Antigen-specific cancer immunotherapy directed toward tumor-nourishing angiogenic blood vessels holds the promise of high efficacy, low toxicity, and ease of application. To evaluate whether the human angiogenic kinase insert domain-containing receptor (KDR) can serve as a target for cellular immunotherapy, 19 peptide sequences with HLA-A*0201 motifs were selected by computer-based algorithms. Five peptides (KDR82-90, KDR288-297, KDR766-774, KDR1093-1101, KDR1035-1044) stimulated specific cytotoxic T lymphocytes (CTLs) from peripheral-blood mononuclear cells (PBMCs) of 3 HLA-A*0201 donors. The decapeptide KDR288-297 was efficient in sensitizing target cells for recognition by a CTL clone at a concentration of 10 nM. More important, KDR288-297 - specific CTLs lysed target cells transfected with HLA-A2/KDR cDNAs and a range of HLA-matched KDR+ angiogenic endothelial cells (aECs) and also recognized CD34+ endothelial progenitor cells. The specificity of CTLs was further confirmed by tetramer assay and cold-target inhibition assay. In addition, ex vivo exposure of aECs to the inflammatory cytokines enhanced CTL reactivity, which is in keeping with up-regulated KDR and HLA class 1 expression. In Matrigel assays, recognition of aECs by specific CTLs triggered an antivascular effect. These findings provide the first proof of the antigenic property of KDR protein and may be useful for devising new immunotherapeutic approaches to human cancers.
Introduction
Angiogenesis is a common attribute of tumors. Without a vascular supply, tumors cannot expand beyond a minimal size and cannot invade locally or metastasize to distant sites.1 Thus, the inhibition of angiogenesis promises a broadly applicable strategy for treating cancer. The discovery of abnormalities of tumor endothelium that express distinct adhesion molecules, receptors, or other cell-surface proteins makes it possible to target therapeutic agents selectively to tumor angiogenic endothelial cells (aECs).2 Indeed, in the wake of such abnormalities, a wide range of pharmacologic inhibitors of angiogenesis have been devised, and many are in the process of incorporation, or have already been incorporated, into clinical trials.1,3
Recently, mouse studies demonstrating that tumor aECs express antigens that are recognized by the immune system of the host further disclosed a new immunobiologic feature of these cells. In these models, highly active and specific CTLs against dividing tumor aECs can be induced in immunized animals by restimulation, either with xenogeneic endothelial cells as a vaccine or with single molecularly defined immunogens based on angiogenic receptors.4-10 Intriguingly, the induction of T-cell immunity to tumor aECs resulted in profound inhibition of tumor angiogenesis and growth, albeit with only modest or no toxicities. Thus, the tumor endothelia could serve as an effective target for T cell-based immunotherapies. Notably, such an immune strategy differs considerably from strategies involving chemical or biologic inhibitors of angiogenesis, which often require constant administration at relatively high dosage levels.1,3 Similarly, this strategy is in contrast to conventional cancer immunotherapies directed against tumor cells because it targets genetically stable endothelial cells of the newly formed tumor vessels; hence, the development of immune escape variants appears unlikely.4-6,11,12
In human counterparts, however, the relative importance of the tumor endothelium in immune recognition by CTLs in vitro or in vivo has not yet been elucidated. In this study, therefore, we sought a suitable antigen on human aECs recognized by CD8+ CTLs. Rather than analyzing tumor endothelia-derived cDNA libraries with specific T-cell clones of patients, we applied a “reverse immunology” approach13,14 in which candidate antigens and their CTL epitopes are deduced from the genes known to be exclusively expressed, or at least highly overexpressed, in target cells. We hypothesized that a tumor endothelial antigen would (1) be highly restricted to the aECs of human cancers, (2) include peptide sequences that bind to MHC molecules, (3) be processed by activated aECs such that antigen-derived peptides are available for binding to MHC molecules, and (4) be recognized by the human T-cell repertoire in an MHC-restricted fashion.
The kinase insert domain-containing receptor (KDR) is an endothelial cell-specific receptor that is strikingly up-regulated in neovasculatures of many human tumors.15-18 It serves a key function during tumor angiogenesis: once bound to its ligand, VEGF, KDR mediates signals for the survival, proliferation, and differentiation of endothelial cells and for vascular permeability.19 Such properties make KDR a valuable target for immunologic intervention. In this study, we identify KDR as a tumor endothelium-associated antigen and report the generation of CTLs specific for a KDR peptide that recognizes KDR+ aECs and their CD34+ progenitor cells in an MHC-restricted manner. Furthermore, we show that endothelial recognition by KDR-specific CTLs could be enhanced by inflammatory conditions and could trigger an antivascular effect in an in vitro angiogenesis assay.
Materials and methods
Synthetic peptides and monoclonal antibodies
KDR and control peptides were synthesized in a peptide synthesizer (model EPS 221; Abimed, Langenfeld, Germany) using Fmoc technology. Identity and purity of the peptides were confirmed by reverse-phase high-performance liquid chromatography (HPLC) and mass spectrometry. PE- or FITC-conjugated mAbs to CD3, CD8, CD14, CD31, CD54 (ICAM-1), CD83, CD106 (VCAM-1), CD62E (E-selectin), and CD144 (VE-Cadherin) were obtained from BD Biosciences (Heidelberg, Germany). Monoclonal antibodies W6/32 (anti-HLA-A, -B, -C), BB7.2 (anti-α2 domain of HLA-A2), and L243 (anti-HLA-DR) were from American Tissue Culture Collection (ATCC; Manassas, VA).
Cell lines
Primary human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs) were isolated from different donors using standard protocols. Their endothelial origin was affirmed by flow cytometry analysis demonstrating 100% of cells positive for CD31 and vascular endothelial cadherin (VE-cadherin), the 2 endothelial lineage cell markers. These cells actively proliferate, express KDR protein, and are the most common ex vivo models of aECs for studying angiogenesis. The EBV-transformed B-cell lines were generated from HLA-A2+ healthy donors and used as feeder cells for T-cell expansion. COS-7 cells stably transfected with HLA-A*0201 cDNA were kindly provided by Dr Jan Mueller-Berghaus (Mannheim, Germany). The human retinal pigment epithelial cell line ARPE-19, the transporter associated with antigen processing (TAP)-deficient T2 cells and major histocompatibility complex (MHC) class 1-negative lymphoma/leukemia cells Daudi and K562, 293-EBNA cells, and HEK-293T cells expressing HLA-A*0201 (60% by fluorescence-activated cell sorter [FACS]) were obtained from ATCC.
