Although the antimicrobial activity of reactive oxygen species (ROSs) is well defined, the role of ROSs in regulating the immune response of the body is not well understood. We now provide evidence that hydrogen peroxide (H2O2), a major component of ROSs, inhibits interleukin-12 (IL-12) p40 and IL-12 p70 induction in murine macrophages and catalase pretreatment prevents H2O2-mediated down-regulation of IL-12. Endogenous accumulation of H2O2/ROSs in macrophages treated with alloxan resulted in IL-12 p40 inhibition. Although nuclear expression of both p50 and p65 NF-κB increased on H2O2 exposure, nuclear c-rel level was inhibited. Overexpression of c-rel restored IL-12 p40 on stimulation with lipopolysaccharide plus IFN-γ during H2O2 treatment. H2O2 did not inhibit c-rel induction in cytosol; however, it prevented the transport of c-rel from cytosol to the nucleus. H2O2 activated calmodulin (CaM) protein in the cytosol, which subsequently sequestered c-rel in the cytosol preventing its transport to the nucleus. The CaM inhibitor trifIuoperazine increased both nuclear c-rel and IL-12 p40 levels in H2O2-treated macrophages, emphasizing a role of CaM in these processes. H2O2/ROSs thus down-regulate IL-12 induction in macrophages by a novel pathway inhibiting c-rel translocation to the nucleus through activation of CaM protein.
Introduction
Accumulated damage due to reactive oxygen species (ROSs) has been suggested to occur during aging1,2 and more clearly demonstrated during the respiratory burst of phagocytes.3,4 Hydrogen peroxide (H2O2), is a primary component of ROSs produced in large amounts by macrophages and granulocytes and is a mediator of innate immunity against the invading pathogens.5,6 H2O2 is known to function as a second messenger7,8 and regulates activation of many important transcription factors, such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1),9,10 that control the inducible expression of genes regulating macrophage-effector functions and cytokine signaling.11-13 The macrophage-induced cytokines determine the fate of the subsequent T-cell responses as type 114,15 or type 2.16,17 Thus, the possibility exists that H2O2 may alter the T-cell immune responses by affecting the macrophage-effector responses. The function of ROSs may not be restricted to its antimicrobial activity alone,18 but ROSs may also play an important role in regulating the immune environment in the body. Such situations may arise during certain pathophysiologic conditions such as tuberculosis.19,20
We earlier reported that activated macrophages from the Bruton tyrosine kinase (btk)-deficient mice (CBA/N) produced lower levels of ROSs as compared to the wild-type mice (CBA/J).21 However, in contrast to CBA/J macrophages, the IL-12 induction was significantly higher in macrophages from CBA/N mice with T-cell responses biased toward type 1.21,22 Although the contribution of btk enzyme in controlling the signal transduction cascades responsible for ROS production independent of IL-12 signal may not be ruled out, it is possible that btk targets the ROS pathway to influence IL-12 production. However, the exact mechanism by which ROSs down-regulate IL-12 production is not well understood. We now provide evidence that H2O2/ROSs affect the signaling events important for IL-12 production, leading to down-regulation of the same in activated macrophages.
Materials and methods
Mice
BALB/c mice were bred and maintained in the animal facility of Indian Immunologicals (IIL; Hyderabad, India). All mice were 6 to 12 weeks old and experimental protocol was approved by the Institutional Review Committee for care and usage of animals of Indian Immunologicals.
Macrophage stimulation assay
The peritoneal exudate cells (PECs) were harvested by injecting 4% thioglycolate broth as described elsewhere.23 The RAW 264.7 macrophages were obtained from NCCS (National Centre for Cell Science, Pune, India) and maintained in Dulbecco minimal essential medium (DMEM; Invitrogen, Grand Island, NY) containing 10% fetal calf serum and antibiotics (DMEM-10). The macrophages were plated at a density of 3 × 106 cells/mL and stimulated with a combination of 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich, St Louis, MO) and 1 ng/mL interferon-γ (IFN-γ; R&D Systems, Minneapolis, MN) in the absence or presence of various concentrations of either H2O2 (Qualigens, Mumbai, India and Sigma-Aldrich) or alloxan (Sigma-Aldrich). In some experiments, H2O2 was treated with catalase (Sigma-Aldrich). After 48 hours of stimulation, the culture supernatants were harvested for the estimation of IL-12 p40 or IL-12 p70 cytokine by an enzyme immunosorbent assay (EIA). In another experiment, the RAW 264.7 macrophages were treated with 5 μM trifIuoperazine (TFP; Sigma-Aldrich) for 20 minutes followed by treatment with H2O2 and LPS plus IFN-γ. The c-rel levels were measured after 1 hour and the IL-12 p40 levels in the culture supernatants were estimated after 48 hours of stimulation.
EIA for measuring IL-12 p40 and IL-12 p70 cytokines
Western blot analysis
The RAW 264.7 macrophages were either left untreated or treated with 250 μM H2O2 for 1 hour followed by stimulation with LPS plus IFN-γ for 1 hour. The cells were harvested after 1 hour and the nuclear extracts were prepared from NP-40-lysed cells as described.24 Equal quantities of nuclear proteins were separated in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred onto nitrocellulose membranes, and incubated with affinity-purified rabbit antibodies to either p50 NF-κB or p65 NF-κB or c-rel (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were further incubated with anti-rabbit immunoglobulin-horseradish peroxidase (HRP) conjugate (Sigma-Aldrich) and detected by enhanced chemiluminescence (ECL; Amersham Biosciences, Little Chalfont, United Kingdom) as described earlier.21 Equal loading of protein was confirmed either by Ponceau S Red stain (Sigma-Aldrich) or by checking for nonspecific signals on the immunoblots.
