Abstract
Insulin-like growth factor 1 (IGF-1) plays a pleiotropic role in multiple myeloma (MM), that is, in survival, proliferation, chemotaxis, and angiogenesis. Strategies targeting the IGF-1 receptor (IGF-1R) may therefore be important to develop efficient anti-MM agents. In this work we investigated the effect of an IGF-1R tyrosine kinase (IGF-1RTK) inhibitor (picropodophyllin or PPP) in the 5T33MM mouse model. In vitro data showed that PPP reduced IGF-1R autophosphorylation and downstream ERK activation, leading to inhibition of IGF-1–stimulated proliferation and vascular endothelial growth factor (VEGF) secretion of MM cells. In an in vivo study, PPP reduced the bone marrow tumor burden and serum paraprotein in 5T33MM mice by 77% and 90%, respectively, compared to vehicle-treated animals. Angiogenesis was assessed by quantifying the microvessel density on CD31-stained paraffin sections and this was reduced by 60% in the PPP-treated group. In a separate survival experiment, Kaplan-Meier analysis demonstrated a significant increase in survival in PPP-treated 5T33MM animals compared to the vehicle controls (28 versus 18 days). These data suggest that the IGF-1RTK inhibitor PPP possesses a marked antitumor activity and strongly points to the possibility of using IGF-1R inhibitors in the treatment of MM.
Introduction
Multiple myeloma (MM) is a deadly B-cell malignancy, characterized by the proliferation of monoclonal plasma cells in the bone marrow (BM). There the MM cells secrete high levels of immunoglobulins, induce osteolysis, and activate angiogenesis.1,2
We have reported that MM cells produce post-switch isotypes of immunoglobulins with clonally fixed hypervariable regions.3 Whether this switch occurs in lymph nodes, circulation, or BM, the cells need to enter or re-enter the BM where they interact with the stromal cells and receive essential survival and proliferation signals. The homing process has been thoroughly described4 and chemokines are required for the directed migration of the MM cells to the extravascular compartment of the BM. Once in the BM, MM cells activate osteoclasts, leading to MM-associated bone disease, and endothelial cells (ECs), leading to neovascularization.5,8 The expression of vascular endothelial growth factor (VEGF), an EC-specific mitogen, stimulating vascular permeability,9 has been reported in human10 MM and by the murine 5TMM cells.11
Insulin-like growth factor 1 (IGF-1) is an endocrine factor involved in metabolic control and normal growth, having crucial roles in many types of cancer cells.12,14 It is known to be produced by different cell types including BM stromal cells.15 IGF-1 has been acknowledged to be an important antiapoptotic and proliferation factor for MM cells, stimulating proliferation of both interleukin 6 (IL-6)–independent and –dependent cell lines.16,20 We and others have shown that IGF-1 also acts as a chemoattractant for MM cells, thus playing a role in the homing of these cells to the BM.21,23 Furthermore, we have previously demonstrated that IGF-1 stimulates VEGF secretion by MM cells, contributing to angiogenesis.24
The principal pathways for transduction of the IGF-1 receptor (IGF-1R)–mediated signal are the ERK and the Akt pathways.12,13,24 On ligand interaction with the IGF-1Rα subunit, residues in the tyrosine kinase domain of the β subunit are autophosphorylated. Additional phosphorylation sites adjacent to these tyrosine residues can serve as a docking site for the adaptor proteins (IRS proteins) mediating activity through the regulatory subunit of PI3K. The receptor can also recruit SHC, leading to activation of the MAPK pathway.12
Because IGF-1 is a pleiotropic factor in different cancers, it is an attractive target for therapeutic intervention. A variety of strategies have been developed to inhibit the IGF-1R signaling pathways in tumor cells. Recently, a member of the cyclolignan family, picropodophyllin (PPP), was developed by molecular modeling to mimic the 3-dimensional structure of the IGF-1R tyrosine kinase domain. It was shown to potently and selectively inhibit the activity of the IGF-1R tyrosine kinase (IGF-1RTK) in solid tumors.25
The aim of the present work was to investigate the in vitro and in vivo effects of PPP on MM using the 5T33MM model. This model originated spontaneously in aging C57BL/KaLwRij mice26 and has been propagated in vivo by transplanting BM into young syngeneic recipients by intravenous injection. It closely represents the human disease with respect to clinical (ie, selective localization in the BM, serum M component, and angiogenesis) and molecular (ie, adhesion and chemokine profile) characteristics.27,30
We here demonstrate that PPP efficiently inhibits the IGF-1RTK activity and downstream signaling. By blocking the IGF-1R pathway using PPP in vitro, chemotaxis, proliferation, and VEGF secretion of MM cells are disrupted. PPP treatment of the mice injected with 5TMM in vivo leads to a strong reduction of MM disease and a significant prolonged survival of the mice.