Purification of CD34+KDR+ cells
Cryopreserved human CD34+ cells purified from cord blood of 2 HLA-A2+ donors (90%-97% purity) were purchased from Cambrex BioScience (Walkersville, MD). After a 10-day expansion period in hematopoietic stem cell medium (Stemline II; Sigma, St Louis, MO) containing SCF, G-CSF, and TPO at 100 ng/mL each (all from Sigma), cells were subjected to positive selection of CD34+KDR+ cells as follows: 3 × 106 nonadherent cells were incubated with anti-KDR scFv (1 μg/mL, clone A7; ReliaTech GmbH, Braunschweig, Germany) on ice. After 30 minutes of mixing, cells were washed and subsequently underwent 2 consecutive incubations (30 minutes each), first with anti-E-tag mouse mAb (Amersham Biosciences Europe, Freiburg, Germany) and then with pan-mouse IgG (Dynabeads, Dynal, Oslo, Norway) at 4°C. Rosetted cells were isolated using magnetic Dynal magnetic particle concentrators (MPCs). After supernatants were removed, the cells were washed and maintained with stem-cell medium for 18 hours to allow for detachment of the beads. The purity of KDR-selected cells (1 × 104 cells) was analyzed by 2-color flow cytometry using a combination of PE-conjugated anti-CD34 mAb (Miltenyi Biotech, Bergisch Gladbach, Germany), anti-KDR scFv, anti-E-tag mAb, and FITC-conjugated F(ab′)2 goat anti-mouse immunoglobulin (Dako, Hamburg, Germany).
HLA-A*0201 binding assay
T2 cells were incubated overnight with saturating amounts of the peptides at 50 μg/mL in the presence of 3 μg/mL β2-microglobulin (Sigma). HLA-A*0201 expression was then measured by flow cytometry using BB7.2 mAb followed by incubation with FITC-conjugated F(ab′)2 goat anti-mouse immunoglobulin.
Generation of CTL lines and clones
Human PBMCs were obtained from HLA-A*0201 donors (M, S, and P) by Ficoll density centrifugation. DCs were then prepared using IL-4 and GM-CSF, as described,20 with more than 85% of the cells CD83+CD14-. CD8-enriched T cells (more than 90% CD8+, more than 95% CD3+) were prepared by CD8+ selection using Dynabeads (Dynal). DCs were harvested after 9 days in culture, pulsed with peptide (50 μg/mL) and β2-microglobulin (3 μg/mL) for 4 hours at 37°C, inactivated with mitomycin C, and added to autologous CD8-enriched T cells at a T/DC ratio of 10:1 in RPMI medium with 10% human AB sera, 2 mM glutamine, 100 U/100 μg/mL penicillin/streptomycin, 25 mM HEPES, and 20 IU/mL IL-2. At day 12 and weekly thereafter, T-cell cultures were restimulated with inactivated, peptide-pulsed autologous monocytes, as described.20 T-cell lines specific for a decamer KDR peptide (KDR288-297) were generated from donors S and M and were further cloned by limiting dilution methods (at 1 cell/well) using inactivated allogeneic peripheral-blood leukocytes (PBLs) (1 × 103 cells/well) as feeder cells in RPMI medium, as described, containing 500 IU IL-2. After 12 days, T-cell clones were expanded in AIM-V medium containing 6000 IU/mL IL-2. To achieve optimal expansion, we used the OKT3 expansion method described by Riddell and Greenberg.21 Briefly, on day 0, 5 × 104 to 5 × 105 T cells were cocultured with HLA-A*0201+ PBLs (PBL/T-cell ratio, 500:1) and inactivated EBV-B cells (EBV/T-cell ratio, 100:1) in 25 mL RPMI medium containing 11% human sera, 30 ng/mL OKT3 Ab, and antibiotics. On day 1, IL-2 was added at a final concentration of 180 IU/mL. On day 5 and every 3 days thereafter, the medium was exchanged with fresh medium containing 11% human sera and 180 IU/mL IL-2. On days 12 through 14, T cells were harvested, counted, and cryopreserved.
Peptide-MHC tetramer assays
To stain CD8+ T-cell clones, soluble tetrameric complexes of HLA-A*0201/peptide were produced using methods described previously22 with the HLA-A*0201-binding peptides KDR288-297 FLSTLTIDGV and EBV/BMLF1259-267 GLCTLVAML. CTLs (2 × 105) were incubated with 10 μg/mL tetramer (PE-conjugated) on ice in 50 μL PBS, 0.01% NaN3, 2 mM EDTA, and 2% FCS. Fifteen minutes later, saturating amounts of FITC-conjugated anti-CD8 mAb were added, and samples were incubated on ice for another 20 minutes. After extensive washing with PBS containing 1% FCS, the samples were analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany).
Antibody blocking and IFN-γ assays
To check for the MHC restriction element used by T cells, T-cell activity was measured in the absence or presence of a variety of antibodies, including W6/32, BB7.2, and L243, that were purified from ATCC hybridoma supernatants. Target cells in 90 μL T-cell assay medium (10% human sera, 50 IU IL-2) were incubated with 10 μL antibody (200 μg/mL) for 60 minutes. After the removal of mAbs, T cells in 200 μL T-cell assay medium were then added and incubated for 18 to 24 hours. IFN-γ released in culture supernatants was measured by standard IFN-γ enzyme-linked immunosorbent assay (ELISA).
Transfection of KDR and HLA-A*0201 expression constructs
The KDR full-length construct was purchased from OriGene Technologies (Rockville, MD). Cells (293-EBNA) were (co)transfected with KDR plasmid or HLA-A*0201 gene20 using LipofectAMINE reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The same protocol was used for transfecting endothelial and nonendothelial cells, which are singly positive for HLA-A*0201 or for KDR.
Cytotoxicity assays
Cloned CTLs were used as effector cells. The cytolytic activity of CTLs was tested in a 6- to 16-hour LDH release assay20 performed in triplicate per condition, using 5 × 103 target cells per well in U-bottomed, 96-well microplates. Percentage specific lysis was calculated from LDH: [(experimental release - target spontaneous release - effector spontaneous release)/(target maximum release - target spontaneous release)].
Cold target competition assays
CTLs were preincubated with excessive numbers of unlabeled cells (cold targets) for 1 hour, after which chromium Cr 51 (51Cr)-labeled cells (hot targets, 5 × 103 cells/well) were added to the wells. Cytotoxicity was assessed in a standard 6-hour 51Cr release assay.22 Percentage inhibition was calculated as follows: [(% specific lysis without cold target - % specific lysis with cold target)/(% specific lysis without cold target)] × 100.
Retroviral transduction of HUVECs and in vitro angiogenesis assays
To directly visualize anti-KDR CTL-mediated antivascular effects in vitro, amphotropic retroviruses AZE-GFP (a gift from Dr Kasahara, Keck School of Medicine, University of Southern California, Los Angeles) were used to transduce HUVECs using standard protocol.23 This MuLV-based vector is highly infectious to proliferating cells and can retain the GFP insert over multiple passages.24 Once a whole transduced cell population turned GFP positive, as demonstrated by flow cytometry, cells were seeded in microwells containing Matrigel (BD Biosciences) and were cultivated for 24 hours for the formation of fluorescent tubular structures. CTLs were then added to the wells and were cocultivated for 18 to 20 hours at 37°C in 5% CO2. To prevent CTLs from viral infection, 10 μM AZT, an inhibitor of reverse transcriptase (Sigma), was included in the culture medium. For blocking studies, CTLs or newly formed capillary tubes on Matrigel were preincubated with 100 μM Granzyme B inhibitor zAAD (Enzyme Systems, Livermore, CA) and 20 μg/mL W6/32 mAb, respectively, before cocultivation. The wells were imaged before and after cocultivation using a fluorescence microscope equipped with a 20 ×/0.40 Korr Ph2 objective lens, a W-PI 10×/23 ocular lens, an Axiocam HRC camera, and Axiovision 3.1 software (all from Zeiss, Oberkochen, Germany). This system could obviate noise images resulting from any nonspecific attachment of CTLs to the HUVECs.