Measurement of endogenous H2O2/ROS level
The intracellular H2O2/ROS level was measured using a fluorescent dye, 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich). DCFH-DA is a nonpolar compound that is readily diffusible into cells, where it is hydrolyzed to the nonfluorescent polar derivative DCFH and thereby trapped within cells.25 In the presence of an oxidant, DCFH is converted into the highly fluorescent 2′,7′-dichlorofluorescein (DCF). For assay, the cells (6-7 × 105) were loaded with 5 μM DCFH-DA in the absence or presence of 1 mM alloxan. After 15 minutes of incubation in the dark, cells were stimulated with LPS plus IFN-γ for another 15 minutes. The stained cells were analyzed on a Becton Dickinson flow cytometer (FACS Vantage SE, Becton Dickinson, San Jose, CA) and the post-flow cytometric data analysis was carried out using CellQuest (Becton Dickinson, San Jose, CA) data analysis software.
Transfection with c-rel overexpression plasmid construct
The c-rel overexpression plasmid construct was a kind gift from Dr Klaus Ruckdeschel (Max von Pettenkofer-Institute for Hygiene and Microbiology, Munich, Germany). Transfection was conducted with 10 μg c-rel plasmid. Expression vector (PRC/CMV) without any insert was used as mock control. The plasmid constructs were transfected into RAW 264.7 macrophages using Lipofectamine 2000 (Invitrogen). Transfection efficiency was scored by selecting cells for neomycin resistance by growth in medium containing G418 (800 μg/mL) for 10 to 20 days and about 20% cells were found to be transfectable (data not shown). In another set, 24 hours after transfection, the cells were either left untreated or treated with H2O2 (250 μM) for 1 hour. The cells were further stimulated with LPS plus IFN-γ either for 1 hour to detect c-rel, p65, and p50 levels in the nuclear extracts by Western blotting or for 48 hours to estimate the amounts of IL-12 p40 secreted in the culture supernatants by EIA.
MTT assay
The RAW 264.7 macrophages were either left unstimulated or stimulated with LPS plus IFN-γ in the absence or presence of various concentrations of H2O2 in 96-well tissue culture plate. After 48 hours 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) was added as 5 mg/mL and incubated further for 4 hours. The cells were lysed overnight using 100 μL lysis buffer (20% SDS and 50% dimethylform-amide [DMF]) and the absorbance determined at 550 nm.
H2O2 colorimetric assay
Around 6 million RAW 264.7 macrophages were stimulated with LPS plus IFN-γ or LPS (5 μg/mL) in the absence or presence of 100 μM N-acetyl-l-cysteine (NAC; Sigma-Aldrich). After 15 minutes, macrophages were lysed by repeated freezing and thawing of the samples. H2O2 content in the cell lysate was measured using H2O2 colorimetric assay kit (Sigma-Aldrich) following the manufacturer's protocol. The absorbance was measured at 550 nm.
Calmodulin EIA
An EIA method involving competitive binding of anti-calmodulin (anti-CaM) monoclonal antibody was used to measure CaM present in the cytoplasmic extracts from various groups as described earlier.26 In brief, the RAW 264.7 macrophages were either left unstimulated or stimulated with LPS plus IFN-γ or LPS for 1 hour in the absence or presence of either 250 μM H2O2 or 100 μM NAC or 100 U/mL catalase. Cells from all the groups were harvested and the cytoplasmic extracts were prepared as described.22 The cytoplasmic extracts were first incubated with the mouse monoclonal anti-CaM antibody (Sigma-Aldrich) at a ratio of 10:1 and then transferred to microtiter plates that were previously coated with recombinant CaM protein (Sigma-Aldrich) at a concentration of 10 ng/well. After incubation at 37°C for 2 hours, all the wells were washed thoroughly with phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween-20 and further incubated for another 1 hour with goat anti-mouse IgG-HRP (Sigma-Aldrich). HRP activity in the EIA plate was detected by using o-phenylenediamine tetrahydrochloride (OPD, Sigma-Aldrich) as the chromogen and H2O2 (Qualigens) as the substrate. OPD was used as 0.5 mg/mL in citrate-phosphate buffer (pH 5.4) containing H2O2 as 1 μL/mL. The reaction was terminated using 1 N H2SO4 and the absorbance values were measured at 492 nm. A standard curve was prepared and CaM levels in the test samples were expressed as the fold changes over unstimulated control.
Immunoprecipitation assay
The immunoprecipitation (IP) assay was carried out as described earlier,27 with slight modifications. The cytoplasmic extracts were incubated with 4 μg/mL anti-CaM antibody (Sigma-Aldrich) for 3 hours at 4°C. Fifteen microliters protein A/G-Sepharose (Santa Cruz Biotechnology) was added to each sample and incubated further for 2 hours at 4°C. Beads were washed extensively with the CaM-IP buffer as described earlier.27 Coimmunoprecipitated c-rel or CaM was detected by Western blotting using anti-c-rel or anti-CaM antibody.
Statistical analysis
Data were analyzed using the Student t test wherever applicable, and P values of less than .05 were considered statistically significant.
