Abstract
Toll-like receptors (TLRs) play a critical role in stimulating innate immunity by recognizing pathogen-associated molecular patterns (PAMPs) on invading microorganisms. Platelets also play a role in innate immunity, and we studied whether they express TLR. Results show that human and murine platelets variably expressed TLR2, TLR4, and TLR9 by flow cytometry and Western blotting. TLR4 expression was confirmed by demonstrating murine platelet binding to lipopolysaccharide (LPS). Thrombin activation of the platelets significantly enhanced the expression of TLR9, suggesting that at least some TLRs may derive from intracellular compartments. When LPS was administered to LPS-sensitive C3H/HeN and LPS-resistant C3H/HeJ mice, functional TLR4 expression in vivo was shown to be responsible for LPS-induced thrombocytopenia. However, when the C3H/HeN mice were first rendered thrombocytopenic by an antiplatelet antibody and then administered LPS, a significant reduction occurred in their ability to produce TNF-α. The decreased cytokine production in the thrombocytopenic mice was restored with platelet transfusion. These results suggest that platelets express various TLRs and that the functional significance of one of these, TLR4, appears to be a role in the modulation of LPS-induced thrombocytopenia and TNF-α production. This work implicates platelets as important mediators of innate immune responses against invading microorganisms.
Introduction
Inflammation is a complex interaction between various soluble factors and inflammatory cells. For example, signals that drive inflammatory events are transmitted through a family of signaling receptors, known as Toll-like receptors (TLRs), which are homologs of the Drosophila protein Toll.1,6 Several human and murine TLRs have been characterized, and they recognize a variety of molecular structures found on bacteria, viruses, and fungi.3,7,9 Recognition of these “danger” molecules leads to TLR signaling primarily through a MyD88-dependent pathway that, in turn, leads to the production of several proinflammatory cytokines.10 It appears that TLRs are sentinels of the innate immune system and are essential for the eventual stimulation of adaptive immunity against invading microorganisms.1
TLR expression has been examined in human and murine tissues. Several cell types have been shown to express TLRs, such as human platelets (TLR1 and TLR6),11 murine platelets (TLR2),12 and CD41+ megakaryocytes (TLR4).13 Platelet expression of TLR is of interest because it has recently been suggested that, in addition to their hemostatic role, platelets play an important role in linking innate and adaptive immune responses.14 In addition, TLR expression on platelets might have a role to play during infectious inflammation and atherosclerotic vascular disease. This is perhaps best exemplified by the clinical observation of severe thrombocytopenia associated with sepsis.15 To address this, we tested the expression of TLR on platelets and their ability to modulate platelet levels and inflammatory cytokine production in LPS-resistant and LPS-sensitive murine strains. The results suggest that functional TLR4 expression on platelets is necessary for thrombocytopenia induction and that it might be responsible for tumor necrosis factor-α (TNF-α) production in LPS-challenged mice and thus may act in the early detection of invading pathogens in vivo.
Materials and methods
Human blood for platelet preparation
Human blood was obtained by venipuncture from healthy laboratory volunteers under a St Michael's Hospital Research Ethics Board (REB)–approved protocol. Informed consent was provided according to the Declaration of Helsinki.
Mice
BALB/c, C3H/HeJ (LPS-resistant), and C3H/HeN (LPS-sensitive) female mice, 8 to 10 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME). Breeding pairs of BALB/c β3 (CD61) knockout (KO) mice were a kind gift from Dr Richard O. Hynes (Massachusetts Institute of Technology, Boston, MA). All mice were housed in the vivarium of St Michael's Hospital.
Reagents
Thrombin, LPS, fluorescein isothiocyanate (FITC)–labeled LPS (Escherichia coli 0111:B4), CPDA, and PMA were obtained from Sigma Chemical (St Louis, MO). Purified anti–mouse CD61 (integrin β3 chain); FITC-labeled anti–murine and anti–human CD61; PE-labeled mouse IgG2a; and rat IgG2a isotypes were obtained from PharMingen (Cedarlane Laboratories, Hornby, ON, Canada). Monoclonal PE-labeled and purified anti–murine TLR2, TLR4, and TLR9 were obtained from eBioscience (San Diego, CA). Anti–human TLR2, TLR4, and TLR9 were obtained from Imgenex (San Diego, CA). Simultest control γ1/γ1 (IgG1/IgG1) and control γ1/γ2a (IgG1/IgG2a) were purchased from BD Biosciences (San Jose, CA). Horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG and anti–mouse IgG were purchased from Caltag Laboratories (Hornby, ON, Canada). Before use, the FITC-LPS was reconstituted in 0.15 M NaCl to a concentration of 5 μg/mL.
