collagen I) measured by ATP release (A) and platelet aggregation (B) are inhibited by CTRP-1 (5 μg/mL). The inhibition of platelet aggregation by CTRP-1 is dose dependent (C). For this experiment, PRP was incubated with 1.25 μg/mL collagen I. RU indicates relative units.
Results
CTRP-1 prevents collagen-induced platelet activation and aggregation
Incubation of PRP with fibrillar collagen type I produced a rapid activation and aggregation of platelets (Figure 1). Both processes were inhibited by CTRP-1 with virtually complete inhibition at CTRP-1 concentrations of 10 μg/mL or more (Figure 1). CTRP-1 had no significant effects on activation of platelets by ADP, ristocetin, or thrombin (data not shown).
Binding of 125I-CTRP-1 to collagen type I under static conditions
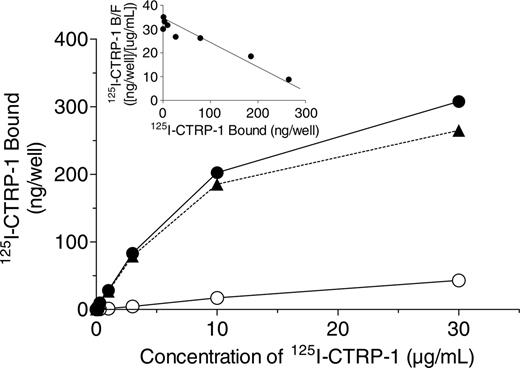
To evaluate whether CTRP-1 inhibition of collagen-induced platelet aggregation was due to CTRP-1 binding to collagen, the binding of 125I-CTRP-1 to immobilized fibrillar collagen type I was investigated. Binding of 125I-CTRP-1 to collagen I was saturable and specific (Figure 2). In contrast, little or no binding of 125I-CTRP-1 was observed to wells coated with an equivalent concentration of BSA (data not shown). Scatchard plots of the specific binding data were linear (Figure 2, inset), consistent with 125I-CTRP-1 binding to a single class of sites. 125I-CTRP-1 bound to fibrillar collagen type I with a Kd and Bmax of 8.78 ± 1.56 μg/mL and 493.3 ± 150.5 ng bound/well, respectively (mean ± SD, n = 4 separate experiments). Thus, 125I-CTRP-1 bound to immobilized collagen type I with a Kd of approximately 50 nM. The stoichiometry of 125I-CTRP-1 binding to collagen could not be readily determined since the amount of collagen bound to each well was not measured.
To evaluate whether the binding of 125I-CTRP-1 to collagen I was reversible, collagen I–coated wells were first incubated for 3 hours at 4°C with blocking buffer containing 125I-CTRP-1 alone and were then washed. Further incubation in the absence of unlabeled CTRP-1 resulted in little (14% of the total) of the previously bound 125I-CTRP-1 being released from the collagen I–coated plates over a subsequent 3-hour period. Incubation with increasing amounts of unlabeled CTRP-1, in contrast, effectively displaced the collagen-bound 125I-CTRP-1 with quantitative recovery of radiolabeled material in the media (data not shown). These data demonstrate that binding of 125I-CTRP-1 to fibrillar collagen type I was saturable, specific, reversible, and of relatively high affinity.
Inhibition of platelet adhesion on collagen I–coated surfaces by CTRP-1 under high shear
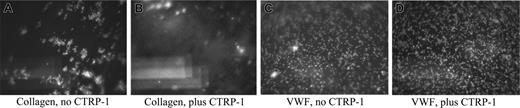
To evaluate whether CTRP-1 can inhibit collagen-induced platelet aggregation under biologic conditions of high shear seen typically in small and large arterial vessels, vehicle alone or vehicle containing 20 μg/mL CTRP-1 was mixed with citrated whole blood from healthy human donors and perfused through a collagen I–coated flow chamber at 13 dyne/cm2 (low shear) and 50 dyne/cm2 (high shear). Flow of whole blood over the collagen-coated surface at high shear immediately produced adhesion and deposition of mepacrine-labeled platelets on the surface, easily visualized under the microscope (Figure 3A). Incubation of whole blood for 5 minutes at room temperature with 20 μg CTRP-1/mL prior to pumping through the flow chamber abolished the adhesion and deposition of platelets on the collagen-coated slide (Figure 3B). Similar results were seen with flow at 13 dyne/cm2 (data not-shown). Thus, CTRP-1 can inhibit collagen-induced platelet deposition under conditions of low and high rates of shear.