Western blot analysis
KDR protein expression was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In brief, freshly isolated PBMCs, EBV-B cells, or the different adherent cells were lysed in 100 μL lysis buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) containing protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Cell lysates corresponding to 30 μg total protein were separated on 7.5% SDS polyacrylamide gels and transferred to nitrocellulose (Hybond ECL; Amersham Biosciences Europe) membranes. After blockage for 1 hour in 3% milk powder in PBS, the membrane was washed and probed with KDR-specific mAb (0.5 μg/mL; Biomol, Hamburg, Germany) diluted in 1% dry milk in PBS. Filters were developed with horseradish peroxidase-conjugated anti-mouse IgG and enhanced by chemiluminescence (Roche).
Results
The entire sequence of the KDR protein (Swissprot accession no. P35968) was reviewed for peptides that could potentially bind to HLA class 1 molecules using T-cell epitope prediction.25 Starting with the most common HLA subtype, HLA-A*0201, we identified 19 nonamer and decamer candidate peptides (Table 1), which cover signal (aa 1-17), extracellular (aa 18-762), transmembrane (aa 763-787), and cytoplasmic (aa 788-1354) domains of KDR. Each contained putative HLA-A*0201 anchor motifs based on the presence of Leu-Ile-Met at position 2 (P2) and Leu-Val-Ile at P9 or P10.26 These peptides were pulsed with β2-microglobulin onto the HLA-A*0201+ T2 hybridoma in a standard functional peptide-binding assay. Based on the increased expression of HLA-A*0201 on pulsed T2 cells, 6 decapeptides and 5 nonapeptides were found to bind strongly to HLA-A*0201 (Table 1). Two positive control peptides derived from tumor antigens TRP2 and Melan-A also bound strongly, but the negative control peptide did not. The TRP2 peptide (TRP2288-296) has previously been identified by the same prediction analysis to bind to HLA-A*0201.20 In addition, induction of TRP2288-296-specific CTLs can lead to the lysis of TRP2+ tumor cells.20,27
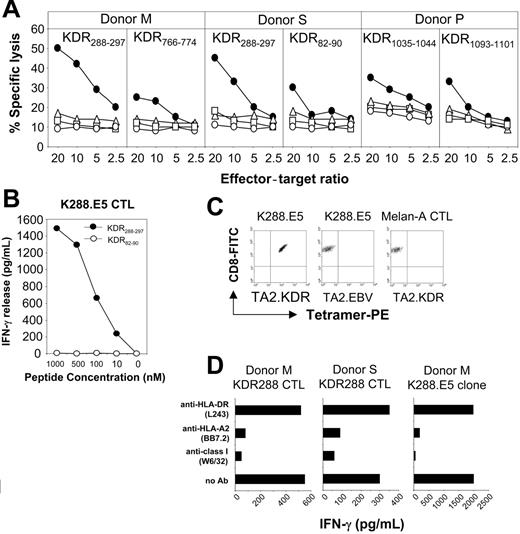
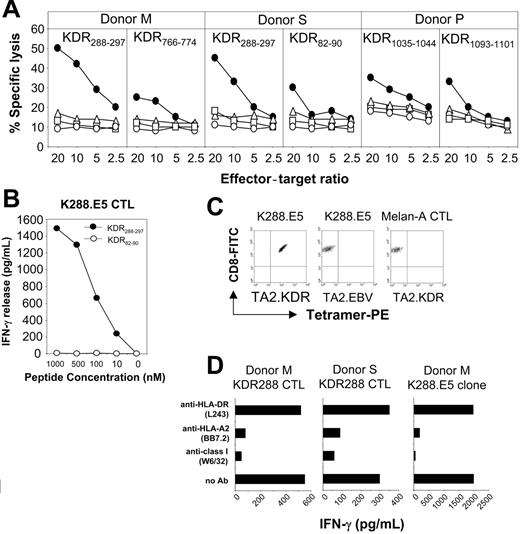
We then generated human CD8+ CTLs specific for the KDR peptides in vitro. Priority was given to 11 good binders because most CTL epitopes display high binding affinity to the MHC class 1 ligands.26 CD8+ T cells from peripheral blood of 3 healthy HLA-A*0201 donors were primed with peptide-pulsed autologous DCs, followed by weekly restimulation with autologous peptide-pulsed monocytes. After 2 rounds of restimulation, more than 95% of the cells in each culture were CD3+ T cells, of which more than 90% were CD8+. CTLs generated from 2 of the 3 donors with decapeptide KDR288-297 significantly lysed peptide-pulsed T2 cells but not the control targets K562, Daudi, or T2 cells alone (Figure 1A). From these, 8 CTL clones with high peptide specificity were generated by limiting-dilution methods. One of these clones, designated K288.E5, reacted with KDR288-297 peptide at a concentration of 10 nM in an IFN-γ release assay (Figure 1B). The purity of this clone was determined by MHC/peptide tetramer assay shown to contain more than 99% of the specific T cells. These CTLs can be stained using a tetrameric fluorescent HLA-A*0201 molecule folded around the KDR288-297 peptide but not by an irrelevant HLA-A*0201 tetramer complexed with an EBV epitope (Figure 1C), confirming peptide-specific recognition of K288.E5. In contrast, a control CTL line raised against a Melan-A HLA-A2 epitope was not stained by this tetramer. In blocking experiments, the reactivity of these CTL lines and clones with peptide-pulsed target cells was inhibited by mAbs specific for HLA class 1 and HLA-A*0201, but not by control mAb (Figure 1D and data not shown), suggesting that T-cell recognition is HLA-A*0201 restricted. CTLs generated with 3 nonapeptides, KDR82-90, KDR766-774, and KDR1093-1101, and another decapeptide, KDR1035-1044, showed weak cytotoxicity against peptide-pulsed T2 cells relative to the control targets (Figure 1A), but the specificity of their bulk cultures was lost during successive restimulations (data not shown). T-cell lines stimulated with other candidate KDR peptides did not show apparent peptide specificity either in cytotoxicity assays or in cytokine release assays, even after 5 restimulations (data not shown).