Materials and methods
Animals
C57BL/KaLwRij mice were purchased from Harlan CPB (Horst, The Netherlands). Mice were used at 6 to 10 weeks of age. They were housed and treated following the conditions approved by the Ethical Committee for Animal Experiments, VUB (license no. LA1230281). The animal ethics meet the standards required by the 1998 UKCCCR (United Kingdom Co-ordinating Committee on Cancer Research) Guidelines.
In vivo MM models
The 5T33MM cells originated spontaneously in elderly C57BL/KaLwRij mice and have since been propagated in vivo, by intravenous transfer of the diseased marrow in young syngeneic mice. The paraprotein was assessed by protein electrophoresis of the obtained serum samples. When the serum concentration reached 10 mg/mL, the mice were humanely killed and the BM was flushed out of the femurs and tibiae and crushed out of the vertebrae. The BM cells were suspended in serum-free medium (RPMI 1640; Gibco, Life Technologies, Ghent, Belgium), supplemented with penicillin-streptomycin, glutamine, and minimal essential medium (MEM) nonessential amino acid (NEAA)–pyruvate (Gibco, Life Technologies). The cells were then purified by Lympholyte M (Cedarlane, Hornby, ON, Canada) gradient centrifugation at 1000g for 20 minutes, generating enriched 5T33MM cells, with a purity reaching 90% to 95%, as measured by flow cytometric analysis (fluorescence-activated cell sorting [FACS] staining with 5T33MM anti-idiotype–specific antibodies)28 and a viability of more than 95%.
In vivo analysis of tumor burden
Three groups of 10 mice were given intravenous injections of 5T33MM cells; one group of 10 naive mice was included as negative control. From the day of injection onward 10 tumor-bearing mice were treated with a high concentration of PPP (20 mg/kg, twice a day, intraperitoneally); 10 mice with a low dose (7 mg/kg, twice a day, intraperitoneally); and the last group with vehicle (dimethyl sulfoxide [DMSO]/oil, 9:1, twice a day, intraperitoneally). At week 3, when the vehicle controls showed signs of morbidity, the mice were killed. Bone marrow was isolated to determine tumor load by FACS staining and one femur was fixed for CD31 staining. The liver and spleen were removed, weighed, and fixed. Blood samples were also obtained to determine serum paraprotein concentrations.
Kaplan-Meier analysis of survival
To determine the effect of PPP on survival, a similar experiment as described was performed. Three groups of 12 mice were given injections of 5T33MM cells: one group remained untreated, one group was treated with vehicle, and the last group was treated with high-dose PPP, twice a day, intraperitoneally. Twelve naive mice were included as negative controls. Treatment continued until each animal showed signs of morbidity, namely, hind limb paralysis, at which point they were humanely killed. Tumor load was confirmed on BM samples.