Results
H2O2 reduces IL-12 p40 and IL-12 p70 induction in macrophages
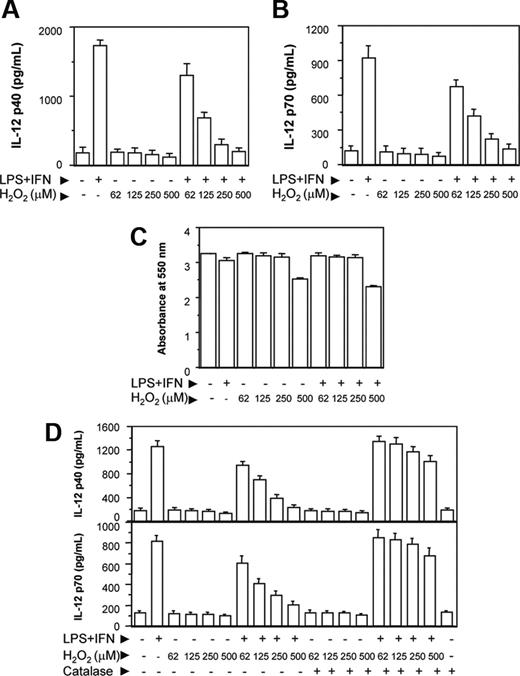
The RAW 264.7 macrophages were activated with LPS and IFN-γ together (LPS plus IFN-γ) in the absence or presence of increasing concentrations of H2O2. After 48 hours, the culture supernatants were harvested and the levels of IL-12 p40 secreted in the culture supernatants were determined by EIA. The IL-12 cytokine is known to be a heterodimeric protein of 70 kDa of which the p35 subunit is constitutively expressed at lower level by a variety of cell types, whereas the p40 subunit is only produced by the cells making biologically active IL-12.28 Therefore, in the present study, we examined whether H2O2 inhibited the levels of IL-12 p40 in RAW 264.7 macrophages. We also determined whether a reduction in the IL-12 p40 resulted in down-regulation of IL-12 p70 production by the activated macrophages. It could be seen that increasing concentrations of H2O2 decreased the levels of IL-12 p40 induction in RAW 264.7 macrophages activated with LPS plus IFN-γ (Figure 1A). A reduction in the IL-12 p40 levels by H2O2 also coincided with the decreased levels of IL-12 p70 (Figure 1B). H2O2 inhibited IL-12 induction without affecting the cell viability except for very high (500 μM) concentration of H2O2 (Figure 1C), indicating that H2O2 specifically interfered with the signal leading to IL-12 gene transcription. Because a concentration of 500 μM (Figure 1C) or more of H2O2 appeared to be toxic to the macrophages, all following experiments were carried out using 250 μM H2O2.
It could be seen that H2O2 down-regulated IL-12 p40 and IL-12 p70 also when H2O2 from Sigma-Aldrich was used (Figure 1D). Further, pretreatment of H2O2 with catalase (detoxify H2O229 ) prevented the H2O2 effect on IL-12 p40/p70 down-regulation (Figure 1D), implicating a role of H2O2 in down-regulating IL-12 in activated macrophages. Macrophages treated with catalase alone yielded no significant difference in IL-12 production compared with untreated macrophages.
H2O2 down-regulates IL-12 p40 and IL-12 p70 induction in RAW 264.7 macrophages. The RAW 264.7 macrophages were either left untreated or treated with varying doses of H2O2 for 1 hour and further stimulated with LPS plus IFN-γ for 48 hours. The IL-12 p40 (A) and IL-12 p70 (B) levels secreted in the culture supernatants were measured by EIA. The cell viability was measured by the MTT assay (C). In another experiment, H2O2 from a different source (Sigma-Aldrich) is either left untreated or pretreated with 100 U/mL catalase for 30 minutes and then added into the macrophage cultures. After 48 hours, both IL-12 p40 and IL-12 p70 levels were measured by EIA (D). This experiment is representative of 3 experiments performed with similar results. Results are expressed as mean ± SD.
H2O2 down-regulates IL-12 p40 and IL-12 p70 induction in RAW 264.7 macrophages. The RAW 264.7 macrophages were either left untreated or treated with varying doses of H2O2 for 1 hour and further stimulated with LPS plus IFN-γ for 48 hours. The IL-12 p40 (A) and IL-12 p70 (B) levels secreted in the culture supernatants were measured by EIA. The cell viability was measured by the MTT assay (C). In another experiment, H2O2 from a different source (Sigma-Aldrich) is either left untreated or pretreated with 100 U/mL catalase for 30 minutes and then added into the macrophage cultures. After 48 hours, both IL-12 p40 and IL-12 p70 levels were measured by EIA (D). This experiment is representative of 3 experiments performed with similar results. Results are expressed as mean ± SD.
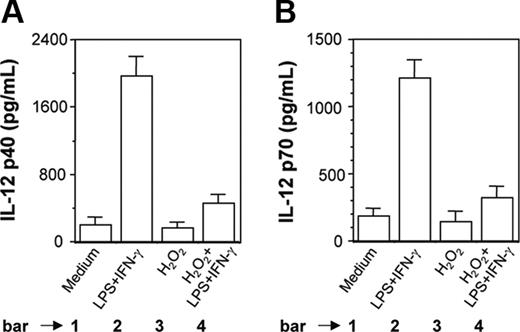
H2O2 inhibits induction of IL-12 p40 and IL-12 p70 in PEC-derived macrophages from BALB/c mice. Peritoneal macrophages from BALB/c mice were cultured with LPS plus IFN-γ without or with H2O2 (250 μM). After 48 hours of stimulation, both IL-12 p40 (A) and IL-12 p70 (B) levels (mean ± SD) were measured in the culture supernatants and are representative of 3 experiments performed with similar results.
H2O2 inhibits induction of IL-12 p40 and IL-12 p70 in PEC-derived macrophages from BALB/c mice. Peritoneal macrophages from BALB/c mice were cultured with LPS plus IFN-γ without or with H2O2 (250 μM). After 48 hours of stimulation, both IL-12 p40 (A) and IL-12 p70 (B) levels (mean ± SD) were measured in the culture supernatants and are representative of 3 experiments performed with similar results.
It was also found that H2O2 inhibited LPS plus IFN-γ-induced IL-12 p40 (Figure 2A, compare bar 4 with bar 2; P = .001) and IL-12 p70 (Figure 2B, compare bar 4 with bar 2; P = .001) production in PEC-derived macrophages from BALB/c mice. This indicated that the results obtained with a transformed mouse macrophage cell line (RAW 264.7) were equally true in primary cultures of mouse macrophages.
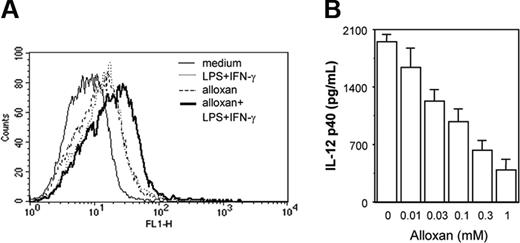
Because exogenous H2O2 treatment was found to affect the macrophage costimulation signal leading to down-regulation of IL-12 p40, we next examined whether endogenous accumulation of H2O2/ROSs could affect IL-12 p40 production. Alloxan,30 a potent H2O2/ROS generator (Figure 3A), was used to examine effect of endogenous H2O2/ROSs on IL-12 p40 secretion. Cells were pretreated with titrating concentrations of alloxan for 1 hour and further stimulated with LPS plus IFN-γ for another 48 hours and the levels of IL-12 p40 secreted by these cells were examined. It was apparent that with increasing concentrations of alloxan, IL-12 p40 induction decreased in a concentration-dependent manner (Figure 3B). These results categorically demonstrate that overexposure of macrophages to H2O2, presented exogenously or endogenously, results in down-regulation of IL-12 p40 induction.