Binding of 125I-CTRP-1 to fibrillar collagen type I. Plates were coated with fibrillar collagen type I and incubated with the indicated concentration of 125I-CTRP-1 in the absence (total binding, •) and presence (nonspecific binding, ○) of 250 μg/mL unlabeled CTRP-1. Specific binding (▴) was calculated as the difference between the total and nonspecific values. Each point represents the mean of duplicate wells and is representative of 4 separate experiments. Inset shows the Scatchard plot of the specific binding data. B indicates bound 125I-CTRP-1; F, free 125I-CTRP-1.
Binding of 125I-CTRP-1 to fibrillar collagen type I. Plates were coated with fibrillar collagen type I and incubated with the indicated concentration of 125I-CTRP-1 in the absence (total binding, •) and presence (nonspecific binding, ○) of 250 μg/mL unlabeled CTRP-1. Specific binding (▴) was calculated as the difference between the total and nonspecific values. Each point represents the mean of duplicate wells and is representative of 4 separate experiments. Inset shows the Scatchard plot of the specific binding data. B indicates bound 125I-CTRP-1; F, free 125I-CTRP-1.
CTRP-1 blocks adhesion of platelets on a collagen type I–coated surface at high shear. CTRP-1 does not affect rolling of platelets on a VWF-coated surface. In A and B, citrated whole human blood was incubated with either vehicle alone (A) or with 20 μg/mL CTRP-1 (B) and was perfused over collagen type I–coated coverslips through a flow chamber at 50 dyne/cm2. In C and D, citrated whole human blood was incubated with either vehicle alone (C) or with 20 μg/mL CTRP-1 (D) and was perfused over VWF-coated coverslips through the flow chamber at 50 dyne/cm2. The pictures shown are the initial frames of brief movies (Videos S1-S4; see the Supplemental Videos link at the top of the online article, at the Blood website) and are representative of repeated experiments.
CTRP-1 blocks adhesion of platelets on a collagen type I–coated surface at high shear. CTRP-1 does not affect rolling of platelets on a VWF-coated surface. In A and B, citrated whole human blood was incubated with either vehicle alone (A) or with 20 μg/mL CTRP-1 (B) and was perfused over collagen type I–coated coverslips through a flow chamber at 50 dyne/cm2. In C and D, citrated whole human blood was incubated with either vehicle alone (C) or with 20 μg/mL CTRP-1 (D) and was perfused over VWF-coated coverslips through the flow chamber at 50 dyne/cm2. The pictures shown are the initial frames of brief movies (Videos S1-S4; see the Supplemental Videos link at the top of the online article, at the Blood website) and are representative of repeated experiments.
Effect of CTRP-1 on the binding of VWF to collagen under static conditions
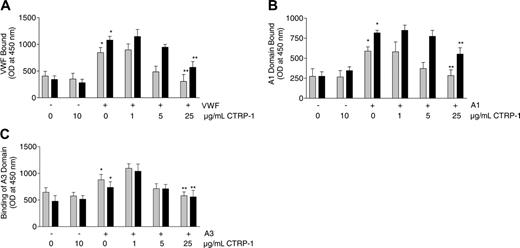
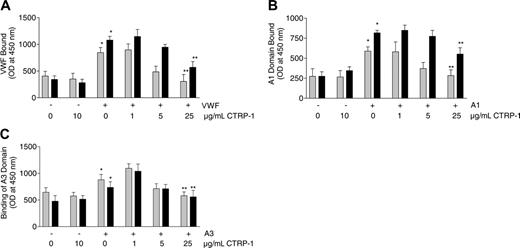
Microtiter plates (96-well) were coated with collagen type I or III, and binding of purified VWF to the plates was evaluated. In the absence of added VWF and in the presence or absence of 10 μg/mL CTRP-1, little or no VWF was detected on plates coated with either collagen I or III (Figure 4A). When VWF was included in the reaction mixtures in the absence of CTRP-1, binding of VWF was observed to both types of collagen. Binding of VWF to collagen I or III was reduced in a concentration-dependent manner by CTRP-1 (Figure 4A). Addition of 25 μg/mL CTRP-1, the highest concentration tested, reduced VWF binding to background levels. Under these conditions, CTRP-1 appeared to be more potent at blocking VWF binding to type I versus type III collagen.