Assessment of microvessel density
Microvessel density (MVD) was determined by CD31 staining as previously described.11 Briefly, one leg was fixed in zinc fixative for 48 hours, decalcified for 48 hours, and embedded in paraffin. After blocking with normal goat serum, sections were incubated with a rat anti-CD31 antibody (PECAM-1, 1:10; PharMingen, San Diego, CA) overnight at 4°C. As a secondary antibody, a biotin-conjugated goat anti–rat antibody was used (1:75; PharMingen). A streptavidin-horseradish peroxidase conjugate in combination with tyramide signal amplification (TSA; NEN Life Science Products, Boston, MA) was used for detection. In the area with the highest blood vessel density (hot spot), the number of blood vessels was counted per 0.22 mm2.
In vitro culturing and reagents
The cells were kept in serum-free medium and stimulated with 100 ng/mL recombinant human IGF-1 (animal/media grade; Grow Pep, Adelaide, Australia), for all experiments excluding the thymidine incorporation assays where 10 ng/mL rIGF-1 was used. The characteristics of the IGF-1RTK inhibitor, PPP, have been previously described in detail.25 PPP was used at a concentration of 1 μM.
IGF-1R tyrosine autophosphorylation
IGF-1R tyrosine autophosphorylation was analyzed by a sandwich enzyme-linked immunosorbent assay (ELISA), essentially as described.25 Briefly, 96-well plates (Immunolon; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 5 μg/well of an antibody to IGF-1R β subunit (H60, Santa Cruz Biotechnology, Santa Cruz, CA). The plates were blocked with 1% BSA in PBS Tween for 1 hour, and then 300 μg/well of total protein lysate from the 5T33MM cells was added. PPP was added in tyrosine kinase buffer without ATP at room temperature for 30 minutes before kinase activation with ATP. As a negative control we used an ATP-free reaction. Kinase assay was performed using the Sigma tyrosine kinase assay kit (Sigma, St Louis, MO).
Western blot
Cell pellets were lysed in lysis buffer containing 50 mM Tris, 150 mM NaCl, 1% NP40, and 0.25% sodium deoxycholate. The following protease and phosphatase inhibitors were added: 4 mM Na3VO4 (Sigma), 1 mM Na4P2O7 (Sigma), 50 mM NaF (VWR International, West Chester, PA), 5 mM EDTA (VWR), 1 mM AEBSF (ICN, Costa Mesa, CA), 2 μg/mL aprotinin (Sigma), 50 μg/mL leupeptin (Sigma), 50 μg/mL pepstatin A (ICN), 500 μg/mL trypsin inhibitor (Sigma), 10 μM benzamidin (Sigma), and 2.5 mM pnp benzoate (Sigma). The cells were then cleared by centrifugation (5 minutes, 13 000g) and sample buffer was added. After boiling, the samples were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were blocked with PBS containing 5% low-fat milk and 0.1% Tween 20 and probed with anti–p-Thr202/Tyr204 ERK and anti–p-S473Ak. For measuring total protein levels, the blots were stripped and reprobed with total ERK and Akt antibody. Anti-p-Thr202/Tyr204 p44/42MAPK, anti–p-44/42MAPK, anti-Akt, and the HRP-coupled secondary antibodies were acquired from Westburg (Leusden, The Netherlands) and anti–p-S473Akt was acquired from Biosource (Camarillo, CA). The bands were visualized using the enhanced chemiluminescence (ECL) system (Amersham, Buckinghamshire, United Kingdom).
Thymidine incorporation assays
Cells (1 × 106/mL) were pretreated with PPP in serum-free medium for 30 minutes and then incubated with or without rIGF-1 for 17 hours. Sixteen hours prior to harvest, cells were pulsed with 1 μCi (methyl-3H) thymidine (0.037 MBq; Amersham). Cells were harvested by a cell harvester (Inotech, Wohlen, Switzerland) on paper filters (Filtermat A; Wallac, Turku, Finland). Filters were dried for 1 hour in a 60°C oven and sealed in sample bags (Wallac) containing 4 mL Optiscint Scintillation Liquid (Wallac). Radioactivity was counted using a 1450 Microbeta Liquid Scintillation Counter (Wallac). Results are expressed as the relative DNA synthesis compared to untreated cells.