Endogenous accumulation of H2O2/ROSs results in down-regulation of IL-12 p40 production by macrophages. The RAW 264.7 macrophages were exposed to alloxan to allow endogenous accumulation of H2O2/ROSs. For measuring endogenous H2O2/ROS induction by alloxan, cells were loaded with 5 μM DCFH-DA in the absence or presence of 1 mM alloxan. After 15 minutes of incubation in the dark, the cells were further stimulated with LPS plus IFN-γ for another 15 minutes. The DCF fluorescence was measured by flow cytometer (A). The IL-12 p40 levels (mean ± SD) were quantified by EIA for LPS plus IFN-γ-activated RAW 264.7 macrophages treated with titrating doses of alloxan (B). This experiment is representative of 3 experiments performed with similar results.
Endogenous accumulation of H2O2/ROSs results in down-regulation of IL-12 p40 production by macrophages. The RAW 264.7 macrophages were exposed to alloxan to allow endogenous accumulation of H2O2/ROSs. For measuring endogenous H2O2/ROS induction by alloxan, cells were loaded with 5 μM DCFH-DA in the absence or presence of 1 mM alloxan. After 15 minutes of incubation in the dark, the cells were further stimulated with LPS plus IFN-γ for another 15 minutes. The DCF fluorescence was measured by flow cytometer (A). The IL-12 p40 levels (mean ± SD) were quantified by EIA for LPS plus IFN-γ-activated RAW 264.7 macrophages treated with titrating doses of alloxan (B). This experiment is representative of 3 experiments performed with similar results.
H2O2 targets c-rel to inhibit IL-12 p40 induction
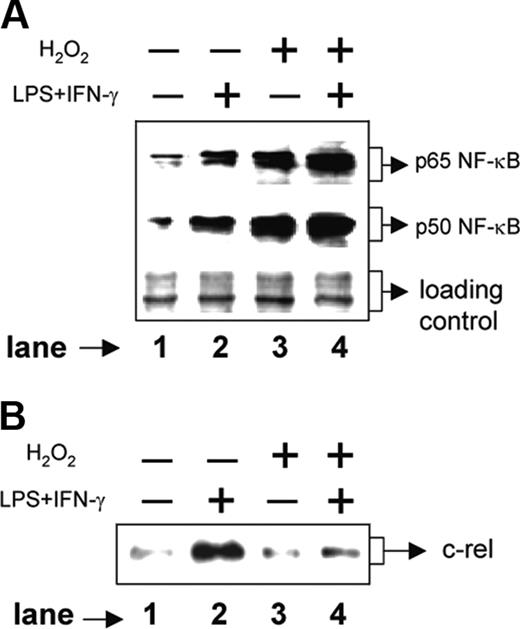
We next examined the signal transduction pathways involved in H2O2-mediated down-regulation of IL-12 p40. The p50/p65 NF-κB rel proteins are known to dominantly regulate the IL-12 p40 transcription.11 Therefore, we examined the expression levels of p50 and p65 NF-κB rel proteins in the nuclear extracts from various experimental groups by Western blotting. The RAW 264.7 macrophages were either left untreated or treated with 250 μM H2O2 for 1 hour followed by stimulation with LPS plus IFN-γ for another 1 hour. Whereas the unstimulated macrophages showed very little of both nuclear p65 and p50 NF-κB rel proteins, the LPS plus IFN-γ-activated macrophages showed significant induction of these proteins (Figure 4A, compare lane 2 with lane 1). Treatment with H2O2, as compared to the unstimulated macrophages, resulted in an increase in both p65 and p50 NF-κB proteins (Figure 4A, compare lane 3 with lane 1). As expected, the LPS plus IFN-γ-activated macrophages displayed very high expression of NF-κB proteins during treatment with H2O2 (Figure 4A lane 4). Because the p50/p65 heterodimer strongly binds to the IL-12 p40 promoter and facilitates IL-12 p40 transcription,11 it is expected that H2O2 would promote IL-12 p40 induction. However, the levels of IL-12 p40 were down-regulated in the presence of H2O2 (Figure 1A), indicating that probably H2O2 targets some other factors crucial for IL-12 p40 production in activated macrophages.
Recent studies have confirmed that the c-rel transcription factor plays a dominant role over p65 NF-κB in activating IL-12 p40 transcription.31 We therefore, examined the c-rel levels in the same nuclear extracts used for studying the p65 and the p50 NF-κB levels. Although the p65 and the p50 levels were increased, the c-rel expression was markedly decreased in the experimental group cotreated with LPS plus IFN-γ and H2O2 as compared to the group treated with LPS plus IFN-γ alone (Figure 4B, compare lane 4 with lane 2). These results, therefore, suggest that although H2O2 up-regulates both p50 and p65 NF-κB proteins, it inhibits c-rel transcription factor and reduces IL-12 p40 expression probably by reducing the nuclear c-rel level in macrophages.
