Abstract
Adult patients with acute lymphoblastic leukemia (ALL) who are stratified into the standard-risk (SR) group due to the absence of adverse prognostic factors relapse in 40% to 55% of the cases. To identify complementary markers suitable for further treatment stratification in SR ALL, we evaluated the predictive value of minimal residual disease (MRD) and prospectively monitored MRD in 196 strictly defined SR ALL patients at up to 9 time points in the first year of treatment by quantitative polymerase chain reaction (PCR). Frequency of MRD positivity decreased from 88% during early induction to 13% at week 52. MRD was predictive for relapse at various follow-up time points. Combined MRD information from different time points allowed definition of 3 risk groups (P < .001): 10% of patients with a rapid MRD decline to lower than 10-4 or below detection limit at day 11 and day 24 were classified as low risk and had a 3-year relapse rate (RR) of 0%. A subset of 23% with an MRD of 10-4 or higher until week 16 formed the high-risk group, with a 3-year RR of 94% (95% confidence interval [CI] 83%-100%). The remaining patients whose RR was 47% (31%-63%) represented the intermediate-risk group. Thus, MRD quantification during treatment identified prognostic subgroups within the otherwise homogeneous SR ALL population who may benefit from individualized treatment.
Introduction
Investigation of minimal residual disease (MRD) has been proven to be a valuable tool for predicting outcome in childhood acute lymphoblastic leukemia (ALL).1-5 In contrast, only a few studies have focused on adult ALL, and they were based mostly on patients with heterogeneous risk profiles and different kinds and intensities of treatment.6-8 However, monitoring homogeneous patient cohorts at different time points during therapy might provide additional insight into the nature and clinical relevance of MRD kinetics in adult ALL, which is particularly relevant for the large population of standard-risk (SR) patients without conventional risk factors. Relapses in this patient group occur in about 40% to 55% of cases and cannot be predicted by any known conventional risk factor.9-11 In a number of clinical studies this led to a policy of stem cell transplantation (SCT) in first remission,12-14 causing overtreatment and additional expenses for those patients who are cured by conventional chemotherapy alone. Therefore, definition of prognostic factors allowing discrimination of SR patients with poor outcome after standard chemotherapy from those with a favorable prognosis is highly warranted. Currently, the most widely used techniques to detect and quantify residual disease in patients with ALL are multiparameter immunophenotypic evaluation of aberrant protein expression2,3,8 and clone-specific polymerase chain reaction (PCR) amplification of immunoglobulin (Ig) and T-cell receptor (TCR) gene rearrangements.1,4,5 Such molecular targets can be identified in more than 90% of patients with ALL by the use of various PCR primer sets. Besides its large applicability and high sensitivity, a main advantage of PCR-based assays is the use of DNA as a stable and easy conveyable specimen, which is particularly relevant in large multicenter studies. Until now, most molecular studies used semiquantitative or qualitative PCR methods for detection of Ig and TCR gene rearrangements.1,5-7 However, in adult ALL there is a high need for precise quantification to define discriminating MRD levels at different sampling time points, as MRD positivity is significantly more frequent in adults than in comparably treated children.15,16 Therefore, we and others have developed different immune gene real-time quantitative (RQ)-PCR assays to permit accurate MRD quantification during the exponential phase of PCR amplification.17-21 This allows the quantitative detection of 1 leukemic cell among 10 000 normal cells with a good quality control, standardization, and comparability of MRD data. Using these RQ-PCR assays, we conducted a prospective study on the predictive significance of MRD monitoring in adult SR ALL within the German Multicenter Study Group for Adult ALL (GMALL) trial with more than 100 participating centers that recruits more than 60% of the German incidence of adult ALL, minimizing the risk of a selection bias and suggesting a high external validity.22 Residual disease was sequentially monitored at multiple time points during therapy of 196 consecutive adults with standard-risk ALL who were treated according to the GMALL 06/99 standard-risk protocol. In a pilot study with 65 patients, logistics and optimal sampling time points as well as threshold levels were established.
Patients, materials, and methods
Patients
A total of 323 adults with ALL were included in the MRD trial at time of diagnosis. The study was divided into 2 phases: (1) a pilot phase within the GMALL 05/93 trial (January 1997-September 1999) to establish logistics, validate quantification methods and required sensitivities, and define sampling time points and MRD threshold levels; and (2) a main phase (October 1999-December 2002) to prospectively prove prognostic significance of MRD in strictly defined adult SR ALL patients who were consecutively enrolled in the GMALL 06/99 trial after written informed consent. Approval for these studies was obtained from the institutional review boards at each of the participating institutions.
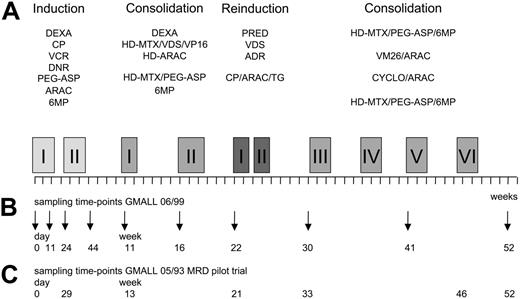
The MRD pilot study comprised 71 adult ALL patients, including 27 patients with high-risk features with available bone marrow samples at diagnosis and at up to 6 times in the first year of therapy (Figure 1).
For the main GMALL 06/99 MRD trial, only standard-risk ALL patients as defined by the following criteria were prospectively investigated: (1) absence of the translocations t(4;11)/MLL-AF4 and t(9;22)/BCR-ABL; (2A) c-/pre-B-ALL with white blood cell (WBC) counts less than 30 × 109/L (30 000/μL) at diagnosis; or (2B) cortical or mature T-ALL with WBC counts less than 100 × 109/L (100 000/μL), since year-2000 restriction to cortical T-ALL regardless of WBC count; (3) aged 15 to 65 years; and (4) achievement of complete remission after phase I of induction therapy. Over the collection period, 291 consecutive GMALL 06/99 patients fulfilled inclusion criteria, and bone marrow samples at diagnosis and at up to 9 follow-up time-points during first-year treatment were prospectively collected from 252 (87%) of these patients. The 9 bone marrow sampling times were defined according to fixed steps along the treatment protocol: mid-induction I (day 11), end of induction I (day 24), end of induction II (day 44), preconsolidation I (week 11) and II (week 16), prereinduction (week 22), preconsolidation III (week 30) and V (week 41), and end of first-year of treatment (week 52) (Figure 1). Thirty-three of 252 patients had to be excluded due to an insufficient amount or quality of DNA in diagnostic and/or follow-up samples; therefore, molecular characterization of TCR/Ig rearrangement patterns was finally performed in 219 patients.