F(ab′)2 fragments were generated from the anti-CD61 antibody, as previously described.16 Briefly, the anti-CD61 molecules (1%-3% wt/vol) were dialyzed against 0.2 M sodium acetate, pH 4.5, and were digested with 2% (wt/wt) pepsin (Sigma) for 24 hours at 37°C. The F(ab′)2 fragments were then purified by Sephadex G150 (Amersham Bioscience, Uppsala, Sweden) gel filtration and Protein G-Sepharose (Amersham Biosciences) adsorption. Final F(ab′)2 purity was determined by high-performance liquid chromatography (HPLC) and was typically greater than 95%. Where indicated, F(ab′)2 fragments of anti-CD61 were used to coat murine platelets.
Platelet preparation
Murine platelets were prepared from either BALB/c or β3 KO mice, as previously described.17 Briefly, blood was drawn by cardiac puncture from donor mice into CPDA. Whole blood was pooled and centrifuged at 150g, and platelet-rich plasma (PRP) was collected. The platelets were washed once in PBS and were adjusted to 109 cells/mL in PBS and CPDA (ratio, 10:1). Leukocytes in the preparation were enumerated by flow cytometry, and their levels in the stock platelet suspension were consistently lower than 1 WBC/μL. For human platelets, citrated blood was centrifuged at 150g for 15 minutes, and PRP was prepared, washed twice with PBS and CPDA (ratio, 10:1), and adjusted to 109 platelets/mL. Where indicated, platelets were pretreated with PMA (1.62 nM), LPS (50 γg), or thrombin (1 U/mL) for 10 minutes at 37°C. In some experiments, murine platelets were bound with F(ab′)2 fragments derived from anti-CD61 to mask the effect of the infused anti-CD61 antibody.
Platelet TLR expression
TLR expression on BALB/c platelets was measured on a FACSort flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon laser emitting 15 mW power using the indicated FITC- and PE-labeled antibodies. Briefly, 105 platelets in staining buffer containing 0.1 mM CaCl2 were incubated with 1 μg of each antibody for 30 minutes at 4°C in the dark and were washed once with PBS; then 10 000 events were acquired through a live gate drawn on forward light scatter (FSC) and side light scatter (SSC). Forward scatter and FL2 fluorescence was analyzed by dot plots using CellQuest software. TLR4 expression was additionally measured by the ability of platelets to bind FITC-LPS by flow cytometry. Briefly, 105 platelets were incubated with titrations of FITC-LPS in the absence or presence of 50 μg unlabeled LPS for 30 minutes. The platelets were washed once, and 10 000 events were acquired.
Western blotting
Western blot analysis of platelet TLR expression was performed by standard methods. Briefly, platelets (2 × 109/mL) were lysed with RIPA-NP40, and 50 μg cell lysate was separated on an 8% sodium dodecyl sulfate (SDS)–polyacrylamide gel and blotted onto nitrocellulose membrane. Membranes were blocked with 5% skim milk in TBST for 1 hour at room temperature, washed, and incubated with primary anti–human or anti–murine TLR mAbs (1/500 dilution) overnight at 4°C. Then the membranes were washed with TBST and incubated with the appropriate HRP-conjugated secondary antibody (1/1000 dilution) for 1 hour at room temperature. Detection of antigen was performed using the enhanced chemiluminescence detection method (ECL-Plus; Amersham Biosciences, Piscataway, NJ).