Two separate domains of VWF, the A1 and A3 domains, appear to mediate collagen binding,17 and their role was tested using purified A1 and A3. In the absence of added A1 domain, and in the absence or presence of 10 μg/mL CTRP-1, little or no A1 was detected on the collagen-coated plates. Addition of A1 led to A1 binding to collagen I and III that was reduced in a dose-dependent manner by CTRP-1 (Figure 4B). The binding of A1 domain to collagen type I was reduced to a greater extent by CTRP-1 than the binding to collagen type III. A1 binding to collagen type I was reduced to background levels by 25 μg/mL CTRP-1 (Figure 4B); similar results were observed for A3 domain binding (Figure 4C).
CTRP-1 blocks intact VWF, VWF A1 domain, and VWF A3 domain binding to fibrillar collagen types I and III under static conditions. Plates were coated with collagen type I (▦) or III (▪) and were incubated with vehicle control or CTRP-1 alone, or the indicated mixtures of CTRP-1 with intact VWF (A), VWF A1 domain (B), or VWF A3 domain (C). Each bar represents the mean ± SD of 6 separate measurements. Differences were significant comparing vehicle and VWF alone (*P < .001), A1 domain alone (*P < .001), or A3 domain alone (*P < .02) by an unpaired, 2-tailed Student t test. Differences were significant (**P < .001) relative to incubation with vehicle alone by analysis of variance (ANOVA). OD indicates optical density.
CTRP-1 blocks intact VWF, VWF A1 domain, and VWF A3 domain binding to fibrillar collagen types I and III under static conditions. Plates were coated with collagen type I (▦) or III (▪) and were incubated with vehicle control or CTRP-1 alone, or the indicated mixtures of CTRP-1 with intact VWF (A), VWF A1 domain (B), or VWF A3 domain (C). Each bar represents the mean ± SD of 6 separate measurements. Differences were significant comparing vehicle and VWF alone (*P < .001), A1 domain alone (*P < .001), or A3 domain alone (*P < .02) by an unpaired, 2-tailed Student t test. Differences were significant (**P < .001) relative to incubation with vehicle alone by analysis of variance (ANOVA). OD indicates optical density.
CTRP-1 does not prevent platelet rolling on a VWF-coated surface under high shear
Platelet adhesion to collagen under high shear requires the interaction of the platelet GPIb receptor with VWF bound to collagen through its A3 domain. The A1 domain of VWF, though able to bind collagen on its own and thought to also mediate the platelet-VWF interaction, has a fast on/off rate producing the well-described rolling of platelets on a VWF-coated surface under high shear. To assess whether CTRP-1 could block the GPIb-VWF interaction, citrated whole blood was incubated for 5 minutes at room temperature with either vehicle alone or with vehicle containing 20 μg/mL CTRP-1 and flowed over a VWF-coated surface at 50 dyne/cm2. Platelet rolling over the VWF-coated surface was observed in the presence (Figure 3D) or absence (Figure 3C) of CTRP-1. Thus, CTRP-1 did not significantly affect the interaction of GPIb with VWF.
Effect of CTRP-1 on the binding of 125I-GPVI to collagen type I under static conditions
To evaluate whether CTRP-1 could inhibit collagen-induced platelet aggregation by blocking the interaction of the platelet GPVI receptor with collagen, a soluble GPVI-Fc4 fusion protein was prepared. The GPVI-Fc4 was active as shown by its potent inhibition of collagen-induced aggregation of PRP (Figure S1; see the Supplemental Materials link at the top of the online article, at the Blood website). In this assay, CTRP-1 was more effective at blocking platelet aggregation than GPVI-Fc4 (EC50, 1.4 μg/mL vs 7.0 μg/mL; Hill slope, –3.3 and –1.3 for CTRP-1 and GPVI, respectively). These data demonstrate that the soluble GPVI-Fc fusion protein was active and could effectively compete with the platelet GPVI receptor for collagen-induced aggregation of platelets.