Recognition of human KDR-derived peptides by CD3+CD8+ CTLs generated from PBMCs of donors S, M, and P. (A) Cytotoxicity was determined in a 6-hour LDH release assay using T2 cells preincubated for 60 minutes with each peptide at 25 μg/mL (•). T2 alone (○), K562 (▵), and Daudi (□) cells were used as negative controls. Percentage of specific lysis is shown at different E/T ratios. (B) Dose-response recognition of KDR288-297 peptide by CTL clone K288.E5. Serial dilutions of KDR288-297 peptide were incubated with 5 × 104 T2 cells for 60 minutes, which were then used as target cells to stimulate T cells. Control peptide KDR82-90 was also used at various concentrations. This experiment was performed twice; results were similar each time. (C) Assessment of peptide-specific recognition of K288.E5 by HLA/peptide tetramer staining. CTLs specific for KDR288-297 peptide (K288.E5) or for Melan-A26-35 epitope (Melan-A CTL) were stained with FITC-anti-CD8 mAb and PE-HLA-A*0201 tetramers folded around either the KDR288-297 peptide (TA2.KDR) or a EBV/BMLF1-derived epitope (TA2.EBV). Data shown are representative of 2 independent experiments. (D) T-cell recognition of KDR288-297-pulsed T2 cells was specifically inhibited by mAbs against HLA class 1 and HLA-A2. Results are expressed as IFN-γ release (picogram per milliliter daily) from 2 × 104 K288.E5 CTLs. All antibodies were used at a final concentration of 20 μg/mL each.
Recognition of human KDR-derived peptides by CD3+CD8+ CTLs generated from PBMCs of donors S, M, and P. (A) Cytotoxicity was determined in a 6-hour LDH release assay using T2 cells preincubated for 60 minutes with each peptide at 25 μg/mL (•). T2 alone (○), K562 (▵), and Daudi (□) cells were used as negative controls. Percentage of specific lysis is shown at different E/T ratios. (B) Dose-response recognition of KDR288-297 peptide by CTL clone K288.E5. Serial dilutions of KDR288-297 peptide were incubated with 5 × 104 T2 cells for 60 minutes, which were then used as target cells to stimulate T cells. Control peptide KDR82-90 was also used at various concentrations. This experiment was performed twice; results were similar each time. (C) Assessment of peptide-specific recognition of K288.E5 by HLA/peptide tetramer staining. CTLs specific for KDR288-297 peptide (K288.E5) or for Melan-A26-35 epitope (Melan-A CTL) were stained with FITC-anti-CD8 mAb and PE-HLA-A*0201 tetramers folded around either the KDR288-297 peptide (TA2.KDR) or a EBV/BMLF1-derived epitope (TA2.EBV). Data shown are representative of 2 independent experiments. (D) T-cell recognition of KDR288-297-pulsed T2 cells was specifically inhibited by mAbs against HLA class 1 and HLA-A2. Results are expressed as IFN-γ release (picogram per milliliter daily) from 2 × 104 K288.E5 CTLs. All antibodies were used at a final concentration of 20 μg/mL each.
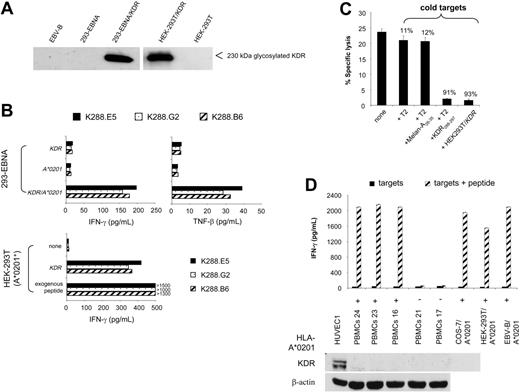
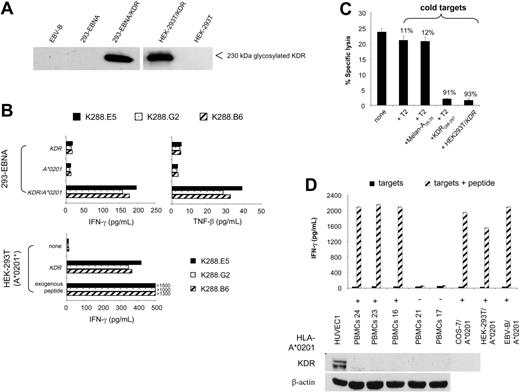
Next, we asked whether KDR288-297 peptide is naturally processed and recognized by specific CTLs in a KDR-dependent fashion. We first created target cells in which KDR-encoding plasmid and HLA-A*0201 cDNA were transiently cotransfected. In separate experimental settings, HEK-293T cells positive for HLA-A*0201 were singly transfected with the KDR gene to achieve a large fraction of KDR+A*0201+ cells in the transfectants. The expression of KDR protein, as represented by a 230-kDa band, was consistently detected in all transfected cells but not in nontransfected cells or in EBV-B cells as control (Figure 2A). Two independent cytokine secretion experiments reproducibly showed that K288.E5 responded to 293-EBNA double transfectants and to KDR-transfected HEK-293T cells but failed to recognize 293-EBNA single transfectants or the parental HEK-293T cells (Figure 2B). Similar results were obtained with the CTL clones K288.G2 and K288.B6, specific for the KDR288-297 peptide. Although the KDR/HEK-293T cell transfectants stimulated a greater cytokine response than 293-EBNA double transfectants, supporting the assumption of a greater number of KDR+A*0201+ cells contained in the former cultures, a much higher level of IFN-γ was induced when CTLs were cultivated with peptide-pulsed cells (Figure 2B). To test whether this CTL recognition occurs through the recognition of a specific KDR288-297 peptide, we performed cold-target inhibition assays using KDR/HEK-293T transfectants. As shown in Figure 2C, lytic activity was blocked by the addition of unlabeled relevant targets (KDR transfectants and T2 cells pulsed with KDR288-297 peptide), emphasizing the specificity of these CTLs. Whereas the presence of either T2 alone or cells pulsed with a control peptide yielded only marginal inhibition, these data indicate that KDR288-297 peptide is indeed naturally processed and presented by HLA-A*0201 on the transfectants. Accordingly, these CTLs failed to respond to a variety of primary cells and cell lines expressing HLA-A*0201 but negative for KDR protein, including PBMCs, EBV-B cells, and several epithelial cell lines (Figure 2A,D).