Treatment
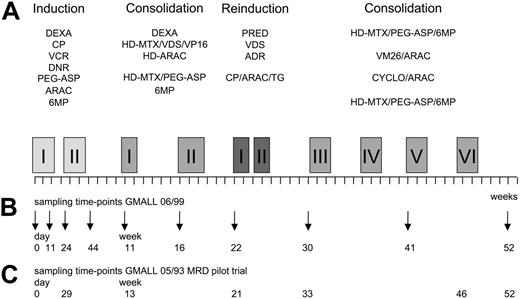
In brief, GMALL 06/99 induction therapy phase I consisted of a 6-drug regimen given over a period of 3 weeks with dexamethasone, cyclophosphamide, vincristine, daunorubicine, asparaginase, and intrathecal methotrexate. After attaining a complete clinical remission, patients received 3 weeks of induction phase II therapy with cyclophosphamide, cytarabine, mercaptopurine, and intrathecal methotrexate. Consolidation therapy started at week 11 and consisted of 6 cycles with alternating combinations of drugs over a period of 40 weeks and was interrupted by a 4-week reinduction therapy at week 22 (Figure 1). All patients received intrathecal therapy with methotrexate, cytarabine, and dexamethasone for 1 year and cranial irradiation (24 Gy). Mediastinal irradiation was added for patients with residual mediastinal tumor mass after induction therapy (24 Gy). The generally recommended maintenance therapy lasted for additional 12 months and consisted of alternating drug combinations similar to the consolidation cycles with intermediary application of mercaptopurine plus methotrexate.
Scheme of treatment and bone marrow sampling time points. (A) Schematic representation of GMALL 06/99 SR first-year treatment. (B) Bone marrow sampling time-points in the GMALL 06/99 trial. (C) Bone marrow sampling time-points in the GMALL 05/93 MRD pilot trial (time-points synchronized according to the treatment phase). ADR indicates adriamycine; CP, cyclophosphamide; DEXA, dexamethasone; DNR, daunorubicine; (HD)-ARAC, (high-dose) cytarabine; (HD)-MTX, (high-dose) methotrexate; 6MP, mercaptopurine; PEG-ASP, PEG-asparaginase; PRED, prednisolone; TG, thioguanine; VCR, vincristine; VDS, vindesine; VM26, tenisposide; and VP16, etoposide. Specifics of drugs and doses used in the main GMALL 06/99 treatment protocol. Induction I (including preinduction treatment): 10 mg/m2 DEXA orally on days 1-5, 11-14; 200 mg/m2 CP intravenously on days 1-3 (CP part of Induction I protocol only until 2000); 1000 U/m2 PEG-ASP intravenously on day 18 (reduced dose intensity in patients older than 55 years); 2 mg VCR intravenously on days 4, 11, and 18; 45 mg/m2 DNR intravenously on days 4, 5, 11, and 12 (reduced dose intensity in patients older than 55 years); and 15 mg MTX intrathecally on day 1. Induction II: 1000 mg/m2 CP intravenously on days 1 and 21; 75 mg/m2 ARAC intravenously on days 3-6, 10-13, and 17-20; 60 mg/m2 6MP orally on days 1-21; 15 mg MTX intrathecally on days 3, 10, and 17. Consolidation I: 10 mg/m2 DEXA orally on days 1-5; 3 mg/m2 VDS intravenously on day 1; 1500 mg/m2 HD-MTX intravenously on day 1; 250 mg/m2 VP16 intravenously on days 4 and 5; and 2 × 2000 mg/m2 HD-ARAC intravenously on day 5. Consolidation II: 1500 mg/m2 HD-MTX intravenously on days 1 and 15; 500 U/m2 PEG-ASP intravenously on days 2 and 16; and 60 mg/m2 6MP orally on days 1-7 and 15-21. Reinduction I and II: 3 × 20 mg/m2 PRED orally on days 1-14; 3 mg/m2 VDS intravenously on days 1 and 7; 50 mg/m2 ADR intravenously on days 1 and 7; intrathecal triple chemotherapy with 15 mg MTX, 40 mg ARAC, and 4 mg DEXA intrathecally on days 1 and 15; 1000 mg/m2 CP intravenously on day 15; 75 mg/m2 ARAC intravenously on days 17-20, 24-27; and 60 mg/m2 TG orally on days 15-28. Consolidation III: see Consolidation II. Consolidation IV: 150 mg/m2 ARAC intravenously on days 1-5; 100 mg/m2 VM26 intravenously on days 1-5; and intrathecal triple therapy (see Reinduction) on day 1. Consolidation V: 1000 mg/m2 CP intravenously on day 1; 500 mg/m2 ARAC intravenously on day 1; intrathecal triple therapy (see Reinduction) on day 1. Consolidation VI: see Consolidation II; and intrathecal triple therapy (see Reinduction I) at week 52.