Binding of 125I-GPVI-Fc4 to immobilized type I collagen was saturable, specific, and of high affinity with nonspecific binding less than 10% of the total binding (data not shown). Binding was concentration dependent, approaching saturation at approximately 20 μg/mL with little or no nonspecific binding. Scatchard plots were curvilinear, suggesting the presence of 2 classes of binding sites. The high-affinity site exhibited a Kd of 0.56 ± 0.18 μg/mL or 5.6 nM and a Bmax of 5.91 ± 2.67 ng bound/well (mean ± SD), whereas the low-affinity site showed a Kd of 17.83 ± 3.12 μg/mL or 178 nM (mean ± SD) and a Bmax of 27.22 ± 4.08 ng bound/well (mean ± SD). Thus, under static conditions, 125I-GPVI-Fc4 bound to a small number of high-affinity sites and a larger number of low-affinity sites on type I collagen–coated plates. Thus, compared with the binding of 125I-CTRP-1 shown in Figure 2, 125I-GPVI-Fc4 bound to collagen type I with a higher affinity but lower capacity.
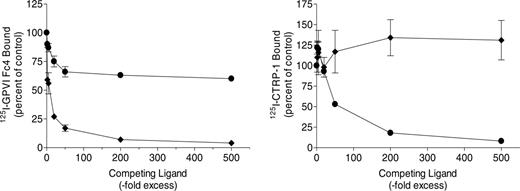
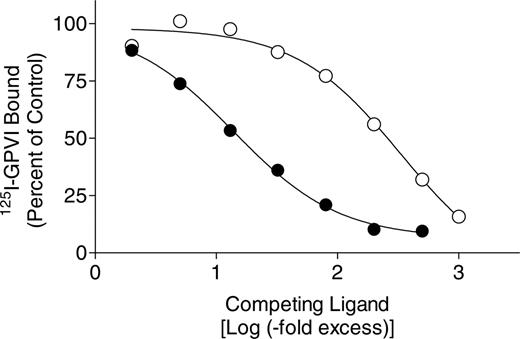
To evaluate whether the binding of CTRP-1 and GPVI-Fc4 is to the same or different sites on the collagen matrix, competition studies were performed using the solid-phase collagen-binding assay. Addition of increasing amounts of unlabeled GPVI Fc4 reduced the amount of 125I-GPVI-Fc4 bound to collagen (Figure 5, left). A 50-fold excess of unlabeled GPVI reduced 125I-GPVI-Fc4 binding by more than 80%, with higher concentrations completely blocking 125I-GPVI-Fc4 binding. Unlabeled CTRP-1, in contrast, was a less effective competitor for 125I-GPVI-Fc4 binding. The maximum competition achieved under these conditions was about 40% at a 500-fold excess of unlabeled CTRP-1 (Figure 5). Addition of increasing amounts of unlabeled CTRP-1 reduced the amount of 125I-CTRP-1 bound to collagen I (Figure 5, right). Over an identical range of concentrations, unlabeled GPVI-Fc4, on the contrary, failed to compete for 125I-CTRP-1 binding (Figure 5). Taken together, these data show that the binding sites for CTRP-1 on immobilized fibrillar type I collagen partially overlap with those of GPVI-Fc4.
Cross competition for GPVI-Fc4 and CTRP-1 binding to fibrillar collagen. Plates were coated with collagen type I as described in “Materials and methods.” (Left) Each well was incubated with 0.3 μg 125I-GPVI-Fc4/mL in the presence or absence of the indicated fold excess of unlabeled GPVI-Fc4 (♦) or CTRP-1 (•). (Right) Each well was incubated with 0.3 μg 125I-CTRP-1/mL in the presence or absence of the indicated fold excess of unlabeled CTRP-1 (•) or GPVI-Fc4 (♦). The 100% values for 125I-GPVI-Fc4 and 125I-CTRP-1 binding in the absence of competitors were 2.87 ± 0.15 and 16.38 ± 2.11 ng bound/well (mean ± SD, n = 8 separate measurements), respectively. Each point represents the mean ± SD of 4 separate measurements from 2 separate experiments.