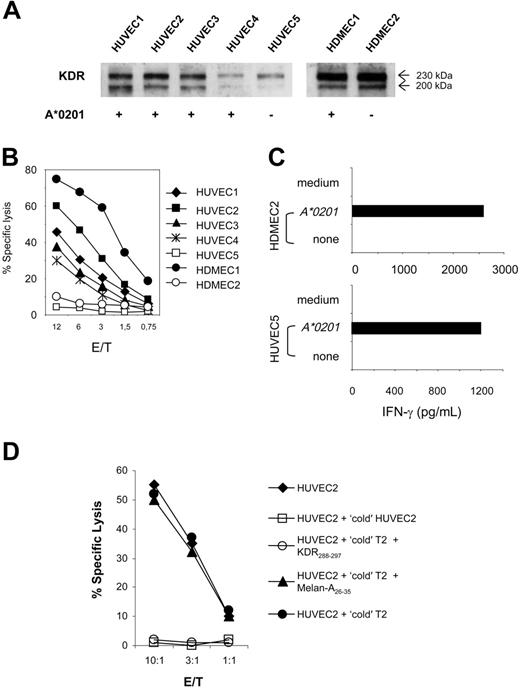
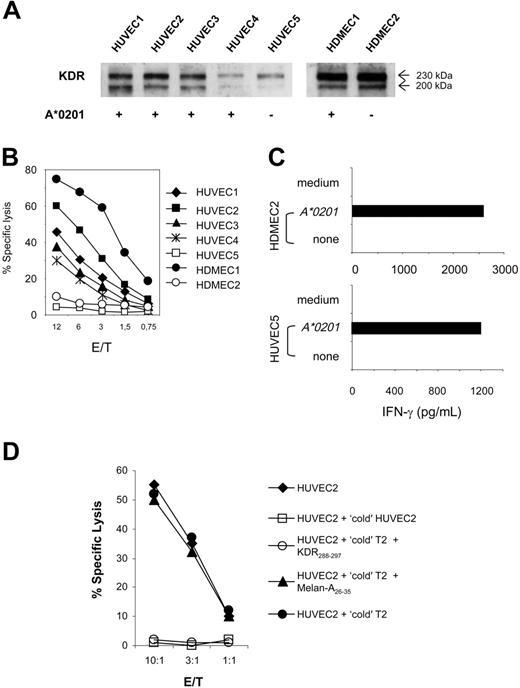
These findings suggest that KDR288-297 peptide fulfills the requirements for an immune antigen capable of triggering CTL response: it can be naturally processed and presented to CTLs in an MHC-restricted fashion. To examine whether KDR288-297-specific CTLs can recognize this antigen in the relevant angiogenesis-involving human aECs, we first evaluated the lytic activity of K288.E5 against a panel of primary endothelial-cell cultures isolated from human umbilical veins and human dermal microvasculatures. We used these cells because they typically exhibit an “activated” endothelial phenotype and are the most widely used types of aECs ex vivo. Each endothelial culture expressed high levels of CD31 and several endothelial activation markers such as KDR, VE-cadherin, E-selectin, and VCAM-1, as demonstrated by flow cytometry and Western blot analysis (Figure 3A and data not shown). Expression of the HLA-A*0201 allele was evaluated by flow cytometry and reverse transcription-polymerase chain reaction (RT-PCR) (data not shown). As shown in Figure 3B, K288.E5 CTLs lysed 4 HUVEC cultures isolated from different HLA-A*0201+ donors but not HUVECs negative for HLA-A*0201. Similarly, these CTLs specifically lysed a primary HDMEC culture in an HLA-A*0201-restricted fashion (Figure 3B). However, the transfection of HLA-A*0201-negative aECs with A*0201 cDNA reconstituted KDR288-297 epitope presentation and sequent CTL recognition (Figure 3C), again demonstrating A*0201 as the restriction element. Next, we assessed the specificity of aEC-specific lysis by these CTLs in cold-target inhibition assays (Figure 3D). Lysis of 51Cr-labeled HUVEC2 cells by K288.E5 was markedly reduced when these effectors were preincubated with unlabeled HUVEC2- or KDR288-297-pulsed T2 cells, demonstrating that KDR288-297 peptide can be naturally processed and can form a CTL epitope on aECs. No such inhibition was observed for T2 alone or for cells pulsed with a control peptide. Altogether, these results demonstrate that in a range of activated, dividing aECs of diverse origin, endogenous KDR is naturally processed and presented in the context of HLA-A*0201 and serves as a target for antigen-specific CTLs.
Natural processing and presentation of KDR288-297 peptide to cloned specific CTLs. (A) KDR protein expression by 293-EBNA and HEK-293T cells transfected with full-length KDR cDNA. Cells were transfected with 24 μg KDR plasmid DNA using LipofectAMINE. KDR expression was determined by Western blot. EBV-B cells and nontransfected cells were used as controls. (B) Recognition of transfectants by cloned CTLs. Cells (1 × 104 293-EBNA or A*0201-expressing HEK-293T) were transfected with 100 ng KDR, A*0201 plasmid DNA, or both using LipofectAMINE. Positive controls were cells pulsed with the exogenous KDR288-297 peptide at 10 μg/mL. T-cell assays were performed using cloned CTLs K288.E5, K288.G2, and K288.B6 specific for KDR288-297 peptide at an E/T ratio of 2:1. Cytokine contents in 24-hour culture supernatants were determined by standard IFN-γ ELISA and TNF-β bioassay.20 (C) Antigen-specific recognition of K288.E5 in cold-target inhibition assay. Effector cells (5 × 104/well) were incubated with 51Cr-labeled KDR/HEK-293T cells (5 × 103/well) in the presence of cold targets, KDR/HEK-293T, T2 alone, or T2 pulsed with KDR288-297 or an irrelevant peptide, at an inhibitor-target ratio of 20:1. Lytic activity was measured by 51Cr release assay. Numbers above bars represent percentage inhibition of lysis. (D) No reactivity of K288.E5 with KDR-negative primary cells and cell lines. Cells loaded with KDR288-297 peptide (10 μg/mL) were used as positive controls. Shown are the mean ± SD of triplicate wells consisting of 2 × 104 CTLs and equal numbers of individual targets. The lack of KDR protein in these cells was affirmed by Western blot analysis using a primary HUVEC culture and the β-actin marker as positive controls. All experiments were performed at least twice, with similar results.
Natural processing and presentation of KDR288-297 peptide to cloned specific CTLs. (A) KDR protein expression by 293-EBNA and HEK-293T cells transfected with full-length KDR cDNA. Cells were transfected with 24 μg KDR plasmid DNA using LipofectAMINE. KDR expression was determined by Western blot. EBV-B cells and nontransfected cells were used as controls. (B) Recognition of transfectants by cloned CTLs. Cells (1 × 104 293-EBNA or A*0201-expressing HEK-293T) were transfected with 100 ng KDR, A*0201 plasmid DNA, or both using LipofectAMINE. Positive controls were cells pulsed with the exogenous KDR288-297 peptide at 10 μg/mL. T-cell assays were performed using cloned CTLs K288.E5, K288.G2, and K288.B6 specific for KDR288-297 peptide at an E/T ratio of 2:1. Cytokine contents in 24-hour culture supernatants were determined by standard IFN-γ ELISA and TNF-β bioassay.20 (C) Antigen-specific recognition of K288.E5 in cold-target inhibition assay. Effector cells (5 × 104/well) were incubated with 51Cr-labeled KDR/HEK-293T cells (5 × 103/well) in the presence of cold targets, KDR/HEK-293T, T2 alone, or T2 pulsed with KDR288-297 or an irrelevant peptide, at an inhibitor-target ratio of 20:1. Lytic activity was measured by 51Cr release assay. Numbers above bars represent percentage inhibition of lysis. (D) No reactivity of K288.E5 with KDR-negative primary cells and cell lines. Cells loaded with KDR288-297 peptide (10 μg/mL) were used as positive controls. Shown are the mean ± SD of triplicate wells consisting of 2 × 104 CTLs and equal numbers of individual targets. The lack of KDR protein in these cells was affirmed by Western blot analysis using a primary HUVEC culture and the β-actin marker as positive controls. All experiments were performed at least twice, with similar results.