Scheme of treatment and bone marrow sampling time points. (A) Schematic representation of GMALL 06/99 SR first-year treatment. (B) Bone marrow sampling time-points in the GMALL 06/99 trial. (C) Bone marrow sampling time-points in the GMALL 05/93 MRD pilot trial (time-points synchronized according to the treatment phase). ADR indicates adriamycine; CP, cyclophosphamide; DEXA, dexamethasone; DNR, daunorubicine; (HD)-ARAC, (high-dose) cytarabine; (HD)-MTX, (high-dose) methotrexate; 6MP, mercaptopurine; PEG-ASP, PEG-asparaginase; PRED, prednisolone; TG, thioguanine; VCR, vincristine; VDS, vindesine; VM26, tenisposide; and VP16, etoposide. Specifics of drugs and doses used in the main GMALL 06/99 treatment protocol. Induction I (including preinduction treatment): 10 mg/m2 DEXA orally on days 1-5, 11-14; 200 mg/m2 CP intravenously on days 1-3 (CP part of Induction I protocol only until 2000); 1000 U/m2 PEG-ASP intravenously on day 18 (reduced dose intensity in patients older than 55 years); 2 mg VCR intravenously on days 4, 11, and 18; 45 mg/m2 DNR intravenously on days 4, 5, 11, and 12 (reduced dose intensity in patients older than 55 years); and 15 mg MTX intrathecally on day 1. Induction II: 1000 mg/m2 CP intravenously on days 1 and 21; 75 mg/m2 ARAC intravenously on days 3-6, 10-13, and 17-20; 60 mg/m2 6MP orally on days 1-21; 15 mg MTX intrathecally on days 3, 10, and 17. Consolidation I: 10 mg/m2 DEXA orally on days 1-5; 3 mg/m2 VDS intravenously on day 1; 1500 mg/m2 HD-MTX intravenously on day 1; 250 mg/m2 VP16 intravenously on days 4 and 5; and 2 × 2000 mg/m2 HD-ARAC intravenously on day 5. Consolidation II: 1500 mg/m2 HD-MTX intravenously on days 1 and 15; 500 U/m2 PEG-ASP intravenously on days 2 and 16; and 60 mg/m2 6MP orally on days 1-7 and 15-21. Reinduction I and II: 3 × 20 mg/m2 PRED orally on days 1-14; 3 mg/m2 VDS intravenously on days 1 and 7; 50 mg/m2 ADR intravenously on days 1 and 7; intrathecal triple chemotherapy with 15 mg MTX, 40 mg ARAC, and 4 mg DEXA intrathecally on days 1 and 15; 1000 mg/m2 CP intravenously on day 15; 75 mg/m2 ARAC intravenously on days 17-20, 24-27; and 60 mg/m2 TG orally on days 15-28. Consolidation III: see Consolidation II. Consolidation IV: 150 mg/m2 ARAC intravenously on days 1-5; 100 mg/m2 VM26 intravenously on days 1-5; and intrathecal triple therapy (see Reinduction) on day 1. Consolidation V: 1000 mg/m2 CP intravenously on day 1; 500 mg/m2 ARAC intravenously on day 1; intrathecal triple therapy (see Reinduction) on day 1. Consolidation VI: see Consolidation II; and intrathecal triple therapy (see Reinduction I) at week 52.
Treatment elements in the GMALL 05/93 study were similar to the GMALL 06/99 protocol and are described elsewhere.9
Detection of residual disease
Mononuclear cells were isolated from bone marrow and stored in liquid nitrogen or at -80°C until extraction. Samples were analyzed at 1 of 2 central laboratories (in Heidelberg and Kiel, Germany). Standardization of screening PCR for detection of clonal markers was performed in the context of the BIOMED-1 and BIOMED-2 Concerted Action.23,24 Rearrangements of the TCR genes TCRB, TCRG, and TCRD, and the Ig genes IGH and IGK-Kde were sought by PCR amplification in samples obtained at diagnosis. Clonality was confirmed either by heteroduplex analysis or by gene scanning.24
After sequencing, allele-specific oligonucleotides (ASOs) were designed for each MRD target on the basis of the sequence data of the junctional regions, using OLIGO 6.3 software (Cascade, CO). Tests for residual disease were conducted by RQ-PCR amplification of 500 ng DNA of follow-up samples, using either TaqMan (Applied Biosystems, Foster City, CA) or LightCycler (Roche Diagnostics, Mannheim, Germany) technology. Both systems have been demonstrated to produce concordant results with the former standards for molecular MRD quantification, ASO-PCR, and dot-blot hybridization.19,21,25 In addition, multicenter quality control of quantification data were achieved by interlaboratory tests in the frame of the European Study Group on MRD detection in ALL (ESG MRD ALL) under the direction of J. J. M. van Dongen (Rotterdam, the Netherlands). Fifteen of the patients investigated in the pilot phase were quantified by conventional ASO-PCR and dot-blot hybridization, as described earlier.5 MRD levels and individual sensitivity threshold values were corrected according to quality of DNA that was checked by amplification of control gene segments (albumin or β-actin). Follow-up samples judged MRD negative were excluded in case of an amplifiability of less than 10%. The LightCycler measured leukemia-specific PCR products, generated by ASO-PCR, at each cycle by staining the PCR product with the DNA-binding dye SYBR Green I.19 For TaqMan RQ-PCR, we and others developed target-specific assays with a set of different germ-line TaqMan probes (13 TCRB-Jβ, 8 TCRG-Vγ, 1 TCRD-Jδ1, 1 TCRD-Dδ2, 3 TCRD-Vδ,4 IgH-JH, and 1 IGK-Kde)17,18,20,21 and ASO primers. MRD levels were stated as proportion of leukemic cells in normal cells. If MRD levels, quantified by 2 or more targets, differed, the higher MRD level was assumed to be more accurate. In case of MRD positivity but with levels below the reproducible range, MRD levels were stated as a range between the limits of reproducibility and sensitivity. If no specific PCR product was detectable, this time-point was considered MRD negative regardless of the PCR-target sensitivity. In total, molecular analysis was performed in 290 patients. In 21 (7%) of 290 patients, no clonal marker was detected; in and additional 8 (3%) of 290 cases, clonal markers were not suitable for MRD quantification. Finally, residual disease was evaluated in 261 adult ALL patients (65 within the pilot study 05/93, and 196 within the 06/99 trial). A total of 418 different allele-specific assays were performed for these patients, 108 of them targeting clonal TCRB gene rearrangements, 79 targeting TCRG, 61 targeting TCRD, 126 targeting IgH, and 44 targeting IGK-Kde rearrangements. In 137 of 261 cases, patients were assayed with 2 (121 of 261) or more than 2 (16 of 261) PCR targets. In 233 (89%) of 261 cases, sensitivity of at least 1 assay reached 10-4 or less, with a sensitivity limit of 10-4 in 77 (30%) of 261 cases, a limit of 5 × 10-5 in 61 (23%) of 261 cases and a limit of 10-5 in 95 (36%) of 261 cases.