In vivo LPS treatment
LPS-sensitive C3H/HeN and LPS-resistant C3H/HeJ mice were prebled 24 hours before the start of the experiment to enumerate basal platelet counts and serum cytokine levels. To induce thrombocytopenia, mice were injected intraperitoneally with 10 μg hamster anti–mouse CD61 monoclonal antibody 48 and 24 hours before the initiation of the LPS experiments. This treatment induces severe thrombocytopenia.18 Mice receiving LPS were given intraperitoneal doses of 1 μg, and mice receiving platelet transfusions were transfused with 108 platelets intravenously; this approximates a human transfusion of 3 × 1011 platelets. Control platelet transfusions from donors into any of the nontreated recipients did not induce thrombocytopenia. Mice were bled at the indicated times, and platelet counts and serum TNF levels were measured.
TNF detection
Serum was prepared for TNF-α determinations and were stored at –70°C until they were analyzed. TNF-α was measured by commercial ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. TNF-α levels were compared with those from control mice that did not receive LPS.
Statistical analysis
Student t test for comparison of means was used to compare groups. P values less than .05 were considered statistically significant.
Results
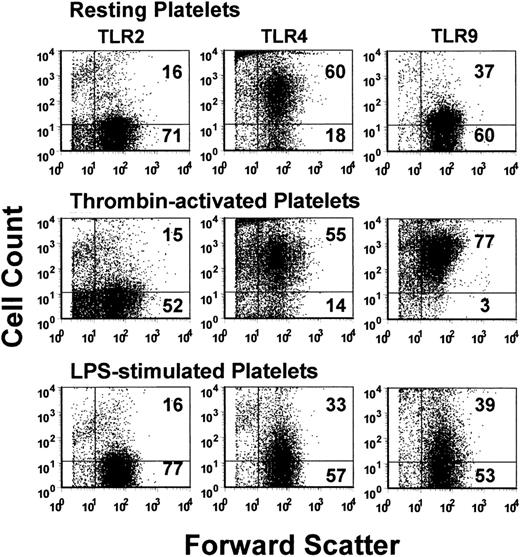
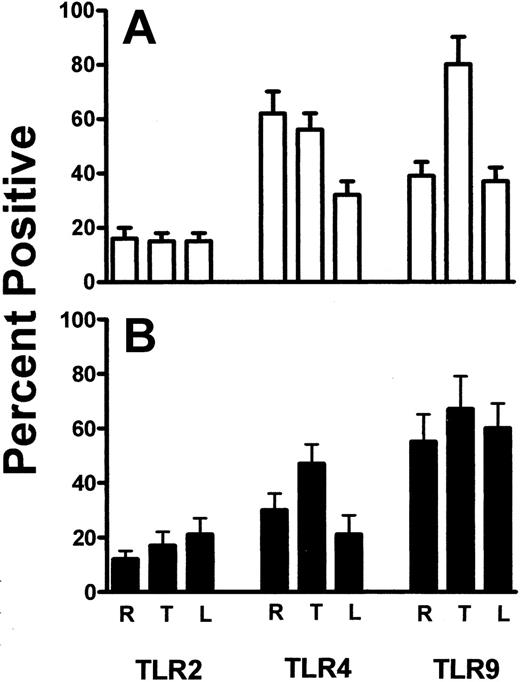
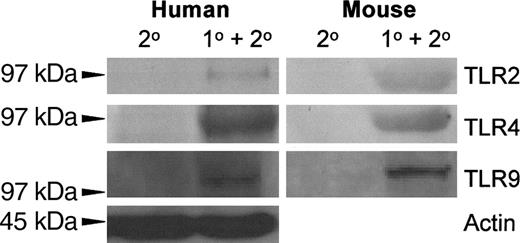
The expression of TLR2, TLR4, and TLR9 on resting, thrombin-activated, or LPS-stimulated human platelets was measured by flow cytometry. Human platelets typically expressed only low levels of TLR2, but levels of TLR4 and TLR9 were significantly (P < .01) expressed (Figure 1). Activation of human platelets with thrombin in the presence of Ca++ did not significantly affect the expression of TLR2 or TLR4 but significantly enhanced (P < .01) expression of TLR9 (Figure 2A). On the other hand, LPS stimulation of human platelets significantly (P < .02) reduced anti-TLR4 fluorescence (Figure 2A). The expression of TLR4 on human platelets was confirmed by Western blot analysis of platelet lysates (Figure 3).