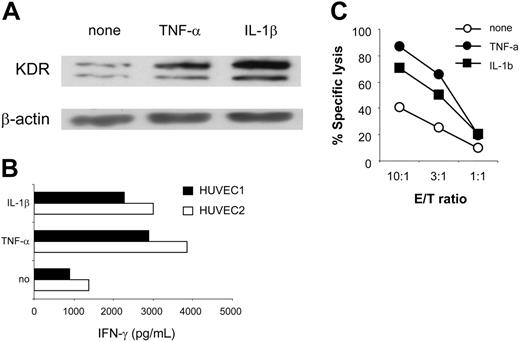
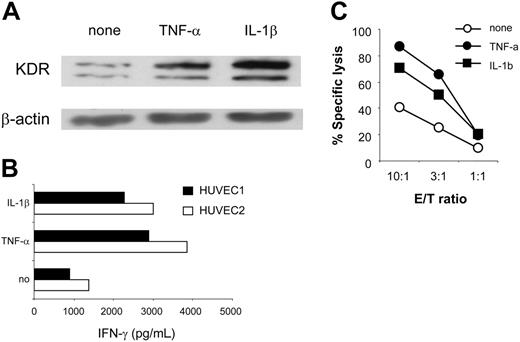
In angiogenically stimulated tissues, aECs are frequently exposed to proinflammatory cytokines, such as TNF-α and IL-1β, that potentiate tumor angiogenesis.28,29 Their intrinsic ability to respond to such molecules with respect to KDR and HLA class 1 expression suggests that an inflamed response may enhance KDR288-297 recognition by CTLs. To test this possibility, we studied CTL reactivity with 2 HUVEC cultures maintained in the presence or absence of cytokines. Consistent with other reports,30-32 treatment of aECs with TNF-α or IL-1β strikingly up-regulated surface levels of HLA class 1, -A2, and adhesion molecules CD54, E-selectin, and VCAM-1. KDR up-regulation was also observed (Figure 4A; Table 2; and data not shown). As a result, cytokine-treated cells were more effective than untreated cells and stimulated up to 3-fold greater IFN-γ production from K288.E5 (Figure 4B), presumably because of better T-cell activation by each KDR+ cell or increased numbers of KDR+ cells, or both. Similarly, lysis of cytokine-treated aECs by K288.E5 was also enhanced; representative data are presented in Figure 4C. Whether enhanced antigen processing of KDR288-297 epitope could be induced in this experimental setting is under investigation.
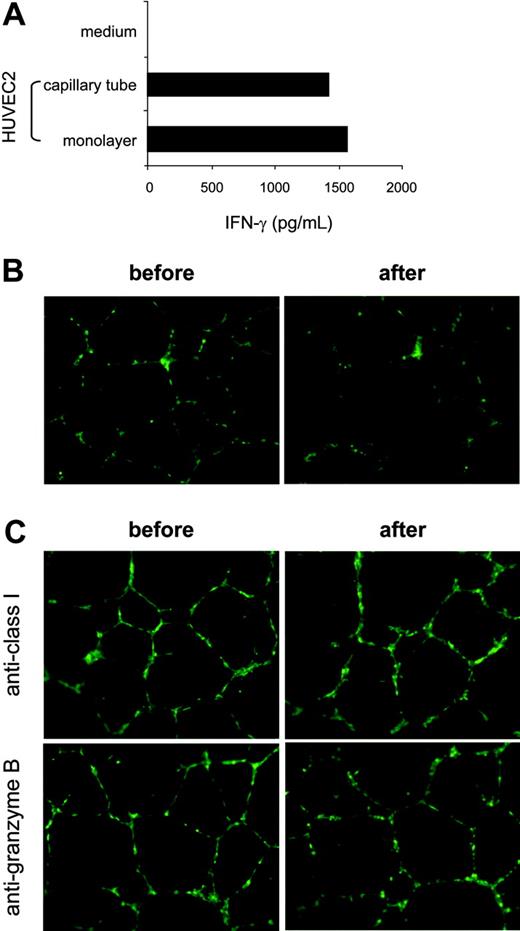
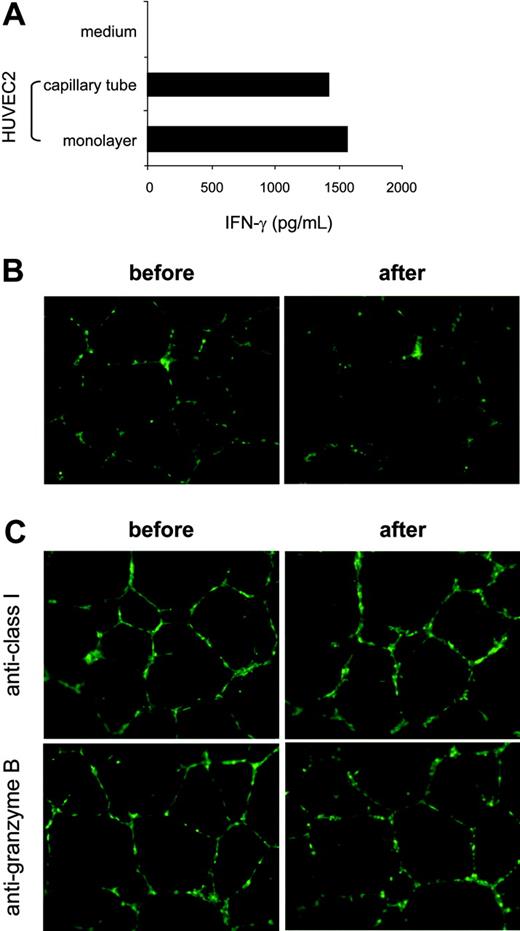
To evaluate the relative efficacy of KDR-specific recognition and hence to assess the significance of the level of CTL responses against preexisting neovasculature, we compared HUVEC2 cells maintained in monolayer with the same target in tubelike capillaries formed in Matrigel basement membrane matrix, in the context of CTL stimulation. The HUVEC2 cells were used as targets because these venous cells displayed a relatively high level of KDR and were recognized by K288.E5 with the strongest activity (Figure 3A-B). Once plated on a Matrigel surface, the HUVEC2 cells spontaneously aggregated and assembled into multicellular capillary-like structures after 24-hour cultivation (Figure 5B-C). As shown in Figure 5A, the monolayered HUVEC2 cells stimulated similar levels of IFN-γ from K288.E5, as did the capillary-forming cells in Matrigel. We then studied the capillary destruction capacity of these T cells using retrovirally transduced GFP-expressing HUVEC2 cells as targets. In this system, HUVEC2 cells can stably express the GFP marker, thereby allowing direct visualization of a CTL-mediated antivascular effect. Figure 5B shows that K288.E5 drastically eliminated KDR+ cells in newly formed tubes on cocultivation. This antivascular effect was completely abrogated when CTLs were pretreated with a Granzyme B inhibitor or if capillary tubes were preincubated with anti-HLA class 1 mAb (Figure 5C), indicating that CTL-mediated endothelial killing and tube destruction are indeed mediated through peptide recognition. Collectively, these results suggest that KDR288-297 recognition by antigen-specific CTLs could efficiently occur on angiogenic KDR+ cells and vessels, eventually mediating neovasculature damage.