Statistical analyses
Distribution of variables between groups was compared using Fisher exact test or Chi-square test; the Mann Whitney U test was used to estimate significance of differences in continuous parameters. The end point to determine the prognostic significance of variables studied was the disease-free survival (DFS), calculated as the interval between first documented complete remission and relapse or end of observation. Patients who underwent SCT in first remission (11 patients in the GMALL 06/99 trial), patients with premature termination of first-year treatment (15 GMALL 06/99 patients), and patients who died in complete remission (4 GMALL 06/99 patients) were included but censored at the date of event. Kaplan-Meier estimates were calculated for the time-to-event variables DFS and overall survival (OS). Comparison between curves was performed using the log-rank test, or the log-rank test for linear trend in case of ordered categorical variables.26 The influence of potential prognostic factors on DFS was estimated with the stepwise Cox proportional hazard model, including age, sex, WBC count, immunophenotype, and MRD levels at different time-points.
All the statistical analyses were performed using the GraphPad Prism version 3.02 for windows (GraphPad Software, San Diego, CA) and SPSS version 11.5 (SPSS, Chicago, IL).
Results
Results of the pilot study: definition of sampling time-points and threshold levels
A total of 148 first-year follow-up samples of 65 ALL patients were evaluable for MRD analysis. Nineteen patients relapsed during the observation period, median follow-up of patients in continuous complete remission was 55 months, and estimated 5-year DFS rate was 55.0% (95% confidence interval [CI] 39.9%-70.1%). Ten of 27 patients with adverse prognostic factors (Table 1) were given transplants in first remission, and another 5 patients died during remission.
Percentage of MRD positivity decreased from 71% (20 of 28) after induction phase I (day 29) to 42% (15 of 36) before start of consolidation (week 13) and to 30% (8 of 27) before start of reinduction (week 21). Afterward, follow-up samples of only 3 patients were MRD positive (1 positive sample of 29 [week 30], 18 [week 46], and 11 [week 52] samples analyzed, respectively).
Presence of MRD with a level of at least 10-4 before start of consolidation (day 29 and/or week 13; Figure 1) was associated with a greater risk of relapse: 5-year DFS rate was 32.6% (95% CI 5.3%-59.9%) in 21 patients with MRD of 10-4 or higher within this period, compared with 71.8% (95% CI 50.0%-93.6%) in 27 patients with low (< 10-4) or undetectable MRD levels (P = .003). Frequency of patients with adverse prognostic factors was significantly higher in the MRD high level group (16 of 21) compared with the group with low or undetectable MRD (6 of 27; P < .001). However, multivariate analysis could not be done because of the small number of patients. During consolidation therapy, frequency of MRD-positive samples and relapse rate was too small for final statements. Six of 8 patients with measurable MRD at week 21 relapsed 3 to 34 months after MRD assessment. In contrast, only 2 of 19 patients without detectable MRD at this time-point relapsed after 33 and 36 months, respectively. Probability of 5-year DFS was 81.7% (95% CI 57.6%-100%) in MRD-negative patients, compared with 0% for MRD-positive patients (P < .001). In conclusion, for MRD evaluation of standard-risk ALL patients in the GMALL 06/99 trial,1 a sensitivity limit of 10-4 or lower was targeted for RQ-PCR assays,2 10-4 was defined as crucial threshold MRD level before start of consolidation treatment,3 and additional MRD evaluation time-points during the early course of therapy were defined to gain more insights into MRD kinetics (Figure 1), because residual disease at later time-points dropped below detection limit in almost all patients.
GMALL 06/99 MRD results for adult standard-risk patients
The 196 standard-risk ALL patients were monitored at up to 9 time-points during the first year of therapy (Figure 1). As the vast majority of all German adult ALL patients are enrolled in the GMALL trials and 87% of all eligible GMALL 06/99 standard-risk patients were included into molecular analysis over the collection period, the main potential bias was availability of material and presence of sensitive clonal markers. The investigated standard-risk ALL study population was compared with the remaining 95 adult GMALL 06/99 standard-risk ALL patients who were not assayed. The clinical features (sex, age, WBC count, immunophenotype, and 3-year cumulative incidence of relapse) did not differ between the 2 groups, with the exception of a higher proportion of cases comprising a T-cell phenotype in the MRD study population (37% vs 22%, P = .02). Within the observation period, 67 patients relapsed, 4 patients died during remission, and for 15 patients, therapy was stopped prematurely during the first year of treatment. In 35 of 63 cases of medullary relapse, bone marrow samples at clinical relapse were available. In 5 (10%) of 52 investigated targets, false-negative results were obtained at relapse, probably reflecting changes in the Ig and TCR gene rearrangements during the disease course. This concerned 2 (13%) of 15 IGH, 1 (25%) of 4 IGK, 1 (6%) of 16 TCRB, 0 (0%) of 8 TCRG, and 1 (11%) of 9 TCRD targets, and led to a failure to detect the relapse by PCR in 4 (11%) of 35 patients. Estimated 3-year DFS was 52.7% (95% CI 43.5%-61.9%), and median follow-up period of the patients in continuous complete remission was 30 months.