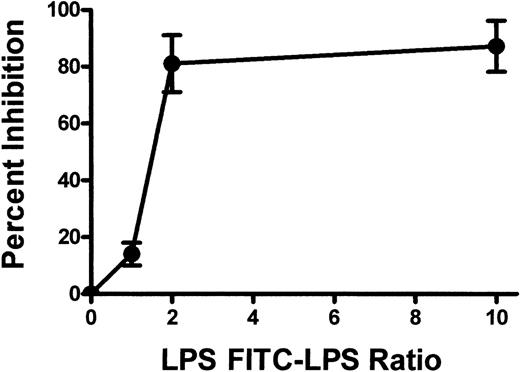
TLR2, TLR4, and TLR9 expression was also confirmed on BALB/c murine platelets by Western blotting (Figure 3). However, compared with human platelets, murine platelets showed differences in the modulation of TLR expression in that TLR9 expression was not affected by thrombin activation (Figure 2B). To further examine the expression of TLR4, its fluorescent ligand, FITC-LPS, was tested for the ability to bind murine platelets. FITC-LPS bound to murine platelets, and the fluorescence could be significantly (P < .01) inhibited by titrations of unlabeled LPS (Figure 4).
Flow cytometric dot-plot analyses. Analyses of TLR2 (left column), TLR4 (middle column), and TLR9 (right column) expression on human platelets. Platelets were stained with the indicated PE-labeled antibody and were acquired through a live platelet gate based on forward and side scatter. Data are presented as dot plots of forward scatter and TLR (FL2) fluorescence. The horizontal marker was set based on background fluorescence using fluorescent isotype controls. The numbers in the top and bottom right quadrants refer to the percentage of cells in each quadrant. A typical example is shown.
Flow cytometric dot-plot analyses. Analyses of TLR2 (left column), TLR4 (middle column), and TLR9 (right column) expression on human platelets. Platelets were stained with the indicated PE-labeled antibody and were acquired through a live platelet gate based on forward and side scatter. Data are presented as dot plots of forward scatter and TLR (FL2) fluorescence. The horizontal marker was set based on background fluorescence using fluorescent isotype controls. The numbers in the top and bottom right quadrants refer to the percentage of cells in each quadrant. A typical example is shown.
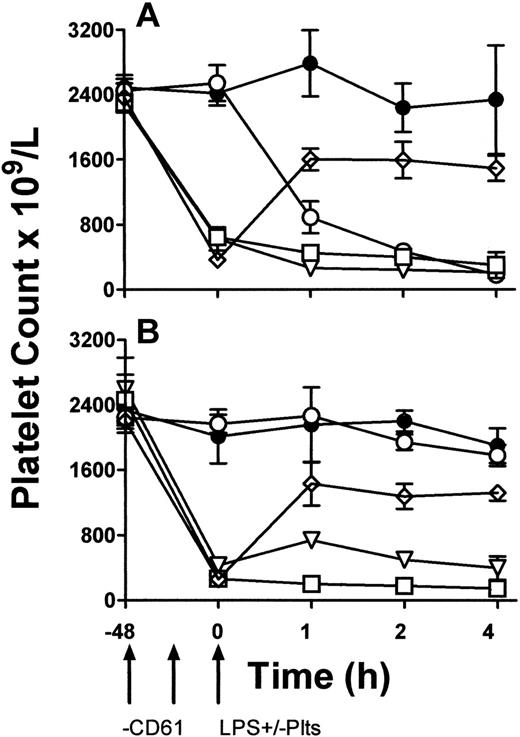
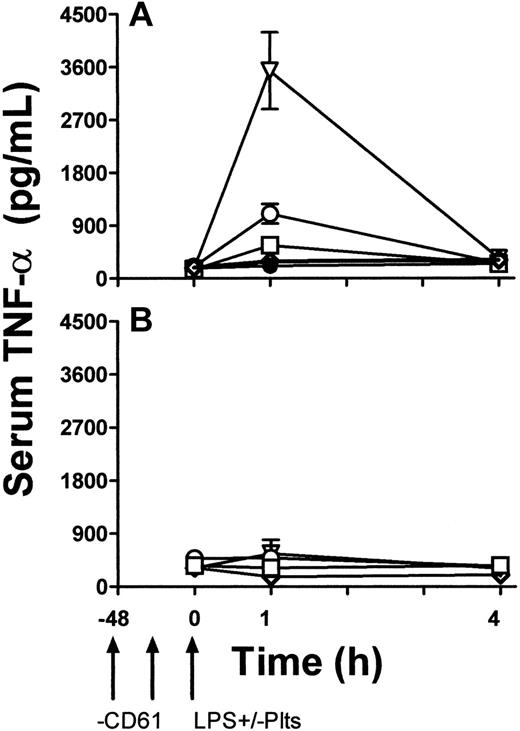
To determine a functional role of platelet TLR4 expression, LPS-induced TNF production in vivo was studied. TLR4 wild-type (LPS-sensitive) C3H/HeN and TLR-defective (LPS-resistant) CH3/HeJ mice were rendered thrombocytopenic by a monoclonal anti-CD61 antibody and then exposed to 1 μg LPS. Some mice were then immediately transfused with 108 platelets