Inhibition of the downstream pathways of the IGF-1R by PPP. (A) IGF-1R isolated from 5T33MM cell lysates was incubated with different concentrations of PPP for 30 minutes, before kinase activation. IGF-1R autophosphorylation was detected by a sandwich ELISA. (B) 5T33MM cells were preincubated with 1 μM PPP for 4 hours before stimulation with IGF-1 (100 ng/mL for 10 minutes). Equivalent amounts of lysates were immunoblotted with anti–P-ERK1/2 and reblotted with anti-ERK1/2 to confirm equal loading. One experiment representative of 3 is shown.
Inhibition of the downstream pathways of the IGF-1R by PPP. (A) IGF-1R isolated from 5T33MM cell lysates was incubated with different concentrations of PPP for 30 minutes, before kinase activation. IGF-1R autophosphorylation was detected by a sandwich ELISA. (B) 5T33MM cells were preincubated with 1 μM PPP for 4 hours before stimulation with IGF-1 (100 ng/mL for 10 minutes). Equivalent amounts of lysates were immunoblotted with anti–P-ERK1/2 and reblotted with anti-ERK1/2 to confirm equal loading. One experiment representative of 3 is shown.
Quantifying F-actin content
The MM cells were pretreated with PPP and then stimulated with rIGF-1 for 10 minutes, followed by fixation, as previously described,31 for 15 minutes with 3% paraformaldehyde in PBS. Cells were washed with PBS and quenched in 0.1 M glycine in PBS for 15 minutes. After a second wash, the cells were permeabilized with 0.2% Triton X-100 in PBS containing 1% BSA (Boehringer Mannheim, Mannheim, Germany) for 10 minutes. The cells were then treated with 0.5 μM fluorescein isothiocyanate (FITC)–conjugated phalloidin (Molecular Probes, Eugene, OR) for 30 minutes. All the incubations were performed at room temperature. After washing, the cells were analyzed by flow cytometric analysis (FACScalibur, Becton Dickinson, Los Angeles, CA).
VEGF ELISA
MM cells were kept in serum-free RPMI 1640 medium at a density of 1 × 106 cells/mL with or without rIGF-1 for 48 hours after pretreatment with PPP. Conditioned media of the MM cells were analyzed with a VEGF ELISA kit according to manufacturer's instructions (Quantikine M, mouse VEGF ELISA; R&D Systems, Minneapolis, MN).
Statistical analysis
For statistical analysis of the in vitro data the Mann-Whitney test was used. For the in vivo (antitumor) data the Student t test was used and for the in vivo survival effect, we used a Kaplan-Meier analysis. P ≤ .05 was considered significant.
Results
PPP inhibits the autophosphorylation of the IGF-1R and the activation of p44/p42 ERK
The effect of PPP on the autophosphorylation of the IGF-1R was examined on captured 5T33MM IGF-1R in vitro. Figure 1A shows, using the ELISA, a substantial reduction of IGF-1R autophosphorylation by PPP at a concentration of 0.5 μM, thereby confirming the activity of the inhibitor.
The effect of 1 μM PPP on the phosphorylation of ERK and Akt, induced by IGF-1, in vitro, was examined. We have previously shown that IGF-1 activates the MEK-ERK and the PI3K-Akt pathways in 5T33MM cells. IGF-1–induced ERK activation, measured as the phosphorylation on Thr202/Tyr204, was completely inhibited after 4 hours of preincubation with PPP, whereas total ERK was unaltered during the period of analysis (Figure 1B). P-Akt (Ser473) was, however, only slightly reduced after 24 hours of incubation with PPP, which was the maximal in vitro incubation using the in vivo growing 5T33MM line (data not shown).
PPP inhibits DNA synthesis, chemotaxis, and VEGF secretion of 5T33MM cells in vitro
We have previously demonstrated that IGF-1 augments the DNA synthesis of the 5T33MM cells via the ERK pathway.24 When the cells were incubated with 1 μM PPP, DNA synthesis induced by IGF-1, as measured by thymidine incorporation, was blocked. Because PPP had no effect on the viability of the MM cells during this period (data not shown), this suggests that PPP effectively blocks IGF-1–induced DNA synthesis of MM cells (Figure 2A).