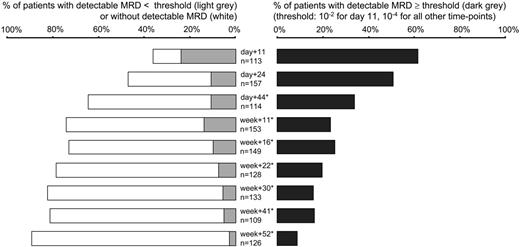
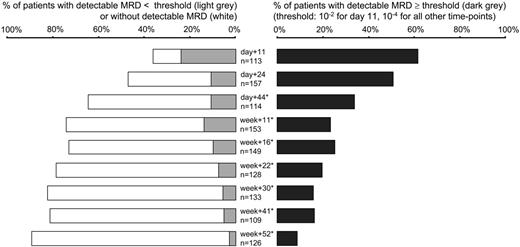
MRD was detected in 470 of 1196 evaluable follow-up samples; an additional 113 samples (8.6% of all samples) were excluded due to insufficient quality or quantity of DNA, in particular at day 11 (29 [20.4%] of 142 samples) and day 44 (22 [16.2%] of 136 samples), when hypoplasia was frequently found. In 79 MRD-negative samples with moderate restriction of quality (control gene amplifiability between 10% and 100%), individual sensitivity thresholds had to be scaled up to more than 10-4 despite sensitive underlying RQ-PCR assays. In case of detectable MRD with levels lower than 10-4 MRD values were mostly not exactly quantifiable as they were outside the reproducible range of the RQ-PCR assays.18,20,21 During induction phase I (day 11), MRD at any level was detectable in most (88%) patients. Percentage of MRD positivity decreased to 63% at day 24 and to 39% at week 11. At week 22, MRD was detected in 29% of the standard-risk patients, and after completion of first-year therapy in 13% of patients (Figure 2). Of note, the percentage of MRD positivity did not differ significantly in patients with T-lineage ALL compared with B-lineage ALL (P > .05 for every single time-point). Median MRD levels of MRD-positive samples were 7 × 10-2 and 1 × 10-3 during and after induction I (day 11 and day 24, respectively). Afterward, median MRD levels ranged between 3 × 10-4 and 7 × 10-4 in patients with detectable disease.
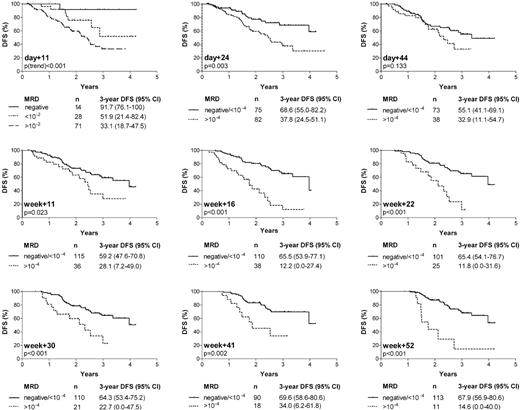
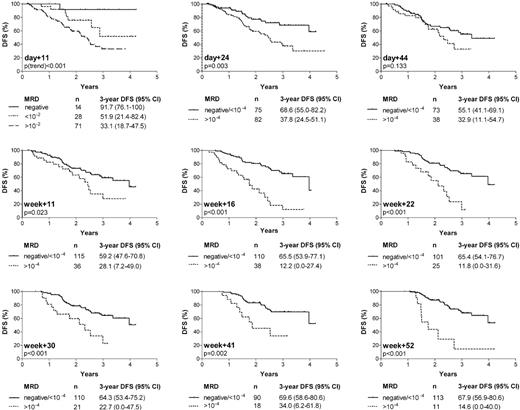
Figure 3 summarizes the estimated 3-year DFS rates for the different time-points depending on MRD levels. According to the results of the pilot study, a threshold level of 10-4 showed the strongest discriminative power after the end of induction I (day 24) until start of consolidation therapy (week 11), and was also used as cut-off point from week 16 to week 52. During induction I (day 11), MRD loads of 10-2 or higher were associated with a 3.2-fold higher incidence of relapse than lower degrees or absence of detectable disease. A tumor load below detection limit (regardless of target sensitivity) allowed a further substratification of patients with respect to a favorable outcome (Figure 3). After the end of induction I (day 24), the relative risk for relapse in patients with MRD levels of 10-4 or higher compared with levels lower than 10-4 or below detection limit was 2.4 (95% CI 1.3-4.2). For day 44 and week 11, the accuracy of MRD in defining patients with relapse was reduced, as ongoing treatment reduced MRD in most patients to levels close to 10-4 regardless of outcome. MRD information during the later course of therapy (week 16 to week 52) narrowed a smaller population of 26% to 9% of patients with persistent disease of 10-4 or higher and a 3- to 5-fold increase in relative relapse rates compared with patients with low (< 10-4) or undetectable MRD (95% CI of relative risks: week 16, 2.3-7.3; week+22, 1.9-7.1; week 30, 1.5-5.9; week 41, 1.5-7.2; and week 52, 2.3-11.4). Since only standard-risk ALL patients within the GMALL 06/99 trial were investigated, the study population was highly homogeneous concerning classical adverse prognostic factors. The remaining clinical and biological variables (sex, age, WBC count, and B-versus T-lineage ALL) were each tested as single variables in the Cox regression model in addition to MRD. The cut-off points for the quantitative variables WBC count and age were identical to those in Table 1. MRD was found to be the only variable that showed a significant impact on outcome.
Frequency of MRD positivity at the different following time points. Percentage of patients without detectable MRD (□), detectable MRD below ( ), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.
), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.
Frequency of MRD positivity at the different following time points. Percentage of patients without detectable MRD (□), detectable MRD below ( ), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.
), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.
Probability of disease-free survival (DFS) according to MRD results at 9 time-points during first year of therapy. Numbers of patients within each group and estimated DFS rates at 3 years (with 95% CI) are also stated.
Probability of disease-free survival (DFS) according to MRD results at 9 time-points during first year of therapy. Numbers of patients within each group and estimated DFS rates at 3 years (with 95% CI) are also stated.
Relation between residual disease on day 24 and MRD during consolidation therapy
Low or undetectable levels of day-24 MRD were related to MRD kinetics during later course of therapy. One hundred and five patients were assayed at day 24, week 16, and beyond week 16. In 47 of 105 patients, day-24 MRD levels were undetectable or lower than 10-4. MRD remained low or undetectable at week 16 in 45 (96%) of these 47 patients, and no MRD value of 10-4 or higher was measured from week 16 to week 52 in 42 (89%) of 47 cases.