from β3 KO mice. Compared with LPS and anti-CD61 nontreated control mice, LPS administration alone induced significant (P < .01) thrombocytopenia within 1 hour in C3H/HeN mice but not in C3H/HeJ mice (Figure 5). In addition, compared with C3H/HeJ mice, LPS administration in C3H/HeN mice induced significant (P < .01) levels of serum TNF-α within 1 hour of administration (Figure 6A). In contrast, however, when C3H/HeN mice were first rendered thrombocytopenic by an anti-CD61 antibody and then administered LPS, their ability to generate TNF-α production (Figure 6A) was significantly reduced (P < .02). The reduced TNF-α production was not restored by a transfusion of anti–CD61-F(ab′)2 fragment–masked platelets from C3H/HeJ mice but was restored to significantly (P < .001) higher levels when the LPS administration was followed by a platelet transfusion from β3 KO mice (Figure 6A). These results were not observed in any of the LPS-resistant C3H/HeJ mice (Figure 6B).
Percentages of platelets. Summary of the percentage of resting (R), thrombin-activated (T), and LPS-stimulated (L) platelets for the indicated human TLR (A) and mouse TLR (B) molecules. Data are presented as the mean ± SD (n = 5) of the percentage of cells positive for the indicated TLR molecules and was calculated from data shown in Figure 1.
Percentages of platelets. Summary of the percentage of resting (R), thrombin-activated (T), and LPS-stimulated (L) platelets for the indicated human TLR (A) and mouse TLR (B) molecules. Data are presented as the mean ± SD (n = 5) of the percentage of cells positive for the indicated TLR molecules and was calculated from data shown in Figure 1.
Autoradiography of the indicated TLR Western blots of human and mouse platelets. Platelet lysates were separated on an 8% polyacrylamide gel, immunoblotted onto nitrocellulose, and probed with alkaline phosphatase–conjugated anti-TLR monoclonal antibodies. A typical result is shown.
Autoradiography of the indicated TLR Western blots of human and mouse platelets. Platelet lysates were separated on an 8% polyacrylamide gel, immunoblotted onto nitrocellulose, and probed with alkaline phosphatase–conjugated anti-TLR monoclonal antibodies. A typical result is shown.