To study chemotaxis we used F-actin quantification as described previously.28 IGF-1 induces an increase in the F-actin content of the 5T33MM cells, correlated to their migration to IGF-1. When PPP was added 30 minutes prior to the stimulation with IGF-1, a partial reduction could be seen (50% as compared to control, data not shown). As we have shown, IGF-1–stimulated F-actin formation is mediated by the PI3K-Akt pathway. This is in agreement with the observed partial reduction of Akt phosphorylation at Ser473.
We have shown previously that IGF-1 induces a 2-fold increase in VEGF secretion by 5T33MM cells through the activation of ERK. When the cells were preincubated for 30 minutes with 1 μM PPP, the VEGF secretion is reduced to that of the control levels, demonstrating that PPP completely blocks IGF-1–mediated VEGF secretion of MM cells (Figure 2B).
The effect of PPP on tumor burden in vivo
We next examined the effect of PPP on the in vivo development of the 5T33MM disease. In the first series of experiments, mice were treated either with a low (7 mg/kg) or a high (20 mg/kg) dose of PPP or with the vehicle from the day of injection with 5T33MM onward.
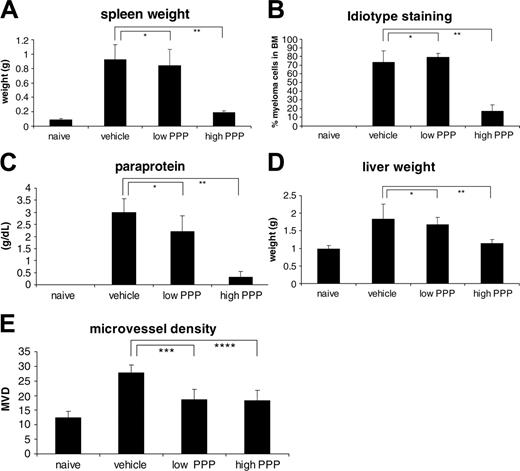
In Figure 3A-B, the effect of PPP on paraprotein and tumor load in the BM is shown. In mice treated with the high dose of PPP, a 90% reduction in serum paraprotein concentrations and a 77% reduction in the percentage of 5T33MM idiotype-positive cells in the BM were monitored. In the 5T33MM model, MM cells also accumulated in spleen and liver, inducing a splenomegaly and hepatomegaly of 10- and 2-fold, respectively. Treatment with a high dose of PPP reduced the splenomegaly by 90% and the hepatomegaly by 80% (Figure 3C-D). These data clearly demonstrate the antitumor effect of the IGF-1RTK inhibitor in MM.
Effects of PPP on IGF-1–induced DNA synthesis and VEGF secretion. The 5T33MM cells were stimulated with or without 10 ng/mL IGF-1 (DNA synthesis) and 100 ng/mL (ELISA) after a 30-minute incubation with 1 μM PPP (▪) or not (□). DNA synthesis (A) was measured by a thymidine incorporation assay and VEGF secretion (B) by ELISA. Results are shown relative to unstimulated cells. The maximum IGF-1–stimulated VEGF secretion reaches 250 pg/mL. Mean values ± SD for 3 independent experiments are shown.*P < .02; **P < .05.
Effects of PPP on IGF-1–induced DNA synthesis and VEGF secretion. The 5T33MM cells were stimulated with or without 10 ng/mL IGF-1 (DNA synthesis) and 100 ng/mL (ELISA) after a 30-minute incubation with 1 μM PPP (▪) or not (□). DNA synthesis (A) was measured by a thymidine incorporation assay and VEGF secretion (B) by ELISA. Results are shown relative to unstimulated cells. The maximum IGF-1–stimulated VEGF secretion reaches 250 pg/mL. Mean values ± SD for 3 independent experiments are shown.*P < .02; **P < .05.