In contrast, 40 (69%) of 58 patients with a high tumor load (≥10-4) on day 24 subsequently achieved low (<10-4) or undetectable MRD at week 16. Eighteen (31%) of 58 patients showed high levels of MRD up to week 16, and 16 of them remained positive at levels higher than 10-4 at later time-points during treatment.
Identification of MRD-based risk-groups
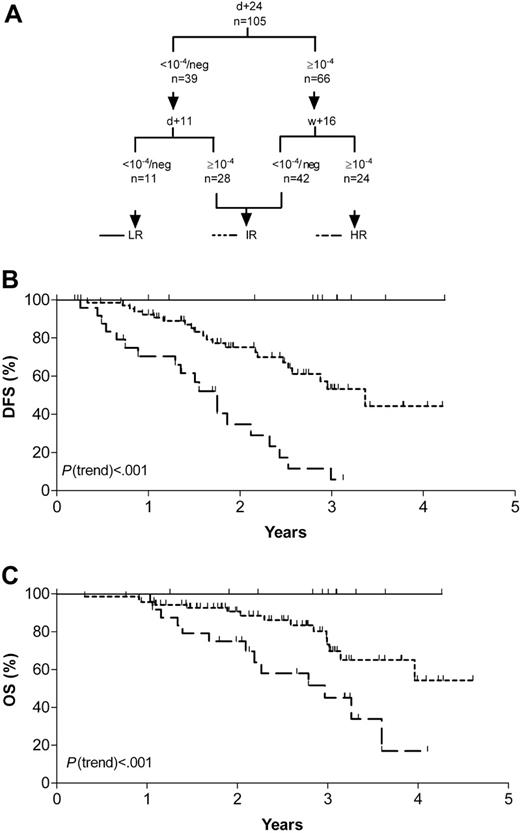
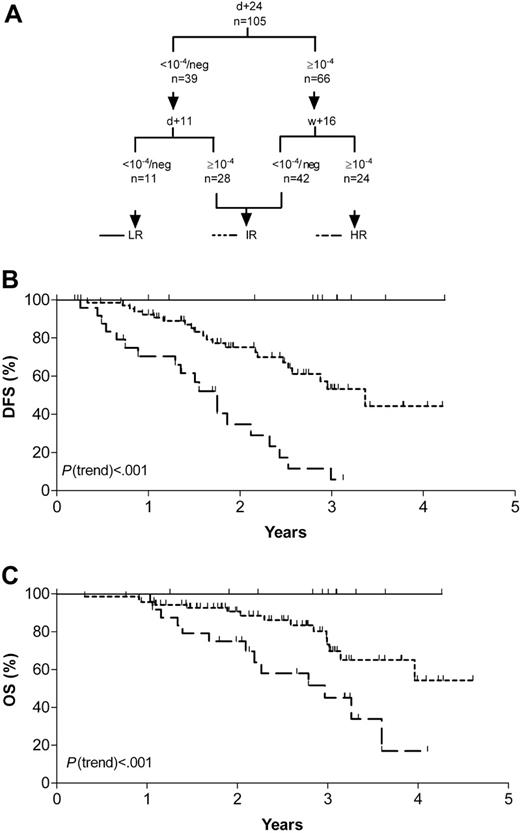
MRD analyses at single follow-up time-points discriminated standard-risk ALL patients with different risks of relapse: patients with a rapid tumor clearance and low relapse rates were recognized by day 11 MRD assessment. In contrast, patients with persistent detectable MRD of 10-4 or higher at week 16 showed an extremely poor outcome. These crucial time-points were therefore adducted to define an MRD-based low (day 11)- and high (week 16)-risk group. However, particularly in case of a reduced amplifiability of an MRD-negative follow-up sample or MRD positivity in the range of 10-4, it might be important to confirm results by a second MRD test. To enhance accuracy of this risk stratification, we therefore added day-24 MRD information because this value predefined 2 large populations of patients of about the same size with different risks of relapse, and generally allowed sensitive and reliable MRD assessment.
This stratification was applicable for 105 patients who did not significantly differ in distribution of sex, age, immunophenotype, and DFS from the group of 91 patients without MRD measurement at crucial time-points.
Eleven patients with low (< 10-4) or undetectable levels of day-11 and day-24 MRD formed the MRD-based low-risk group and had a 3-year DFS and OS rates of 100%. Median follow-up of the patients was 33 months (range, 12-51 months). Pretherapeutic clinical features were similar to that of the whole study population. No patient relapsed during the observation period, with 1 patient being censored after 1 year, as treatment was prematurely stopped prior to consolidation V due to severe treatment-related complications. However, this patient is still in complete remission after an additional 15 months. Twenty-four patients with MRD levels of 10-4 or higher at both day 24 and week 16 were classified as high risk. They showed a 3-year DFS rate of only 5.8% (95% CI, 0.0%-16.7%) and a 3-year OS rate of 45.1% (95% CI, 22.2%-68.2%). Their risk of relapse was increased by the factor 1.4 (95% CI, 1.2-1.7) compared with the whole study population. Until the end of observation, 19 of these patients relapsed, and 3 were censored due to individual treatment modification (in 1 patient treatment was prematurely stopped, and 2 patients received transplants in first remission). Only 2 patients were in continuous complete remission at the end of follow-up at 21 and 38 months, respectively. The remaining 70 patients were combined into an intermediate-risk group. They had a DFS rate of 53.2% (range, 36.9%-69.5%) (P(trend) < .001) and an OS rate of 69.8% (range, 54.8%-84.8%) (P(trend) < .001; Figure 4). The risk of relapse for this MRD-based risk group did not significantly differ compared with the whole study population. A further substratification of this patient group adding other time-points and/or MRD threshold levels using the data set acquired within this study was not successful (data not shown).