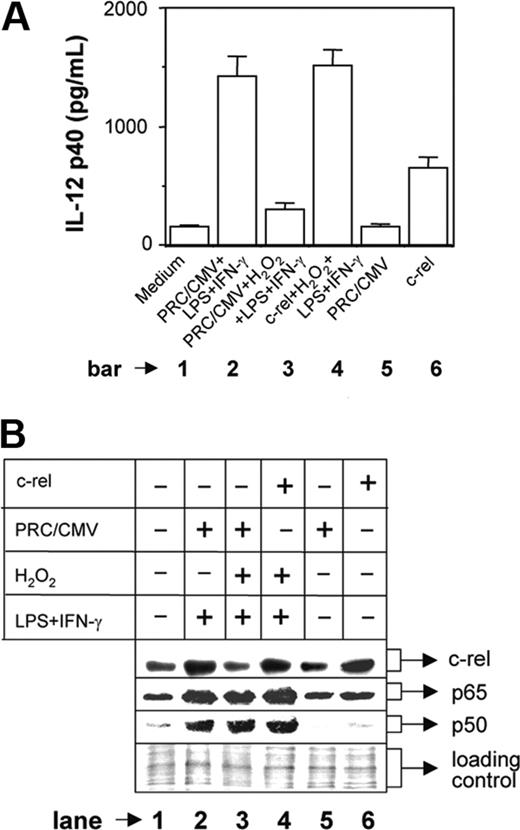
To confirm whether c-rel plays a critical role in H2O2-mediated inhibition of IL-12 p40, we overexpressed c-rel in the cells stimulated with LPS plus IFN-γ during H2O2 treatment. The RAW 264.7 macrophages were transfected with either mock plasmid (PRC/CMV) or c-rel-overexpressing plasmid construct. After 24 hours, the macrophages were washed and stimulated with LPS plus IFN-γ in the presence of 250 μM H2O2 and the IL-12 p40 production was measured at 48 hours after stimulation. The results reveal that the IL-12 p40 production is inhibited by H2O2 in the mock group (Figure 5A, compare bar 3 with bar 2; P = .001) as expected, whereas transfection with c-rel could significantly restore IL-12 p40 induction (Figure 5A, compare bar 4 with bar 3; P < .001) demonstrating an important role of c-rel in IL-12 p40 regulation by H2O2. It is pertinent to mention that only about 20% cells are transfectable and therefore only 20% enhancement of IL-12 p40 is expected in the c-rel-transfected group (Figure 5A bar 6). However, when LPS plus IFN-γ and H2O2 were added in the c-rel-transfected group, IL-12 p40 induction was increased (Figure 5A, compare bar 4 with bar 6) and was equivalent to the group treated with LPS plus IFN-γ (Figure 5A, compare bar 4 with bar 2). This indicates that in addition to c-rel, other transcription factors that are activated during treatment with H2O2 and LPS plus IFN-γ are responsible for increased IL-12 p40 induction in this group. Because H2O2 activated both p65 and p50 NF-κB transcription factors (Figure 4A) and because these rel proteins dominantly regulate IL-12 p40 transcription,11 we compared the expression level of nuclear p65 and p50 NF-κB between these 2 groups. It could be seen that although c-rel level was comparable (Figure 5B lanes 4 and 6) and was equivalent to the LPS plus IFN-γ-activated group (Figure 5B lane 2), both p65 and p50 NF-κB levels were increased in the c-rel-transfected group treated with H2O2 and LPS plus IFN-γ as compared to the c-rel-transfected group that was left untreated (Figure 5B, compare lane 4 with lane 6). Thus, c-rel alone caused 20% enhancement in IL-12 p40 induction in the c-rel-transfected group (Figure 5A bar 6), but the presence of higher levels of all c-rel, p65, and p50 factors in the c-rel-transfected group treated with H2O2 and LPS plus IFN-γ resulted in higher induction of IL-12 p40 (Figure 5A bar 4), equivalent to that induced in response to stimulation with LPS plus IFN-γ (Figure 5A bar 2).
H2O2 increases nuclear p50 and p65 NF-κB expression but inhibits c-rel levels in the nucleus. The RAW 264.7 macrophages were treated with 250 μM H2O2 for 1 hour followed by stimulation with LPS plus IFN-γ for another 1 hour. Nuclear extracts were prepared and the expression of p65 (A, top lane) and p50 (A, middle lane) component of NF-κB was examined by immunoblot analysis. As a loading control identical amounts of total proteins were loaded (A, bottom lane). The same nuclear extracts were used to examine c-rel expression by Western blotting (B). This experiment is representative of 5 experiments performed with similar results.
H2O2 increases nuclear p50 and p65 NF-κB expression but inhibits c-rel levels in the nucleus. The RAW 264.7 macrophages were treated with 250 μM H2O2 for 1 hour followed by stimulation with LPS plus IFN-γ for another 1 hour. Nuclear extracts were prepared and the expression of p65 (A, top lane) and p50 (A, middle lane) component of NF-κB was examined by immunoblot analysis. As a loading control identical amounts of total proteins were loaded (A, bottom lane). The same nuclear extracts were used to examine c-rel expression by Western blotting (B). This experiment is representative of 5 experiments performed with similar results.
Overexpression of c-rel restores IL-12 p40 induction in H2O2-treated macrophages. The RAW 264.7 macrophages were transfected with either the mock plasmid (PRC/CMV) or a c-rel-overexpressing plasmid construct using Lipofectamine 2000. Cells were incubated for 24 hours, washed, and further stimulated with LPS plus IFN-γ in the absence or presence of 250 μM H2O2. After 48 hours, the amounts of IL-12 p40 (mean ± SD) secreted in the culture supernatants were measured by EIA (A). The c-rel, p65, and p50 levels in the nuclear extracts were detected by Western blotting after 1 hour of stimulation with LPS plus IFN-γ (B). The equal loading of protein was confirmed by staining with Ponceau S stain (B). This experiment is representative of 3 experiments performed with similar results.
Overexpression of c-rel restores IL-12 p40 induction in H2O2-treated macrophages. The RAW 264.7 macrophages were transfected with either the mock plasmid (PRC/CMV) or a c-rel-overexpressing plasmid construct using Lipofectamine 2000. Cells were incubated for 24 hours, washed, and further stimulated with LPS plus IFN-γ in the absence or presence of 250 μM H2O2. After 48 hours, the amounts of IL-12 p40 (mean ± SD) secreted in the culture supernatants were measured by EIA (A). The c-rel, p65, and p50 levels in the nuclear extracts were detected by Western blotting after 1 hour of stimulation with LPS plus IFN-γ (B). The equal loading of protein was confirmed by staining with Ponceau S stain (B). This experiment is representative of 3 experiments performed with similar results.