Cross competition for GPVI-Fc4 and CTRP-1 binding to fibrillar collagen. Plates were coated with collagen type I as described in “Materials and methods.” (Left) Each well was incubated with 0.3 μg 125I-GPVI-Fc4/mL in the presence or absence of the indicated fold excess of unlabeled GPVI-Fc4 (♦) or CTRP-1 (•). (Right) Each well was incubated with 0.3 μg 125I-CTRP-1/mL in the presence or absence of the indicated fold excess of unlabeled CTRP-1 (•) or GPVI-Fc4 (♦). The 100% values for 125I-GPVI-Fc4 and 125I-CTRP-1 binding in the absence of competitors were 2.87 ± 0.15 and 16.38 ± 2.11 ng bound/well (mean ± SD, n = 8 separate measurements), respectively. Each point represents the mean ± SD of 4 separate measurements from 2 separate experiments.
Synthetic collagen-related peptide (CRP) cross-linked with glutaraldehyde was used as functional agonist for GPVI.16,26 In preliminary studies, cross-linked CRP (CRPxl) was a potent inducer of platelet aggregation (EC50, 0.26 ± 0.07 μg/mL), which could be blocked by GPVI-Fc4. Saturation analysis revealed that binding of 125I-GPVI-Fc4 to CRPxl was saturable at 10 to 30 μg/mL, specific, and of high affinity (data not shown). Little or no specific binding of 125I-GPVI-Fc4 to an unrelated control peptide was observed. Scatchard plots of the specific binding data were curvilinear, indicating 2 classes of sites. The Kd and Bmax for the high-affinity site were 0.076 ± 0.016 μg/mL and 2.45 ± 0.19 ng bound/well, respectively, and 2.43 ± 0.58 μg/mL and 15.4 ± 4.0 ng bound/well, respectively, for the low-affinity site. Binding of 125I-GPVI-Fc4 to CRPxl was reduced by unlabeled GPVI-Fc4 in a dose-dependent manner (Figure 6). Unlabeled CTRP-1 also reduced the amount of 125I-GPVI-Fc4 bound, but compared with GPVI-Fc4 it was a less effective competitor (Figure 6). These data demonstrate that both CTRP-1 and GPVI-Fc4 recognize and bind to CRPxl. The relative affinity of GPVI-Fc4 binding to CRPxl is, however, more than 20-times higher than that observed for CTRP-1.
Effect of CTRP-1 on the binding of collagen to α2β1 integrin under static conditions
Platelets also interact with collagen through the α2β1-platelet receptor, an interaction mediated by the I-domain of α2β1.16,22 To evaluate whether CTRP-1 can inhibit the α2β1 integrin pathway, the ability of CTRP-1 to compete for I-domain binding to collagen was examined. In the absence of added I-domain, and in the presence or absence of 10 μg/mL CTRP-1, little or no I-domain was detected on plates coated with either collagen I or III. Exogenous I-domain bound to both types of collagen as detected by the anti–I domain–specific antibodies. In contrast to the inhibition of intact VWF or VWF A1 and A3 domain binding to collagen, under identical conditions, CTRP-1 failed to compete for I-domain binding (Figure S2). These data suggest that the collagen-binding domains of α2β1 integrin and CTRP-1 are distinct.