Discussion
The progress in treatment of adult ALL patients without conventional risk factors has been hampered by the inability to predict relapse after patients achieved a complete remission. Prospective MRD trials in large cohorts of homogeneously treated adult SR patients to define complementary prognostic markers are lacking. Within the GMALL 06/99 trial we were able to demonstrate that sequential monitoring of residual disease is a powerful indicator of treatment outcome. Using the combined information on day-11, day-24, and week-16 MRD, patients with a rapid tumor clearance and favorable outcome were discriminated from those with persistent disease and a particularly high relapse rate (Figure 4). Since MRD was monitored prospectively in the GMALL trial, which includes more than 60% of the incidence of adult ALL (15-65 years) in Germany, and all patients without adverse conventional risk factors were intended to be investigated, a selection bias within the SR-ALL population was effectively minimized. The definition of adverse factors emerged from the more than 3000 adult ALL patients homogeneously treated in the GMALL trials over a period of 20 years.9 The 3-year relapse rate of 47% in our SR-ALL study population was in keeping with the results of other adult ALL trials,9 and relapses were not predictable by conventional clinical and biological factors. Therefore, the only known variable potentially influencing outcome in the study population was MRD.
MRD-based risk-groups. (A) Categorization schematic representation according to combined MRD results of day 11, day 24, and week 16. (B) Probability of disease-free survival (DFS). (C) Probability of overall survival (OS). LR indicates low-risk group; IR, intermediate-risk group; and HR, high-risk group.
MRD-based risk-groups. (A) Categorization schematic representation according to combined MRD results of day 11, day 24, and week 16. (B) Probability of disease-free survival (DFS). (C) Probability of overall survival (OS). LR indicates low-risk group; IR, intermediate-risk group; and HR, high-risk group.
With regard to MRD kinetics, frequency of MRD positivity tended to be higher than that reported for childhood ALL. In our study 63% of adult patients had measurable MRD at day 24 and 47% at day 44, whereas in 5 large prospective studies on childhood ALL, residual disease was detectable in 25% to 58% of patients after 4 to 6 weeks of induction therapy.1,4,5 Also at later time-points, percentage of MRD positivity in adult patients was higher. MRD was detected in 23% of cases at week 30, compared with only 10% to 13% of pediatric ALL.1-5 This probably reflects the higher in vivo drug resistance of adult ALL and was also reported by Mortuza et al.7
MRD quantification during induction (day 11) identified patients with a very rapid molecular response and an excellent prognosis (3-year DFS, 92%) in line with reports on childhood ALL.27,28
Also, the extent of residual disease after induction therapy predicted treatment outcome, although patients who reached the condition of MRD clearance down to 10-4 or below detection limit only at this treatment phase still had a poorer prognosis when compared with those who achieved an early (day 11) profound MRD reduction. Therefore, the same MRD status, but differing lengths of time to achieve it, resulted in a different prognosis. The failure to identify a subgroup with an excellent prognosis by postinduction MRD assessment is most likely explained by the PCR sensitivity limit of about 10-4 to 10-5 in the present study. Considering that most patients became RQ-PCR negative after induction treatment, and the number of residual blasts in these patients might vary between 0 and 108, additional subgroups with different MRD kinetics could probably be identified using more sensitive detection techniques.
In our study, postinduction MRD provided important information by identifying patients with an extremely poor prognosis. Single MRD tests at week 16 and week 22 narrowed a population of 38 (26%) of 148 patients and 25 (20%) of 126 patients, respectively, with a 3-year DFS of 12%. This is in keeping with findings of Vidriales et al8 in an immunophenotypic analysis of 102 adolescent and adult ALL patients who demonstrated a high discriminative power of day-35 MRD but a relapse rate of about 50% even in patients with MRD levels lower than 0.05%. Similarly, Brisco et al15 analyzed MRD in 27 adults by PCR, reporting that 8 (73%) of 11 patients with MRD higher than 10-3 relapsed compared with 6 (38%) of 16 with an MRD lower than 10-3 after the end of induction (days 22 to 68). Mortuza and colleagues7 investigated 85 adult patients with B-lineage ALL. DFS for patients with detectable MRD 3 to 5 months and 6 to 9 months after diagnosis was 11% and 0%, respectively, compared with 74% and 80% in MRD-negative patients.
Molecular MRD assessment using Ig/TCR gene rearrangements as PCR targets might be hampered by the occurrence of continuing rearrangements.29 In our study population this is the most likely explanation for false-negative PCR results in 11% of the investigated samples obtained at relapse, and stresses the importance of the use of 2 molecular targets for MRD quantification.
Percentages of MRD positivity did not significantly differ in patients with T-lineage ALL compared with B-lineage ALL for every single time-point which was in line with the observation that relapses were not predictable by immunophenotype. For childhood ALL, Willemse et al identified differences in MRD kinetics between T- and B-lineage ALL, with a higher frequency of MRD positivity in T-lineage ALL,30 but children with T-ALL generally have a poorer prognosis than those with precursor B-ALL, whereas in adult patients different clinical studies showed a poorer outcome for B-lineage ALL.11,31,32 However, results of different trials are not fully conclusive,33-36 and comparability of data are hampered, as we exclusively investigated standard-risk patients.
In this study we have shown that molecular MRD quantification in adult ALL is feasible even in large multicenter studies. Early MRD assessment allowed the identification of patients with a high chance for cure by chemotherapy alone, persistent detectable disease of 10-4 or higher during consolidation was associated with a high risk of relapse. Therefore, day-11 and week-16 MRD information was adducted to define an MRD-based low (day 11)- and high (week 16)-risk group. To enhance accuracy for MRD-based risk stratification, results were approved by a second MRD test (day 24). This stratification was applicable to 105 patients who did not differ in presenting features from the group of patients without MRD measurement at crucial time-points. The relatively high percentage of exclusions due to missing samples is explained by the fact that this was the first prospective MRD trial within the GMALL studies checking a considerable number of follow-up time-points and recruiting patients from more than 100 participating centers. The fraction of evaluable patients was comparable with the results of the first large prospective MRD studies on pediatric ALL.1-5 However, looking at the distribution of MRD-based risk groups, percentages substantially differed: in pediatric patients, MRD-defined low-risk groups made up 40% to 90% of patients, whereas only 5% to 15% of patients belonged to the MRD-based high-risk groups.1-3,5 Differences probably would have been even more pronounced if adult patients with high-risk features were added into our analysis, because median MRD levels in high-risk ALL appear to exceed that of standard-risk ALL,5,8,37 as the results of the GMALL 05/93 MRD pilot study also indicate. The small size of the MRD-based low-risk group in our study compared with pediatric trials might reflect differences in biology of the disease, as even SR-ALL in adults has a much poorer prognosis than childhood ALL. In addition, we applied extremely strict criteria to define the MRD-based low-risk group as specifically as possible, accepting a loss of sensitivity, in order to minimize the risk of relapse for the individual patient being assigned to the MRD-based low-risk group. The fraction of patients that belong to the MRD-based intermediate-risk group is higher compared with childhood ALL. Although these patients will receive standard therapy further on, they might profit from establishment of patient-specific MRD assays, as they can easily be monitored during and after maintenance therapy. Whether a disease recurrence can be identified in time to allow early intervention prior to a clinical relapse is currently the subject of another prospective trial.