Discussion
Toll-like receptors are critical receptors that transmit danger signals to the innate immune system and that mediate inflammatory events that can eventually recruit and activate cells of the adaptive immune system to respond against invading pathogens.19 TLRs recognize pathogen-associated molecular patterns not found on host cells. TLR1 and TLR6 have been observed on human platelets,11 and TLR2 and TLR4 have been observed on murine platelets.12,13 Despite these findings, however, the functional significance of platelet TLR expression is unknown. We demonstrate here that not only do platelets express various TLRs, TLR4 expression, in particular, correlates with the ability of platelets to significantly modulate TNF-α production on challenge with LPS. These results suggest that platelets may play an important role in host defense by perhaps acting as circulating pathogen sentinels to initially alert cells of the innate immune system.20,22
TLR expression was found on human and murine platelets. Although the platelet agonist thrombin did not affect TLR2 or TLR4 expression on human platelets, it significantly enhanced TLR9 (Figures 1, 2A), suggesting that at least some of the TLR9 may arise from intracellular compartments. However, it is unknown whether platelet expression of TLR might have been caused by the adsorption of free TLR in the plasma or the carryover expression from megakaryocytopoiesis. Andonegui et al13 demonstrate that megakaryocytes express TLR4, and Coppinger et al23 demonstrate that TLR5 is released from human platelets on thrombin activation, suggesting that at least some of the platelet TLR expression may originate from megakaryocytopoiesis and from within alpha granules. In murine platelets, altered TLR9 expression on thrombin activation was not observed. The reasons for this are unclear, but one suggestion is that there are species differences in the ability of platelets to modulate TLR expression. Of interest, LPS stimulation of platelets significantly reduced the ability of the fluorescence-labeled anti-TLR4 antibody or LPS to bind to human and murine platelets (Figures 2, 4).
Summary of the binding of FITC-conjugated LPS to murine platelets. Murine platelets were incubated with FITC-LPS, and titrations of nonlabeled LPS and FL1 fluorescence were measured by flow cytometry. Results are presented as the percentage inhibition of fluorescence binding and were calculated by the formula (1 – MCF test/MCF control) × 100, where MCF indicates mean channel fluorescence and MCF control indicates no unlabeled LPS added.
Summary of the binding of FITC-conjugated LPS to murine platelets. Murine platelets were incubated with FITC-LPS, and titrations of nonlabeled LPS and FL1 fluorescence were measured by flow cytometry. Results are presented as the percentage inhibition of fluorescence binding and were calculated by the formula (1 – MCF test/MCF control) × 100, where MCF indicates mean channel fluorescence and MCF control indicates no unlabeled LPS added.
Support is increasing for the notion that platelets not only mediate hemostasis, they play an important role in linking innate and adaptive immune systems. For example, Henn et al24 demonstrate that platelets express CD40L, and Elzey et al25 demonstrate that platelet CD40L expression may act as a bridge between innate and adaptive immunity by allowing platelets to act as helpers for CD8+ T-cell responses against certain viral infections. These observations suggest that platelets may have a dynamic proinflammatory interplay between leukocytes under a variety of physiologic and pathologic conditions. Our results demonstrating the expression of TLR on platelets may extend this possibility in that they appear to be linked to the modulation of LPS-induced thrombocytopenia and TNF-α production. The source of TNF-α, however, is unknown, though it was recently demonstrated that LPS-induced platelet sequestration to the lungs of mice is dependent on the presence of neutrophils.13 Perhaps the interaction of platelets with neutrophils within the lungs after LPS administration has a role to play in TNF-α production. These results are of interest because, in clinical settings, endotoxemia is often accompanied by microvascular inflammatory responses.26,28 A number of mediators, including cytokines and bacterial products such as LPS, are potential candidates in triggering such pathologic events, possibly through the activation of platelets, leukocytes, or both.29,31
The data in Figure 5 demonstrate that LPS only caused significant thrombocytopenia in LPS-sensitive C3H/HeN mice but not in LPS-resistant C3H/HeJ mice. This suggests that a functioning TLR4 molecule is required for LPS to mediate platelet removal from the circulation and confirms the recent observations of Andonegui et al,13 who demonstrate that LPS administration induced profound thrombocytopenia only in wild-type mice but not in TLR4 KO mice.
Peripheral blood platelet counts. C3H/HeN LPS–sensitive (A) and C3H/HeJ LPS–resistant (B) mice. LPS and anti-CD61 nontreated control group (•), LPS-treatment alone group (○), anti-CD61–treated, LPS-treated group (□), anti-CD61–treated group with a transfusion of 108 platelets from CD61-KO mice (⋄), and anti-CD61–treated, LPS-treated group with a transfusion of 108 platelets from CD61-KO mice group (▿) are shown. Platelet counts were measured by flow cytometry at the indicated times on the x-axis. Arrows indicate treatment schedule times. Data are expressed as the mean ± SD platelet count in 5 mice per group.