CaM is involved in H2O2-mediated inhibition of c-rel and IL-12 p40 levels
In previous experiments, we have shown that the nuclear accumulation of c-rel, but not the p65 NF-κB, was poorer in macrophages treated with H2O2 (Figure 4A-B). The question of H2O2 differentially regulating nuclear expression of p65 and c-rel is an interesting one. It has been already shown that H2O2 has little effect on IκBα degradation,32 indicating the possibility of involvement of some other signaling pathways in the differential regulation of nuclear p65 and c-rel factors by H2O2 as observed by us. Recently,27 it was demonstrated that CaM binds to c-rel and p65 NF-κB after their release from IκB and can inhibit nuclear transport of c-rel while allowing p65 NF-κB to translocate to the nucleus.27 These investigators have shown that CaM protein is involved in the differential regulation of p65 and c-rel transcription factor in the nucleus. Therefore, we next examined whether expression of CaM protein was increased by H2O2 and was responsible for sequestering a higher amount of c-rel in the cytosol, thus preventing its transport to the nucleus. To determine the role of CaM in the H2O2-mediated inhibition of c-rel and IL-12 p40 induction, the CaM levels were compared in LPS plus IFN-γ-activated macrophages either left untreated or treated with 250 μM H2O2 for 1 hour. As compared to the unstimulated group, the LPS plus IFN-γ activation led to an increase in the CaM level and on combined stimulation with H2O2 and LPS plus IFN-γ, the CaM level was significantly increased (Figure 6A, compare bar 2 with bar 1; P < .001). Interestingly, the CaM induction was up-regulated by H2O2 treatment alone (data not shown) indicating that H2O2 could alone activate CaM induction and accumulation of oxidative stress could result in increased induction of CaM protein.
As described, we have observed that H2O2 inhibits c-rel level in the nucleus (Figure 4B). Because CaM is known to sequester c-rel in the cytoplasm27 and also H2O2 increases CaM level in activated macrophages (Figure 6A), it is expected that more c-rel will be retained in the cytoplasm as levels of CaM protein are increased in the macrophages treated with H2O2. To test this, the cytoplasmic extracts were made from LPS plus IFN-γ-activated macrophages receiving no (H2O2-) or 250 μMH2O2 (H2O2+) and were subjected to IP with a mouse monoclonal anti-CaM antibody. The coimmunoprecipitated c-rel was detected by Western blot analysis using c-rel-specific antibody. The results reveal that a higher amount of c-rel is immunoprecipitated as CaM-c-rel complex in H2O2+ group as compared to H2O2- group (Figure 6B upper panel; compare lane 3 with lane 2). When the same extracts were used to detect the CaM protein level in Western blot using anti-CaM antibody, expectedly a larger amount of CaM could be immunoprecipitated in the H2O2+ group as compared to the H2O2- group (Figure 6B middle panel; compare lane 3 with lane 2). This further coincided well with the poorer nuclear c-rel level in the H2O2+ group as compared to H2O2- group (Figure 6C upper panel; compare lane 3 with lane 2). These data indicate that the nuclear, but not the cytosolic c-rel level is reduced by H2O2. Furthermore, the results demonstrate that H2O2 prevents nuclear translocation of c-rel indirectly by targeting the CaM protein.
To further confirm the role of CaM in H2O2-mediated down-regulation of c-rel and IL-12 p40, the LPS plus IFN-γ-activated RAW 264.7 macrophages were exposed to H2O2 in the absence or presence of 5 μM TFP, a known inhibitor of CaM activity.33 Nuclear accumulation of c-rel was detected by immunoblot analysis (Figure 6D) and the IL-12 p40 levels secreted in the culture supernatants were measured by EIA (Figure 6E). It could be seen that as compared to the LPS plus IFN-γ control, c-rel (Figure 6D, compare lane 3 with lane 2) and IL-12 p40 (Figure 6E, compare bar 3 with bar 2) levels were poorer in the group treated with H2O2 and LPS plus IFN-γ, which was expected. It could be seen that TFP increased the nuclear c-rel level (Figure 6D, compare lane 4 with lane 3) in the LPS plus IFN-γ-activated macrophages exposed to H2O2. The increase in c-rel was found to be correlated with a concomitant increase in IL-12 p40 induction in the H2O2-treated macrophages (Figure 6E, compare bar 4 with bar 3; P = .001). TFP alone did not show any effect on the c-rel (Figure 6D lane 5) and the IL-12 p40 (Figure 6E bar 5) expression in macrophages. Although the c-rel was increased, the p65 NF-κB level remained unaltered by TFP treatment (data not shown). These results emphasize a direct role of CaM in H2O2-mediated down-regulation of nuclear c-rel level and IL-12 p40 induction in RAW 264.7 macrophages.
Involvement of CaM in H2O2-mediated down-regulation of c-rel. The RAW 264.7 macrophages were stimulated with LPS plus IFN-γ in the absence or presence of 250 μM H2O2. After 1 hour of stimulation, the cells were harvested and both the cytoplasmic and the nuclear extracts were prepared. CaM contents (mean ± SD) in the cytoplasmic extracts were determined by EIA as described in “Materials and methods” and expressed as the fold changes over unstimulated control (A). The same cytoplasmic extracts were incubated with anti-CaM antibody for 3 hours at 4°C and then protein A/G-Sepharose was added to the mixture and was further incubated for 2 hours at 4°C. Coimmunoprecipitated c-rel was detected by Western blot (B, top lane). The same preparation was also used to detect CaM level using anti-CaM antibody, respectively (B, middle lane). The equal loading of protein was confirmed by staining with Ponceau S stain (B, bottom lane). The c-rel levels in the nuclear extracts were measured by immunoblot analysis (C, top lane) of identical amounts of total proteins loaded in the blot (C, bottom lane). This experiment is representative of 3 experiments performed with similar results. In another experiment, the RAW 264.7 macrophages were either treated with LPS plus IFN-γ alone or cotreated with H2O2 and LPS plus IFN-γ in the absence or presence of 5 μM TFP, which is a known inhibitor of CaM activity. The nuclear accumulation of c-rel was detected after 1 hour by immunoblot analysis (D, top lane). The equal loading of protein was confirmed by Ponceau S staining (D, bottom lane). The IL-12 p40 levels (mean ± SD) secreted in the culture supernatants were determined after 48 hours by EIA (E). Results shown are representative of 3 independent experiments.