Discussion
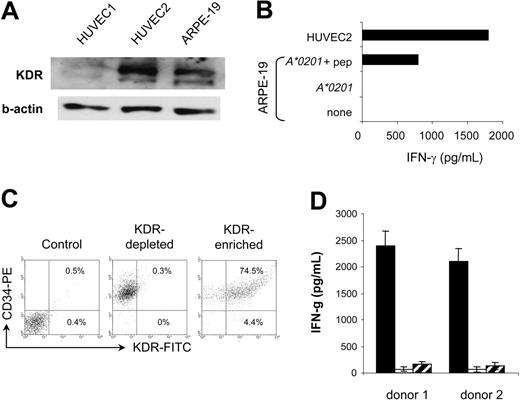
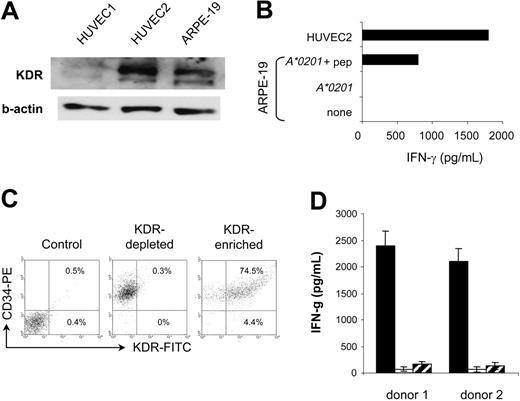
Because angiogenic KDR+ aECs represent unique therapeutic targets with respect to their genetic stability and easy accessibility to circulating T cells, immunotherapeutic strategies targeting this antigen may have the advantage of obviating problems associated with conventional therapies, such as emergement of antigen loss variants and poor T-cell penetration within tumor tissues.11,12,33,34 The major concern with such an approach is the possible destruction of the few normal cell types in which KDR can be substantially expressed. In adults, KDR is largely restricted to vascular endothelial cells and is overexpressed once these cells proliferate during angiogenesis.2,17,35-38 However, the retinal pigment epithelial cells (HRPEpic) and a subset of CD34+ cells, namely a common progenitor of endothelial and hematopoietic cells, have recently been reported to express KDR.39,40 We therefore examined the reactivity of K288.E5 toward these normal cells, starting with HRPEpic as potential targets. A human retinal pigment epithelial cell line, ARPE-19, transfected with the HLA-A*0201 gene, was used as the target. Although ARPE-19 cells expressed a detectable level of KDR (Figure 6A), expression was much weaker than in aECs. Interestingly, these ARPE-19 cells were not recognized by K288.E5, in contrast to a HUVEC culture (Figure 6B), suggesting that KDR does not readily function as an autoantigen on HRPEpic cells. This lack of CTL recognition may be caused by a low KDR expression level and, accordingly, only scarce numbers of KDR288-297-peptide/MHC complexes on the cell surface. Alternatively, assuming that the level of KDR in ARPE-19 cells is not in fact rate limiting for CTL activation, these cells may be unable to process this antigen and present it in the groove of MHC class 1. However, given that peptide-loaded ARPE-19 cells stimulated a weaker IFN-γ response than did HUVEC2 cells (Figure 6B), we cannot exclude the possibility of a low transfection efficiency of the A*0201 gene into the ARPE-19 cells in this system. Further studies using other HRPEpic primary cells are necessary to clarify this point. Next, we tested whether K288.E5 recognizes a subset of CD34+ stem cells. Because of the rarity of KDR+CD34+ cells in the body, the CD34+ cells purified from cord blood of 2 HLA-A*0201+ donors were first expanded ex vivo for 10 days in the presence of cytokines and were then subjected to positive selection using KDR-specific scFv.41 Coexpression of CD34 and KDR by the selected cells was demonstrated by flow cytometry analysis (Figure 6C). On mixing with CTLs, KDR+CD34+-enriched cells exerted the strong stimulatory activity and induced IFN-γ in an MHC class 1-restricted manner, whereas the KDR- cell fractions stimulated little cytokine production (Figure 6D). These results are in line with those shown in Figures 2 and 3, again demonstrating the antigen specificity of CTL responses. They suggest that the KDR+CD34+ cells can be efficient stimulators and targets for KDR-specific CTLs. Obviously, this apparent recognition of KDR+CD34+ cells by CTLs might influence normal hematopoiesis if it were occurring in vivo. However, in mouse models, the presence of a strong CTL immunity induced by a vaccine to mouse Flk-1—the homologue of human KDR—did not result in an abnormal hematopoietic profile.4 Clinical attempts to target KDR as a tumor endothelia antigen in cancer patients will provide major insight into this issue.
Recognition of HLA-A*0201-matched KDR+ aECs by K288.E5. (A) HLA-A2 and KDR phenotypes are summarized for each HUVEC and HDMEC primary culture. KDR protein in 30 μg cell lysate was assessed by Western blot. (B) Lysis of individual aEC cultures by K288.E5 at different E/T ratios. Cytolysis was determined in a 16-hour LDH release assay. (C) T-cell recognition of HLA-mismatched aECs on transfection with the HLA-A*0201 gene. Transfection and T-cell assays were performed as described in Figure 2. All experiments were performed 3 times, with similar results. (D) Cold-target inhibition assays containing unlabeled targets (1 × 105/well), 51Cr-labeled HUVEC2 cells (5 × 103/well), and K288.E5 at indicated E/T ratios. Cytotoxic activities were determined in a 6-hour 51Cr release assay. Data shown are representative of 2 independent experiments.
Recognition of HLA-A*0201-matched KDR+ aECs by K288.E5. (A) HLA-A2 and KDR phenotypes are summarized for each HUVEC and HDMEC primary culture. KDR protein in 30 μg cell lysate was assessed by Western blot. (B) Lysis of individual aEC cultures by K288.E5 at different E/T ratios. Cytolysis was determined in a 16-hour LDH release assay. (C) T-cell recognition of HLA-mismatched aECs on transfection with the HLA-A*0201 gene. Transfection and T-cell assays were performed as described in Figure 2. All experiments were performed 3 times, with similar results. (D) Cold-target inhibition assays containing unlabeled targets (1 × 105/well), 51Cr-labeled HUVEC2 cells (5 × 103/well), and K288.E5 at indicated E/T ratios. Cytotoxic activities were determined in a 6-hour 51Cr release assay. Data shown are representative of 2 independent experiments.
Enhanced CTL recognition of HLA-A2+ aECs on exposure to proinflammatory cytokines. (A) Up-regulated expression of KDR in HUVEC1 and 2 cells on treatment with TNF-α (20 ng/mL) or IL-1β (10 ng/mL) for 24 hours. KDR protein in 30 μg HUVEC1-cell lysates was determined by Western blot. The β-actin control is shown for each extract. (B) Enhanced recognition of TNF-α- or IL-1β-treated aECs by K288.E5. HUVEC1 and HUVEC2 cells were pretreated as described for 24 hours. After washing, they were cocultured with T cells at an E/T ratio of 3:1. Results are presented from 24 hours of IFN-γ production (pg/mL). (C) Enhanced specific lysis of TNF-α- or IL-1β-treated HUVEC1 cells by K288.E5 at different E/T ratios. Cytolysis was assessed in a 16-hour LDH release assay. Data shown are representative of 2 independent experiments.