The type of the treatment protocol, timing of the follow-up samples, and the applied MRD technique might influence the definition of the MRD-based risk groups. Therefore, precise MRD threshold levels for risk-group assignment have to be defined carefully for each treatment protocol before MRD-based risk stratification can be implemented.
Appendix
Participating centers (30 centers selected according to number of patients recruited, listed in alphabetical order):
Berlin: B. Dörken, W. D. Ludwig, U. Peters (Charité Universitätsmedizin); Dresden: G. Ehninger, R. Naumann (Klinikum Carl Gustav Carus); Düsseldorf: R. Haas, S. Knipp (Universitätsklinikum); Essen: U. Dührsen, S. Mahlmann (Universitätsklinikum); Esseni W. Heit, K. H. Baur (Kliniken Essen Süd); Frankfurt: D. Hoelzer, N. Gökbuget (Universitätsklinikum); Giessen: H. Pralle, M. Dörner (Universitätsklinikum); Hamburg: N. Schmitz, J. Rutjes (Allgem. Krankenhaus St Georg); Hamm: L. Balleisen, A. Grote-Metke (Evangelisches Krankenhaus); Hannover: A. Ganser, H. Diedrich (Medizinische Hochschule); Homburg/Saar: M. Pfreundschuh, F. Hartmann (Universitätsklinikum); Jena: K. Höffken, U. Wedding (Universitätsklinikum); Karlsruhe: J. T. Fischer, S. Wilhelm (Städt. Klinikum); Kiel: M. Kneba, M. Lamprecht (Universitätsklinikum Schleswig-Holstein); Köln: M. Hallek, P. Staib (Universitätsklinikum); Mainz: C. Huber, J. Beck (Universitätsklinikum); Marburg: A. Neubauer, M. Jänike (Klinikum Lahnberge); Minden: H. Bodenstein, H. Lampe (Klinikum Minden); München: C. Nerl, T. Lipp (Krankenhaus München-Schwabing); C. Peschel, F. Schneller (Klinikum Rechts d. Isar); W. Hiddemann, G. Lenz (Universitätsklinikum Grosshadern); Münster: W. E. Berdel, M. Stelljes (Universitätsklinikum); Nürnberg: M. Wilhelm, J. Neteler (Klinikum Nürnberg Nord); Oldenburg: H. J. Illiger, B. Metzner (Klinikum Oldenburg); Potsdam: R. Pasold, A. Gerhardt (Klinikum Ernst von Bergmann); Stuttgart: W. Aulitzky, L. Leimer (Robert Bosch-Krankenhaus); Tübingen: L. Kanz, M. Schmalzing (Universitätsklinikum); Ulm: H. Döhner, M. Schmid (Universitätsklinikum); Wiesbaden: N. Frickhofen, C. Gerlach (Dr-Horst-Schmidt-Kliniken).
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-07-2708.
Supported by the Wilhelm Sander-Stiftung (grant no. 2001.074.1), Deutsche Forschungsgemeinschaft (grant no. 422/1-1), Deutsche Krebshilfe (grant no. 70-2657-Ho2), and by the Bundesministerium für Bildung und Forschung (BMBF), Competence Network Leukaemias (grant no. 01GI 9971).
M.B. participated in the development of PCR assays, was responsible for the data collection and interpretation, and drafted the manuscript. T.R. was responsible for molecular MRD analysis and participated in interpretation of the data and manuscript preparation. T.F. and M.N. did the testing and standardization of the PCR techniques and assessed the data in Heidelberg. J.D., S.L., C.P., and M.R. optimized the PCR assays and did the MRD analyses in Kiel. N.G. is the coordinator of the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL); she provided the information on presenting clinical, immunophenotypic, and genetic features, and contributed to the preparation of the paper. U.S. organized the collection, storage, and distribution of many follow-up samples. H.-A.H. performed morphologic analyses of bone marrow aspirates. E.T. did the immunophenotyping for classification of the leukemias. D.H. is the chairman of the GMALL trials; he was responsible for the treatment protocol design and the overall clinical conduct of the studies. C.R.B. and M.K. were responsible for the overall conduct of the MRD study, and participated in manuscript preparation.
A list of participating institutions and principal investigators of the GMALL appears in the “Appendix.”
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the participants of the German Multicenter Study Group for adult ALL for their close collaboration in the MRD study. We also thank R. Reutzel and S. Hug for their outstanding logistic support, J. U. Siebmann for undertaking the statistical analyses, and B. Brix, L. Lorenzen, F. Hemken, H. Seppelt, and N. Passow for their excellent technical assistance.


 ), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.
), and above or equal to (▪) defined threshold values (10-2 for day 11, 10-4 for all other time points). Total number of patients varied at different time-points because sufficient follow-up material was not available from all patients for each time-point (frequency of low quality/quantity DNA was relatively high at time-point day 11 and day 44). *For time-points day 44 to week 52, 1 (week 16 and week 41), 2 (week 11, week 22, week 30, week 52), or 3 (day 44) MRD-positive samples could not be assigned to the MRD levels lower than 10-4 or 10-4 or higher because values below quantitative detection limit and range between quantitative detection limit and sensitivity limit spanned 10-4.