Involvement of CaM in H2O2-mediated down-regulation of c-rel. The RAW 264.7 macrophages were stimulated with LPS plus IFN-γ in the absence or presence of 250 μM H2O2. After 1 hour of stimulation, the cells were harvested and both the cytoplasmic and the nuclear extracts were prepared. CaM contents (mean ± SD) in the cytoplasmic extracts were determined by EIA as described in “Materials and methods” and expressed as the fold changes over unstimulated control (A). The same cytoplasmic extracts were incubated with anti-CaM antibody for 3 hours at 4°C and then protein A/G-Sepharose was added to the mixture and was further incubated for 2 hours at 4°C. Coimmunoprecipitated c-rel was detected by Western blot (B, top lane). The same preparation was also used to detect CaM level using anti-CaM antibody, respectively (B, middle lane). The equal loading of protein was confirmed by staining with Ponceau S stain (B, bottom lane). The c-rel levels in the nuclear extracts were measured by immunoblot analysis (C, top lane) of identical amounts of total proteins loaded in the blot (C, bottom lane). This experiment is representative of 3 experiments performed with similar results. In another experiment, the RAW 264.7 macrophages were either treated with LPS plus IFN-γ alone or cotreated with H2O2 and LPS plus IFN-γ in the absence or presence of 5 μM TFP, which is a known inhibitor of CaM activity. The nuclear accumulation of c-rel was detected after 1 hour by immunoblot analysis (D, top lane). The equal loading of protein was confirmed by Ponceau S staining (D, bottom lane). The IL-12 p40 levels (mean ± SD) secreted in the culture supernatants were determined after 48 hours by EIA (E). Results shown are representative of 3 independent experiments.
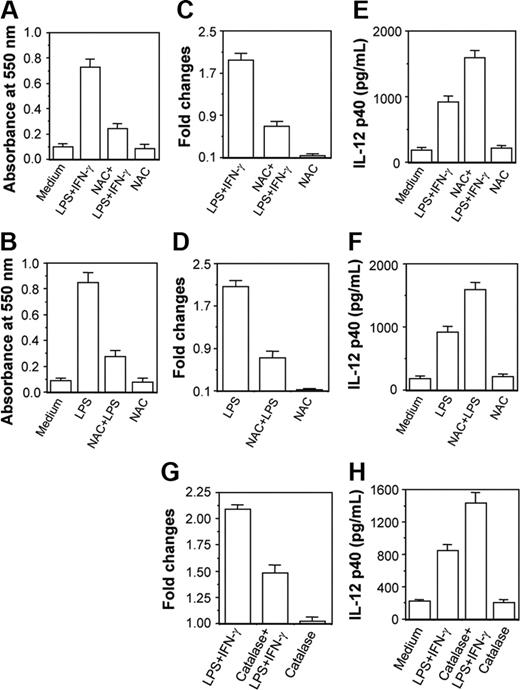
NAC and catalase down-regulate CaM expression leading to up-regulation of IL-12 p40 induction in activated macrophages
Results in Figure 6 clearly reveal that 250 μM H2O2 activated the CaM level causing down-regulation in IL-12 p40 induction. However, the amount of H2O2 used was much higher than produced in activated macrophages during respiratory burst. The physiologic significance of this model was further examined by checking whether reduction of H2O2 level in activated macrophages on treatment with NAC34,35 resulted in decreased CaM expression leading to up-regulation of IL-12 p40. The RAW 264.7 macrophages were therefore stimulated with LPS plus IFN-γ or LPS36,37 in the absence or presence of 100 μM NAC. Figure 7 reveals that H2O2 levels are increased during stimulation of macrophages with LPS plus IFN-γ or LPS, which is expected, and NAC decreases H2O2 levels in these macrophages38 (Figure 7A-B; P < .001). Enhancement of H2O2 resulted in increased CaM protein levels in LPS plus IFN-γ- and LPS-stimulated macrophages, which was prevented by NAC (Figure 7C-D; P < .001). Reduction in CaM production by NAC was found to correlate well with enhancement of IL-12 p40 induction in groups treated with LPS plus IFN-γ (Figure 7E; P = .001) and LPS (Figure 7F; P < .001). NAC alone at 100 μM concentration did not change CaM and IL-12 p40 levels.
Both NAC and catalase inhibit CaM activation leading to subsequent up-regulation of IL-12 p40 induction in activated RAW 264.7 macrophages. The RAW 264.7 macrophages were stimulated with either LPS plus IFN-γ (A,C,E) or LPS (B,D,F) in the absence or presence of 100 μM NAC. The intracellular H2O2 contents (A-B) were measured using H2O2 colorimetric assay kit and the CaM levels (C-D) were estimated by EIA. The IL-12 p40 levels secreted in the culture supernatants (E-F) were measured by EIA. Results shown are representative of 4 independent experiments. In another experiment, RAW 264.7 macrophages were stimulated with LPS plus IFN-γ in the absence or presence of 100 U/mL catalase and inductions of CaM (G) and IL-12 p40 (H) were measured by EIA. Results shown are representative of 3 independent experiments.