In vivo antithrombotic effects of CTRP-1 in a Folts vascular injury model
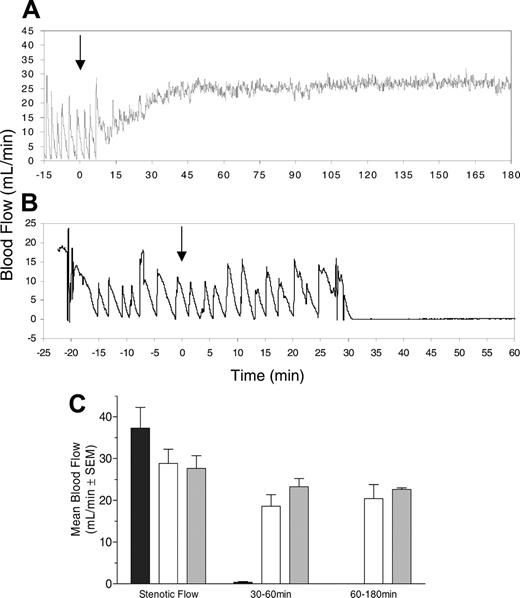
A mechanical crush injury was made to the carotid artery of nonhuman primates (Macaca fascicularis), and blood flow through the injured, highly thrombogenic blood vessel was monitored. Reduced blood flow in this model indicates blockage of the artery by platelet-rich thrombus.25 A typical tracing of blood flow in a CTRP-1–treated animal is shown in Figure 7A. The cyclic flow pattern recorded during the pretreatment phase indicates rapid occlusive thrombus formation that requires mechanical manipulation (tapping) in order to dislodge the occluding platelet thrombus. This manipulation re-establishes blood flow only for a brief period before renewed thrombus forms. A single intravenous injection of CTRP-1 (0.5 mg/kg) re-established blood flow, prevented thrombus formation, and maintained patency of the blood vessel for the complete duration of the 3-hour experiment. A control animal, injected with BSA (Figure 7B), showed similar initial cyclic flow variation; however, the platelet thrombus became permanently occlusive once mechanical intervention stopped 30 minutes after treatment. The results of all 9 animals studied summarized in Figure 7C show that permanent occlusion of the injured blood vessel developed in all control animals (n = 3), whereas both doses of CTRP-1 (0.5 and 1.0 mg/kg, n = 3 each) prevented thrombus formation and maintained blood flow.
Competition for 125I-GPVI-Fc4 binding to cross-linked CRP by unlabeled GPVI-Fc4 or by CTRP-1. Plates were coated with CRP and each well was incubated with 0.05 μg/mL 125I-GPVI-Fc4 in the absence or presence of the indicated fold excess of unlabeled GPVI-Fc4 (•) or unlabeled CTRP-1 (○). The 100% values for 125I-GPVI-Fc4 binding in the absence of competitors were 0.94 ± 0.01 ng bound/well. Each point represents the mean of triplicate measurements and is representative of 2 separate experiments.
Competition for 125I-GPVI-Fc4 binding to cross-linked CRP by unlabeled GPVI-Fc4 or by CTRP-1. Plates were coated with CRP and each well was incubated with 0.05 μg/mL 125I-GPVI-Fc4 in the absence or presence of the indicated fold excess of unlabeled GPVI-Fc4 (•) or unlabeled CTRP-1 (○). The 100% values for 125I-GPVI-Fc4 binding in the absence of competitors were 0.94 ± 0.01 ng bound/well. Each point represents the mean of triplicate measurements and is representative of 2 separate experiments.
To evaluate effects of CTRP-1 treatment on bleeding, we measured the activated clotting time (ACT) and time-to-hemostasis (TTH) following template cuts to the forearm of the nonhuman primates used in this study between 5 and 60 minutes after treatment. ACT and TTH values remained unchanged by treatment with CTRP-1. ACT values before treatment were 120 seconds and remained at 116 seconds and 126 seconds in the low- and high-dose CTRP-1 group, respectively. Baseline TTH values were 171 seconds, 215 seconds, 162 seconds, and 144 seconds in the BSA, 0.5 mg CTRP-1, 1.0 mg CTRP-1, and abciximab (0.25 mg/kg) groups, respectively. TTH values in the same groups after treatment were 154 seconds, 208 seconds, 158 seconds, and more than 1200 seconds. (All SDs were < 10%.)
Discussion
CTRP-1 is a novel member of the C1qTNF-related protein family, and similar to other family members,2,27 the basic structural unit of the recombinant protein is a homotrimer, which can dimerize to form a hexamer. Both forms are maintained by disulfide bonds and remain highly stable in vitro. The expression of CTRP-1 was examined in different species by Northern blot, in situ hybridization, and immunohistochemical analysis and was found to be expressed in a number of tissues, most prominently in vascular wall tissue. The majority of CTRP-1 in the vasculature originated from smooth muscle cells with endothelial cells contributing a smaller proportion (Woerner P. Meehan, Glenn Knitter, K.L., Marc Penn, Ulla M. Marzel, Stephen R. Hanson, P.B., and J.F., manuscript in preparation).