Effect of PPP in vivo. (A) Serum paraprotein concentrations as determined by serum electrophoresis. (B) Tumor load as determined by flow cytometric analysis. Data are expressed as percentage 5T33MM cells of total cell number. (C-D) Weight of spleen and liver in grams of naive and treated or untreated 5T33MM-bearing mice. (E) Microvessel density. The number of microvessels in the tibiae and femora of the mice, counted by CD31 staining. Mean values ± SD for groups of 10 mice are shown.*P > .05; **P < .001; ***P < .05; ****P < .02.
Effect of PPP in vivo. (A) Serum paraprotein concentrations as determined by serum electrophoresis. (B) Tumor load as determined by flow cytometric analysis. Data are expressed as percentage 5T33MM cells of total cell number. (C-D) Weight of spleen and liver in grams of naive and treated or untreated 5T33MM-bearing mice. (E) Microvessel density. The number of microvessels in the tibiae and femora of the mice, counted by CD31 staining. Mean values ± SD for groups of 10 mice are shown.*P > .05; **P < .001; ***P < .05; ****P < .02.
As a control, several parameters including pancreas and liver function were monitored in the serum samples of the mice. The median serum levels and ranges of these parameters are shown in Table 1. As a good indication of PPP selectively inhibiting RTK of the IGF-1R and not the highly homologous insulin receptor, the levels of insulin-dependent metabolic parameters (glucose and albumin) are not elevated in PPP-treated animals. They seem to be normalized by PPP treatment compared with untreated tumor-bearing animals, suggesting a positive effect of PPP on metabolism. The levels of the liver marker alanine aminotransferase (ALAT) are higher in the vehicle-treated mice than in the naive, likely caused by the hepatic infiltration of myeloma cells. In the group treated with a high dose of PPP these levels are normalized to the levels of naive mice. Creatinine (muscle/kidney marker) and calcium levels are practically unaltered between all groups. Because 5T33MM cells do not induce MM-associated bone disease, calcium levels are similar in naive, vehicle-, and PPP-treated groups.
PPP inhibits angiogenesis in vivo
We have previously shown that 5T33MM cells stimulate angiogenesis in vivo, as assessed by quantifying the MVD.11
Because IGF-1 induces secretion of VEGF, one of the key players in angiogenesis, we also investigated the effect of PPP on in vivo angiogenesis. Immunohistochemical staining for CD31 (Figure 3E) demonstrated the increased MVD in tumor-bearing mice (2.2-fold). When mice were treated with either a low dose or high dose of PPP, the MVD was reduced by 60%. This demonstrates that inhibition of the IGF-1R in vivo reduces myeloma-stimulated angiogenesis.
PPP prolongs the overall survival of treated mice
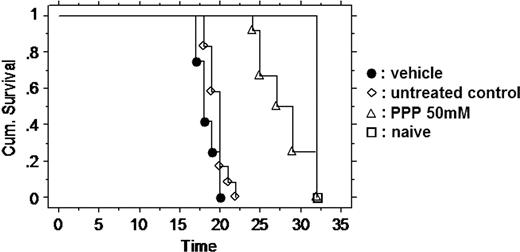
Because inhibiting the IGF-1R had a major effect on tumor burden and angiogenesis, we next studied the effect of PPP on the overall survival of the mice by Kaplan-Meier analysis (Figure 4). The mice were treated either with the vehicle or with the high dose of PPP or remained untreated. The mice treated with PPP had clearly a prolonged survival (28 days as compared to 18.4 days treated with vehicle only, P < .001) according to Kaplan-Meier analysis. As is a common complication in long experiments with intraperitoneal injections,32 we found that, after 2 weeks, the animals (both PPP and vehicle treated) had developed chronic peritonitis with fibrosis in the peritoneal fatty tissue and abdominal wall.