Both NAC and catalase inhibit CaM activation leading to subsequent up-regulation of IL-12 p40 induction in activated RAW 264.7 macrophages. The RAW 264.7 macrophages were stimulated with either LPS plus IFN-γ (A,C,E) or LPS (B,D,F) in the absence or presence of 100 μM NAC. The intracellular H2O2 contents (A-B) were measured using H2O2 colorimetric assay kit and the CaM levels (C-D) were estimated by EIA. The IL-12 p40 levels secreted in the culture supernatants (E-F) were measured by EIA. Results shown are representative of 4 independent experiments. In another experiment, RAW 264.7 macrophages were stimulated with LPS plus IFN-γ in the absence or presence of 100 U/mL catalase and inductions of CaM (G) and IL-12 p40 (H) were measured by EIA. Results shown are representative of 3 independent experiments.
The role of H2O2 in CaM activation and IL-12 p40 down-regulation was further confirmed by treatment of macrophages with catalase.29 When the cytoplasmic extracts were examined, it could be seen that the LPS plus IFN-γ-induced CaM level was poorer in the group cotreated with catalase and LPS plus IFN-γ as compared to the group treated with LPS plus IFN-γ alone (Figure 7G; P = .001). The decrease in CaM in the LPS plus IFN-γ plus catalase-treated group resulted in concomitant up-regulation in IL-12 p40 induction as compared to the LPS plus IFN-γ-treated control group (Figure 7H; P = .001). These data clearly indicate that H2O2 down-regulates IL-12 p40 induction by increasing CaM protein in activated macrophages.
Discussion
Several findings strongly point to a role of ROSs in intracellular signaling pathways,39-41 and H2O2 is known to be the major component of ROSs that triggers activation of multiple signaling pathways in cells.7,42-45
In this study, we observed that IL-12 p40 induction in macrophages could be inhibited by H2O2. The rel family proteins that are involved in IL-12 p40 induction are mainly the p65 NF-κB rel protein and the c-rel transcription factor. However, the relative contribution of these rel proteins in regulating IL-12 p40 promoter remains an enigma. Our findings demonstrate that the nuclear translocation of c-rel transcription factor is inhibited in macrophages treated with H2O2 resulting in decreased IL-12 p40 production in response to stimulation with LPS plus IFN-γ. The presence of reduced levels of c-rel in the nucleus probably limits IL-12 p40 transcription in H2O2-treated macrophages even in an environment otherwise rich in p65 NF-κB protein. A dominant role of c-rel over p65 NF-κB in IL-12 p40 activation is supported by the fact that IL-12 p40 mRNA and proteins remain quite abundant in p65 NF-κB knock-out macrophages despite the apoptotic phenotype of these cells.31 In contrast, the levels of IL-12 p40 mRNA and proteins are reduced to nearly undetectable levels in c-rel knockout macrophages.31 This reveals that the p40 gene is under the direct regulation of the c-rel transcription factor. The reason of such selectivity of the c-rel for the IL-12 p40 transcription is, however, poorly understood. Although many previous reports strongly point to the regulation of IL-12 p40 promoter by rel transcription factors,12,13,46 the information is scanty regarding factors modulating rel activity. In this regard, the present investigation highlights a role of ROSs in regulating particularly the c-rel factor in macrophages. It is pertinent to mention that although we demonstrate that H2O2 down-regulates nuclear c-rel level in RAW 264.7 macrophages, H2O2 is shown to increase nuclear c-rel level in endothelial cell line.47
The calcium sensor CaM protein is known to play a crucial role in various signal transduction events.48,49 Recently, a role of CaM kinase in macrophage activation and cytokine production was reported.50 Recent studies implicate the involvement of IκBα/NF-κB-independent pathway in redox-dependent regulation of inflammatory cytokines.51 We demonstrate that CaM plays an important novel role in the redox regulation of macrophage cytokine signaling. We document that H2O2 down-regulates IL-12 p40 induction in macrophages through inhibition of nuclear transport of c-rel through the participation of CaM protein. H2O2 was found to increase the CaM level, which subsequently sequestered the c-rel in the cytosol preventing its transport to the nucleus. A decrease in the nuclear c-rel level resulted in poorer induction of IL-12 p40 in H2O2-treated macrophages. Because the CaM inhibitor, TFP, intercepted the H2O2-mediated inhibition of nuclear c-rel and IL-12 p40 levels (Figure 6D-E), it can be concluded that H2O2 targets CaM protein to inhibit IL-12 p40 induction. The observations that both NAC and catalase reduce CaM expression and up-regulate IL-12 p40 production further strengthen our conclusion that H2O2 probably down-regulates IL-12 p40 via activating CaM expression. However, the involvement of other pathways in the IL-12 p40 regulation by H2O2 cannot be ruled out. Our data suggest the possibility that the c-rel translocation to the nucleus is inhibited by H2O2 via the direct involvement of CaM protein.
We have also observed that H2O2 treatment causes down-regulation of IL-12 p40 induction in PEC-derived macrophages. IL-12 is an important biologic adjuvant and boosts the type 1 T-cell responses.14,15 Therefore, ROSs are likely to participate in modulating the type 1/type 2 T-cell cytokine balance in vivo. Few preliminary reports indicate that oxidative stress can repress T-cell immune responses.52 Although a direct effect of ROSs on T-cell responses can never be ruled out, it is possible that H2O2/ROSs can repress T-cell response indirectly through down-regulating IL-12 induction in macrophages. It must, however, be pointed out that it is not clear if H2O2/ROS signal is induced in macrophages in vivo during the early stages of an immune response. It is therefore, likely that the early IL-12 production in macrophages in vivo may not be modulated through H2O2/ROSs. In conclusion, our results for the first time provide important clues that H2O2/ROSs can modulate effector function of macrophages by inhibiting the IL-12 p40 induction, an observation that will have a bearing on the development of new strategies for resistance against oxidative stress-induced pathogenesis.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-04-1707.
Supported by a Fellowship from the Council of Scientific and Industrial Research, New Delhi, India (N.K., S.S.R.) and by grants from Department of Biotechnology (DBT), India and Third World Academy of Science (TWAS), Italy. Sheikh Showkat Rahim died on March 3, 2004. N.K. and S.S.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Gayatri Ramakrishna for critically reviewing the manuscript. The authors also thank Ms S. Sivasankari and S. M. Jegadeeswaran for technical assistance.