Data from saturation and competition binding studies presented show CTRP-1 specifically binds to collagen. Furthermore, we show that CTRP-1 inhibits collagen-induced platelet activation and aggregation. This activity might have clinical significance, because collagen is known to elicit platelet thrombosis following damage to the inner lining of the arterial wall—caused either by spontaneous rupture of atherosclerotic plaque or by medical intervention—which can lead to life-threatening thrombogenic conditions including myocardial infarction (MI) and stroke.28
Interaction of platelets and collagen can occur along at least 3 different receptor-mediated pathways (GPIb, GPVI, integrin α2β1).12,14,16,18,22,29-31 The ability of CTRP-1 to interfere with any of these 3 receptor pathways was investigated under static and under flow conditions simulating shear flow conditions seen typically in smaller human arteries. The inhibition of platelet activation in the static and flow studies occurred over a similar range of CTRP-1 concentrations, suggesting a similar mechanism in both assay paradigms. The data show that CTRP-1 binds to immobilized fibrillar collagen type I with virtually all the hallmarks of a receptor-mediated process. Of importance, the results from the competition studies suggest that CTRP-1 binds to sites on collagen that are largely distinct from those that bind the soluble GPVI fusion protein. GPVI is thought to mediate collagen-induced platelet activation by binding to the CRP domain in fibrillar collagen.16,18,30,31 Indeed, the present results show that cross-linked CRP activates platelets, that GPVI-Fc4 inhibits collagen- and CRP-induced platelet activation, and that GPVI-Fc4 binds specifically to immobilized, cross-linked CRP or collagen. It is of interest that the apparent affinity and binding capacity of CTRP-1 for collagen I was different from that for GPVI-Fc4: the binding of CTRP-1 was of lower affinity but higher capacity. Since the amount of available collagen on the plates was not measured in these studies, the stoichiometry of CTRP-1 and GPVI-Fc4 binding could not be accurately assessed. On a mass basis, however, the number of binding sites on collagen I for CTRP-1 is approximately 80 times the number of high-affinity sites for GPVI-Fc4 measured under identical conditions. This finding is consistent with our observation that GPVI-Fc4 did not compete for the binding of CTRP-1. Although CTRP-1 and GPVI appear to recognize different sites on fibrillar collagen I, CTRP-1 did exhibit a low affinity for a portion of GPVI-Fc4 collagen-binding sites, suggesting a partial overlap of their collagen-binding domains. The dose-dependent inhibition of GPVI-Fc4 binding to CRP observed with CTRP-1 further supports this notion. Thus, CTRP-1 appears to bind to collagen to at least 2 distinct sites, one that overlaps with and also recognizes GPVI, and a second site that is largely independent of this pathway.
CTRP-1 prevents platelet thrombosis in the nonhuman primate Folts vascular injury model and re-establishes and maintains blood flow. Panels A and B show typical individual flow traces recorded. Treatment with CTRP-1 (0.5 mg/kg) prevents platelet thrombosis and re-establishes blood flow through the injured carotid artery (A). No treatment effect was seen in a BSA-treated control (B). Arrows indicate the time of treatment. (L) The result of the complete study (n = 3, each group; shown is the mean ± SEM). Pretreatment stenotic blood flow was similar in all 3 groups. BSA-treated control animals showed permanent occlusion with no residual blood flow (▪); treatment with 0.5 mg/kg (□) and 1.0 mg/kg CTRP-1 (▦) reestablished blood flow, which was maintained throughout the study. Average blood flow is shown over 2 time intervals: a 30-minute period immediately following cessation of intervention and a subsequent 2-hour time window indicating longer-term effects.
CTRP-1 prevents platelet thrombosis in the nonhuman primate Folts vascular injury model and re-establishes and maintains blood flow. Panels A and B show typical individual flow traces recorded. Treatment with CTRP-1 (0.5 mg/kg) prevents platelet thrombosis and re-establishes blood flow through the injured carotid artery (A). No treatment effect was seen in a BSA-treated control (B). Arrows indicate the time of treatment. (L) The result of the complete study (n = 3, each group; shown is the mean ± SEM). Pretreatment stenotic blood flow was similar in all 3 groups. BSA-treated control animals showed permanent occlusion with no residual blood flow (▪); treatment with 0.5 mg/kg (□) and 1.0 mg/kg CTRP-1 (▦) reestablished blood flow, which was maintained throughout the study. Average blood flow is shown over 2 time intervals: a 30-minute period immediately following cessation of intervention and a subsequent 2-hour time window indicating longer-term effects.