Peripheral blood platelet counts. C3H/HeN LPS–sensitive (A) and C3H/HeJ LPS–resistant (B) mice. LPS and anti-CD61 nontreated control group (•), LPS-treatment alone group (○), anti-CD61–treated, LPS-treated group (□), anti-CD61–treated group with a transfusion of 108 platelets from CD61-KO mice (⋄), and anti-CD61–treated, LPS-treated group with a transfusion of 108 platelets from CD61-KO mice group (▿) are shown. Platelet counts were measured by flow cytometry at the indicated times on the x-axis. Arrows indicate treatment schedule times. Data are expressed as the mean ± SD platelet count in 5 mice per group.
TNF-α levels. TNF-α levels in the sera of C3H/HeN LPS–sensitive (A) and C3H/HeJ LPS–resistant (B) mice. LPS and anti-CD61 nontreated control group (•), LPS-treatment alone group (○), anti-CD61–treated, LPS-treated group (□), anti-CD61–treated, LPS treated group with transfusion of 108 platelets from CD61-KO mice (▿), and anti-CD61–treated, LPS-treated group with a transfusion of 108 anti-CD61 F(ab′)2-masked platelets from C3H/HeJ mice (⋄) are shown. TNF-α levels were measured using a commercial ELISAkit. Arrows indicate treatment schedule times in the treated groups. Data are expressed as the mean ± SD concentration (pg/mL) of TNF-α in 5 mice per group.
TNF-α levels. TNF-α levels in the sera of C3H/HeN LPS–sensitive (A) and C3H/HeJ LPS–resistant (B) mice. LPS and anti-CD61 nontreated control group (•), LPS-treatment alone group (○), anti-CD61–treated, LPS-treated group (□), anti-CD61–treated, LPS treated group with transfusion of 108 platelets from CD61-KO mice (▿), and anti-CD61–treated, LPS-treated group with a transfusion of 108 anti-CD61 F(ab′)2-masked platelets from C3H/HeJ mice (⋄) are shown. TNF-α levels were measured using a commercial ELISAkit. Arrows indicate treatment schedule times in the treated groups. Data are expressed as the mean ± SD concentration (pg/mL) of TNF-α in 5 mice per group.
The infusion of LPS into animals and humans has been shown to induce severe thrombocytopenia and platelet aggregation formation in the lung and liver microvascular circulation,31 followed by degradation of the platelets and acute inflammation accompanied by tissue destruction.32,33 LPS has also been shown to inhibit human platelet aggregation stimulated by agonists by inhibiting phosphoinositide breakdown and intracellular Ca++ mobilization.34 Furthermore, LPS triggers the production of several proinflammatory cytokines (eg, TNF, IL-1) and the adherence of monocytes to endothelial cells.35 In our studies, when LPS-sensitive C3H/HeN mice were first rendered thrombocytopenic and then challenged with LPS, there was a significant reduction in their ability to produce TNF-α (Figure 6). However, platelets in the form of a transfusion, together with LPS challenge, significantly elevated TNF-α production (Figure 6). These results suggest that platelets in the circulation are critical in mediating the magnitude of LPS-induced inflammatory cytokine production and that they might have a role in focusing LPS to tissue sites rich in inflammatory leukocytes, such as lung and spleen. Taken together, these results suggest that platelets, through the expression of TLR, are important mediators of endotoxin-induced inflammatory responses.
In summary, we present evidence that human and murine platelets express TLR2, TLR4, and TLR9 and that platelet-associated TLR4 expression may significantly modulate LPS-induced thrombocytopenia and TNF-α production in vivo. These results suggest that platelets have the ability to interact quickly with invading microorganisms and that they are critically linked to proinflammatory innate immune responses.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-06-2202.
Supported by grants from the Canadian Institutes of Health Research (CIHR) and Canadian Blood Services.
R.A. designed and performed the research and wrote the first draft. E.R.S. performed the research. M.K. performed the research. A.R.C. performed the research. K.W.A.B. performed the research and analyzed the data. F.P.N. designed the research and analyzed the data. H.N. contributed the mice and analyzed the data. A.H.L. designed the research and analyzed the data. J.F. designed the research and analyzed the data. J.W.S. designed the research, analyzed data, and wrote the second draft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.