Discussion
Despite extensive research and emerging novel therapies, MM remains a fatal malignancy. One of the main characteristics of the disease is the restricted localization of the malignant plasma cells to the BM. This is most likely a consequence of a selective homing and a selective survival of the MM cells in the BM where unique proliferation and survival signals are provided by the stromal cells.27 The importance of IGF-1 as an MM survival factor15,16 is suggested by the correlation of high serum levels of IGF-1 with poor prognosis of the disease33,34 and the identification of IGF-1 as a potent proliferation and survival factor for MM cells in vitro.16,20 IGF-1 was also suggested to be a chemoattractant for MM cells, involved in the process of homing.21,23 We have, moreover, recently demonstrated that IGF-1 induces VEGF secretion in MM cells, thereby stimulating angiogenesis.24 Taken together, the multifunctional role of IGF-1 and its receptor in MM biology provide the basis for targeting these molecules in drug development, especially because IGF-1R signaling is not an absolute requirement for maintenance of normal adult cell homeostasis, implicating its safe use in vivo in the absence of severe side effects.35
Effect of PPP on disease-free survival. Mice were either untreated or treated with 50 mM PPP or the vehicle (DMSO/oil, 9:1) from the day of injection with 5T33MM onward. The first day of onset of morbidity was in the vehicle group on the 17th day. All naive mice were killed on the last day. The PPP group lived 10 days longer (P < .001 between vehicle group and PPP group).
Effect of PPP on disease-free survival. Mice were either untreated or treated with 50 mM PPP or the vehicle (DMSO/oil, 9:1) from the day of injection with 5T33MM onward. The first day of onset of morbidity was in the vehicle group on the 17th day. All naive mice were killed on the last day. The PPP group lived 10 days longer (P < .001 between vehicle group and PPP group).
The IGF-1R is a tyrosine kinase receptor that may activate 2 important pathways for proliferation and survival: the PI3K-Akt and the MAPK pathways. A variety of strategies have been applied to inhibit the IGF-1R signaling pathways in tumor cells. These consist of small-molecule competitive binding antagonists, blocking antireceptor antibodies, antisense or small interfering RNA strategies, introduction of a dominant-negative IGF-1R, and RTK inhibitors.34,36,40 Mitsiades et al41 have recently demonstrated the effect of an IGF-1RTK inhibitor in several malignancies, including MM. In the present study, we use a novel and selective IGF-1RTK inhibitor, the cyclolignan PPP, to specifically target the IGF-1R and to inhibit IGF-1 signaling.
We first demonstrated that autophosphorylation and downstream signaling of the IGF-1R was inhibited by PPP. The kinase assay in vitro on IGF-1Rs isolated from 5T33MM cells showed that RTK activity of the IGF-1R was substantially reduced. This was associated with a down-regulation of IGF-1–induced phosphorylation of ERK at 4 hours of incubation with PPP (this was in concordance with Stromberg et al42 ), whereas the phosphorylation of Akt was only slightly reduced in these cells. This differs from solid tumor cell systems in which Akt phosphorylation was strongly down-regulated by PPP.25,43 Stromberg et al42 have further deciphered that PPP decreased phosphorylation of CDK1/cdc2 in human MM cells. The expression of mcl-1 and survivin were also reduced.