A key component of the mechanism by which CTRP-1 inhibits collagen-dependent platelet functions appears to be blockade of VWF binding to collagen. The binding of VWF to collagens I and III is clearly inhibited by CTRP-1 under static as well as under flow conditions simulating arterial shear stress. The major binding site of VWF for collagen I is located in the A3 domain, while the A1 domain is thought to play a key role in binding to the platelet GPIb receptor, it also binds to collagen VI.16,17,29,31-35 Using isolated VWF A1 and A3 domains, we were able to show that under static conditions CTRP-1 blocked their binding to fibrillar collagen I. Conversely, platelets rolling on a VWF-coated surface were not significantly inhibited by CTRP-1, suggesting that CTRP-1 does not interfere with the interaction of VWF and platelets, which is mediated by the A1 domain of VWF and the platelet GPIb receptor. Furthermore, we were not able to show any direct interaction of platelets with CTRP-1.
Using the purified I-domain16 of the α2β1 receptor, we observed that CTRP-1 had no measurable effects on I-domain binding to collagen, suggesting that inhibition of collagen-mediated platelet functions by CTRP-1 is independent of the α2β1 platelet receptor.
Taken together, these data suggest a dual mechanism for the inhibition of collagen-induced platelet function by CTRP-1; a major mode of action appears to be the blockade of VWF binding sites on collagen leading to the prevention of platelet interaction with collagen in particular under arterial, high-shear blood flow conditions. Blockade of GPVI binding sites on collagen might represent a secondary and additive mechanism for the action of CTRP-1. However, this does not sufficiently explain the mechanism by which CTRP-1 can prevent platelet aggregation under static conditions in vitro. Although unlikely, it is possible that CTRP-1 interacts directly with VWF, CRP, and GPVI to prevent their interaction with collagen. Certainly, the partial blockade of the GPVI binding site on collagen could account for some of the effects of CTRP-1 under these conditions. In addition, a recent report by Bernardo et al36 demonstrates that fibrillar collagen as used in the current studies is contaminated with small amounts of VWF. Although the role of VWF clearly is to mediate the initial interaction of platelets with injured vasculature and exposed collagen under high-shear conditions seen in arterial vasculature, a contribution to platelet aggregation under static in vitro conditions cannot be excluded. Lastly, static conditions might increase the inhibitory activity of CTRP-1 compared with high-shear flow by allowing less-specific binding to interfere with platelet-collagen interaction.
While the exact mechanism contributing to the observed activity of CTRP-1 in vitro is not yet established, the data collected under dynamic flow conditions clearly show that CTRP-1 can prevent collagen-induced platelet adhesion on a collagen matrix by preventing VWF from binding to collagen. The antithrombotic effect of CTRP-1 seen in nonhuman primates shows that CTRP-1 can act as a potent antithrombotic, preventing platelet thrombosis on an otherwise highly thrombogenic injured carotid artery. Of interest, 2 measures of hemostasis indicated normal hemostasis in the treated animals. Adverse bleeding, seen when using current antithrombotic agents, represents a small, but real, clinical problem, and the search for a “magic bullet” (ie, antithrombotic therapy with no or only negligent effect on general hemostasis) is a topic of ongoing investigations.37,38
Further investigations into efficacy and possible adverse bleeding events are needed to better understand the potential of CTRP-1 as a site-specific antithrombotic agent and to address whether this activity is related to its biologic function.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-04-1425.
Supported in part by research funding from ZymoGenetics to J.A.L. Several of the authors (G.L., J.L.E., P.B., P.S., K.L., and J.F.) are employed by ZymoGenetics, whose potential product was studied in the present work.
G.L., J.L.E., P.B., and J.F. designed and/or performed research and analyzed data; J.F. wrote the paper; P.S. and K.L. contributed vital reagents and tools; and P.G., M.A.C., and J.A.L. designed and performed all studies done under flow conditions, in addition to studies involving α2β1 and selected studies under static conditions. In addition, J.A.L. provided valuable expertise to the general design.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the Protein Biochemistry Department at ZymoGenetics for their valuable contribution producing CTRP-1. The contributions of Paul Meehan, Glenn Knitter, Kien Khuu, and Secil Oguz are gratefully acknowledged.