Enhanced CTL recognition of HLA-A2+ aECs on exposure to proinflammatory cytokines. (A) Up-regulated expression of KDR in HUVEC1 and 2 cells on treatment with TNF-α (20 ng/mL) or IL-1β (10 ng/mL) for 24 hours. KDR protein in 30 μg HUVEC1-cell lysates was determined by Western blot. The β-actin control is shown for each extract. (B) Enhanced recognition of TNF-α- or IL-1β-treated aECs by K288.E5. HUVEC1 and HUVEC2 cells were pretreated as described for 24 hours. After washing, they were cocultured with T cells at an E/T ratio of 3:1. Results are presented from 24 hours of IFN-γ production (pg/mL). (C) Enhanced specific lysis of TNF-α- or IL-1β-treated HUVEC1 cells by K288.E5 at different E/T ratios. Cytolysis was assessed in a 16-hour LDH release assay. Data shown are representative of 2 independent experiments.
The potential significance of immune targeting of tumor endothelia using KDR288-297 peptide is 2-fold: its exquisite specificity attributed to the host immune system and its potentially sustained intervening effects, as indicated by animal studies targeting angiogenic receptors.4-12 It may, therefore, be less toxic than pharmacologic inhibitors and more convenient and cost-effective for patients by reducing the frequency of interventions required in conventional strategies. Moreover, immune elimination solely depends on the expression of the target antigen and thus will not be affected by other angiogenesis-associated products with overlapping functions, distinguishing it from pharmacologic intervention.12,42,43
Efficient recognition of the capillary tube comprising HLA-A2+ aECs by K288.E5. (A) Comparison of T-cell recognition of aECs cultivated under different conditions. HUVEC2 cells (2 × 104/well) plated in tissue-culture wells (forming a monolayer) or in polymerized Matrigel (10 mg/mL, assembling into tubular structures) were mixed with K288.E5 at a 1:2 ratio and were cocultured overnight. IFN-γ release from CTLs was measured by IFN-γ ELISA. (B) Destruction by K288.E5 of capillary tubes formed in Matrigel. Tube-forming GFP+ HUVEC2 cells (1 × 104/well) were cultivated overnight with CTLs (3 × 104/well) in the presence of AZT (10 μM). Fluorescent structures were imaged before and after cocultivation using an inverted microscope. (C) Capillary destruction by K288.E5 was blocked by anti-HLA 1 mAb and Granzyme B inhibitor. T-cell assays were performed exactly as described in panel B except that, before cocultivation, tubes and CTLs were treated for 1 hour with anti-HLA 1 mAb (W6/32, 20 μg/mL) and a Granzyme B inhibitor (zAAD, 100 μM; Enzyme Systems), respectively. All experiments were repeated twice, with similar results. Original magnification, × 20 for B and C.
Efficient recognition of the capillary tube comprising HLA-A2+ aECs by K288.E5. (A) Comparison of T-cell recognition of aECs cultivated under different conditions. HUVEC2 cells (2 × 104/well) plated in tissue-culture wells (forming a monolayer) or in polymerized Matrigel (10 mg/mL, assembling into tubular structures) were mixed with K288.E5 at a 1:2 ratio and were cocultured overnight. IFN-γ release from CTLs was measured by IFN-γ ELISA. (B) Destruction by K288.E5 of capillary tubes formed in Matrigel. Tube-forming GFP+ HUVEC2 cells (1 × 104/well) were cultivated overnight with CTLs (3 × 104/well) in the presence of AZT (10 μM). Fluorescent structures were imaged before and after cocultivation using an inverted microscope. (C) Capillary destruction by K288.E5 was blocked by anti-HLA 1 mAb and Granzyme B inhibitor. T-cell assays were performed exactly as described in panel B except that, before cocultivation, tubes and CTLs were treated for 1 hour with anti-HLA 1 mAb (W6/32, 20 μg/mL) and a Granzyme B inhibitor (zAAD, 100 μM; Enzyme Systems), respectively. All experiments were repeated twice, with similar results. Original magnification, × 20 for B and C.
Evaluation of the recognition of KDR+ HRPEpic and CD34+ cells by K288.E5. (A) KDR expression by ARPE-19 cells was determined in Western blot. Two HUVEC-cell lysates were used as positive controls. The β-actin control is shown for each extract. (B) Failure of K288.E5 to recognize ARPE-19 cells transfected with the HLA-A2 gene. In contrast, efficient T-cell recognition was observed on peptide-pulsed cells (10 μg/mL). Recognition of HUVEC2 cells is also shown for comparison. (C) KDR expression by ex vivo selected human cord blood CD34+ cells analyzed by flow cytometry. Percentages represent the number of KDR+ cells in anti-KDR scFv-enriched and -depleted CD34+ cell fractions. Results from 1 of 2 representative A*0201+ donors are shown. (D) HLA class 1-restricted recognition of KDR+CD34+ cells by K288.E5. KDR scFv-enriched (▪) or -depleted ( ) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.
) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.
Evaluation of the recognition of KDR+ HRPEpic and CD34+ cells by K288.E5. (A) KDR expression by ARPE-19 cells was determined in Western blot. Two HUVEC-cell lysates were used as positive controls. The β-actin control is shown for each extract. (B) Failure of K288.E5 to recognize ARPE-19 cells transfected with the HLA-A2 gene. In contrast, efficient T-cell recognition was observed on peptide-pulsed cells (10 μg/mL). Recognition of HUVEC2 cells is also shown for comparison. (C) KDR expression by ex vivo selected human cord blood CD34+ cells analyzed by flow cytometry. Percentages represent the number of KDR+ cells in anti-KDR scFv-enriched and -depleted CD34+ cell fractions. Results from 1 of 2 representative A*0201+ donors are shown. (D) HLA class 1-restricted recognition of KDR+CD34+ cells by K288.E5. KDR scFv-enriched (▪) or -depleted ( ) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.
) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.
In summary, we demonstrate that a peptide derived from human angiogenic receptor KDR can be naturally processed by activated, dividing endothelial cells, presented in an HLA-A*0201-restricted fashion, and can serve as a target for antigen-specific CTLs. T-cell recognition and cytotoxicity were achieved against a range of KDR+ aECs of different origin. These findings, together with the demonstration of KDR overexpression in most tumor microvessels, identify KDR as a potentially important tumor endothelia antigen not yet described in humans. This discovery of KDR as a T-cell target not only provides a novel perspective in the design of innovative immunotherapeutic strategies that target tumor vessels; it is also helpful in our understanding of the T-cell/endothelium interaction and the quantitative assessment of antigen-specific CTL responses, either naturally occurring or vaccine induced, to this target.
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-05-1912.
Supported by grant 10-2250-Su 1 from the Deutsche Krebshilfe (Y.S., K.C.).
We thank Dr Jan Mueller-Berghaus for stable transfectants COS-7/HLA-A*0201, Dr Wolfram Osen for 51Cr release assays, and Lynne Yakes for editing the manuscript.


 ) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.
) CD34+ cells (1 × 104/well) were cocultured overnight with CTLs (2 × 104/well) in the presence (□) or absence of W6/32 (20 μg/mL). IFN-γ released in culture supernatants was determined by IFN-γ ELISA. Each value represents the mean ± SD of 6 cultures from 2 different experiments.