We next determined the direct effect of PPP on the IGF-1 function of the 5T33MM cells in vitro. This was performed at a concentration of 1 μM, the same dose used by Stromberg et al to block IGF-1R in human MM cells. PPP completely blocked IGF-1–induced DNA synthesis and VEGF secretion. Similar to the effects of PPP on human MM cell lines42 we also observed a slight G2/M cell cycle arrest (results not illustrated). PPP had only minor effects on F-actin assembly in 5T33MM cells. This is in concordance with the protein analysis and with our previous findings stating that proliferation and VEGF secretion are MEK-ERK mediated, whereas F-actin assembly is mainly Akt mediated.24
As a control for the specificity of the RTK inhibitor, we also analyzed the effect of PPP on the stroma-independent 5T33MMvt cell line, clonally identical to the 5T33MMvivo line but lacking the IGF-1R.44 In a Western blot analysis, ERK was not activated in the 5T33MMvt cells after incubation with IGF-1 nor did PPP have any effect (data not shown). The phosphorylation of other downstream substrates was also unaffected by PPP treatment in this cell line.42 In contrast to the 5T33MMvv line, IGF-1 showed no stimulatory effect on the thymidine incorporation of the 5T33MMvt cells and, as expected, therefore PPP did not block their proliferation (data not shown). In conclusion, these data suggest that PPP is selectively inhibiting the IGF-1RTK. However, due to the fact that this is a slight overdose according to the in vitro kinase assay, we cannot rule out the possibility that PPP has some off-target effects, other than those previously monitored by Girnita et al.25
The effect of continuous PPP treatment on the tumor burden was assessed in vivo in the 5T33MM model. PPP had a dose-dependent effect: a high dose of PPP was needed to induce a 77% reduction of tumor burden and a 90% reduction in serum paraprotein concentration. On the other hand, a low dose of PPP was already effective to induce a 60% reduction in MVD (a measure of in vivo angiogenesis). This reduction could result from a diminished VEGF secretion by the MM cells as demonstrated by the in vitro ELISA, but also indirectly from a reduction in tumor load. Speaking in favor of a direct effect on VEGF synthesis, the reduction of microvessel density was already detected at a low dose of PPP, a dose where no significant effect on the tumor load could be seen. We can conclude that low doses of PPP seem to influence IGF-1–induced angiogenesis, but higher doses are required to inhibit the IGF-1–stimulated growth of MM cells.
Finally, we performed a long-term survival test to analyze whether blocking the IGF-1R function resulted in a prolonged overall survival of the mice. The mice treated with PPP survived 1.5 times (10 days) longer than untreated vehicle mice. Eventually, the myeloma tumor reappeared. A possible explanation for this is the reduced uptake of the drug through the peritoneum into the bloodstream, caused by accumulating fibrosis. This fibrosis reaction (apparent after 2 weeks) is a result of repeated injection trauma and chemical inflammatory reaction due to the repeated DMSO/oil treatment.32 In naive mice, we found that after 2 weeks of treatment, significant fibrosis occurred. Furthermore, maximum serum PPP levels, measured 2 hours after injection (pmax), dropped after 2 weeks of treatment, from an effective maximum dose of 10 μM to an ineffective pmax of ± 3 μM, which would lead to mean concentrations lower than 0.5 μM over 24 hours. This low level of PPP is incapable of inhibiting IGF-1R function, which would result in the regrowth of the MM cells. It is unlikely that the tumor cells have acquired resistance during this relative short treatment because a remarkably limited resistance to PPP in tumor cells is observed after a 2-year exposure to increasing concentration of the compound (Vasilcanu et al, personal communication September 2004).
To exclude the possibility that PPP affected the insulin receptor, serum albumin and glucose levels were measured, showing no difference between mice treated with or without PPP, indicating that PPP did not influence the insulin receptor signal and metabolism.
In summary, we conclude that IGF-1 is involved in the development of MM disease and can be efficiently blocked by PPP. It targets the IGF-1RTK, blocks the IGF-1R function in vitro and in vivo, reduces tumor burden, and is associated with prolonged survival and this in the absence of apparent in vivo toxicity. To study the treatment value of PPP, a more clinical setting is needed in a slower growing model with osteolytic lesions where treatment studies can be performed. Targeting the IGF-1R could also be helpful in increasing the efficacy of other antineoplastic treatments designed to induce apoptosis by eliminating IGF-1R–induced survival signals.
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-01-0293.
Supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO-Vl), Belgische Federatie tegen Kanker, Fortis, Onderzoeksraad Vrije Universiteit Brussel (OZR-VUB), and the Swedish Cancer Society. K.V. and K.A. are postdoctoral fellows and E.M. a research assistant of FWO-Vl. H.D.R. has a clinical doctoral grant from FWO-Vl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank A. Willems, C. Seynaeve, G. Vrolix, and F. Rylant for technical assistance and the laboratory of Prof Gorus (AZ VUB, Brussels) for serum paraprotein analysis.