The inability of the immune system to recognize and kill malignant plasma cells in patients with multiple myeloma (MM) has been attributed in part to the ineffective activation of natural killer (NK) cells. In order to activate and target NK cells to the malignant cells in MM we designed a novel recombinant bispecific protein (ULBP2-BB4). While ULBP2 binds the activating NK receptor NKG2D, the BB4 moiety binds to CD138, which is overexpressed on a variety of malignancies, including MM. ULBP2-BB4 strongly activated primary NK cells as demonstrated by a significant increase in interferon-γ (IFN-γ) secretion. In vitro, ULBP2-BB4 enhanced the NK-mediated lysis of 2 CD138+ human MM cell lines, U-266 and RPMI-8226, and of primary malignant plasma cells in the allogenic and autologous setting. Moreover, in a nude mouse model with subcutaneously growing RPMI-8226 cells, the cotherapy with ULBP-BB4 and human peripheral blood lymphocytes abrogated the tumor growth. These data suggest potential clinical use of this novel construct in patients with MM. The use of recombinant NK receptor ligands that target NK cells to tumor cells might offer new approaches for other malignancies provided a tumor antigen-specific antibody is available.
Introduction
Despite significant advances in the treatment of patients with multiple myeloma (MM), this malignancy is still an incurable disease with a median survival of 3 to 7 years after diagnosis.1,2 Most recently developed immunomodulatory drugs such as bortezomib and thalidomide have been shown to overcome resistance of MM cells to conventional chemotherapy and to improve clinical outcome.3 The molecular mechanism of thalidomide is at least partly based on an interleukin-2 (IL-2)–dependent activation of natural killer (NK) cells triggering tumor cell lysis.3-5 The antitumor potential of NK cells has also been demonstrated for myeloid tumor cells and cell lines in vitro,6,7 and suggests that the graft-versus-myeloma effect after allogenic stem cell transplantation may be related to NK cell activity.6,7
NK cells, a component of the innate immune system, attack virus-infected and malignant cells without prior antigen stimulation, mediate cellular cytotoxicity, and produce cytokines such as interferon gamma (IFN-γ) upon stimulation. There is growing evidence that NK cells also participate directly in adaptive immune responses, mainly by cross-talk with dendritic cells.8-10 The recognition strategies used by NK cells to discriminate between normal cells and virus-infected or malignant cells are diverse. Depending on the balance of inhibitory and activating NK receptors the cytotoxicity is either blocked or enhanced.9 In humans, inhibitory receptors include the immunoglobulin (Ig)–like receptors (KIRs) and the lectin-like CD94/NKG2A heterodimer detecting different human leukocyte antigen (HLA) class I alleles.11,12 NK cell activation requires a second signal delivered by activating receptors such as the natural cytotoxicity receptors (NCRs) NKp30, NKp46, NKp44, or NKG2D.13 These receptors can induce target-cell lysis upon engagement through corresponding ligands on tumor or virus-infected cells even in the presence of inhibitory signaling. While the cellular ligands that interact with the NCRs are still unknown, the homodimeric C-type lectin-like receptor NKG2D has been shown to recognize the diversified major histocompatibilty complex (MHC) class I–related molecules MICA, MICB, and the glycosylphophatidylinositol (GPI)–anchored UL16-binding proteins (ULBPs).14-16 NKG2D is not exclusively expressed on NK cells, but also found on T cells, where it is associated with DAP10-mediated costimulation.17,18
The expression of the NKG2D-specific ligands on tumor cells correlates directly with their sensitivity to NK cell–mediated lysis.19,20 Therefore, proteolytic shedding of the ligands may represent an escape strategy to circumvent tumor surveillance by NK cells.21-23 Based on these data, novel strategies for NK cell–mediated immunotherapy aim at enhancing the expression of NKG2D ligands on tumor cells. This approach has been shown to overcome NK cell inhibition by MHC class I engagement, including tumor rejection and immunity in preclinical tumor models.24-26 Overexpression of NKG2D ligands has been achieved by transfection of tumor cell lines with the corresponding cDNA using retroviral constructs. Since this approach has major clinical limitations, we designed a novel NKG2D ligand-antibody fusion construct (ULBP2-BB4) with the aim to enhance NK-mediated cell lysis of malignant target cells. The BB4 antibody fragment recognizes CD138 (syndecan1), a tumor antigen frequently overexpressed on MM cells but absent on other hematopoietic cells.27-31 CD138 is a member of the syndecan family that includes cell-surface heparan sulfate proteoglycans involved in cell adhesion, maturation, and proliferation.31 Here we report that the novel bispecific protein ULBP2-BB4 binds to both NK and malignant plasma cells and has potent antitumor effects. To our knowledge this is the first approach that targets an activating NK receptor using its recombinant ligand. We provide a novel principle that might be applied to other malignancies provided a restricted antigen can be targeted for construction of the corresponding bispecific fusion protein.
Materials and methods
Expression constructs encoding BB4, ULBP2-BB4 and ULBP2
The single chain BB4 is derived from a mouse hybridoma producing the anti-CD138 (syndecan 1) monoclonal antibody27 and was a generous gift from Diaclone (Besancon, France). The BB4 polymerase chain reaction (PCR) product was restricted with SfiI and NotI and cloned into the corresponding sites into the eukaryotic expression vector pMS, which has been described previously.32,33 In brief, pMS is a derivative of the pSecTag2 plasmid (Invitrogen, Karlsruhe, Germany) that contains the IVS/IRES-EGFP sequence of the pIRES-EGFP plasmid from Clontech (Palo Alto, CA).
To clone ULBP2 (UL-binding protein 2) whole-cell RNA was isolated from K562 cells using the RNeasy RNA Extraction Kit (Qiagen, Hilden, Germany) as recommended by the manufacturer and used for first-strand cDNA synthesis (SMART PCR cDNA synthesis kit; Clontech, Palo Alto, CA). For amplification of the ULBP2 cDNA (GenBank accession no. NM 025217)16 gene-specific primers (gagctagctccttaatggcagca [upstream] and cactagtcctgagttgggt [downstream]) were used. The PCR products were restricted with NheI and SpeI (restriction sites are marked in bold) for in-frame cloning upstream of BB4 into pMS-(BB4). The resulting construct pMS-ULBP2-BB4 contains the human Ig kappa light-chain signal peptide in front of the inserts, a glycine/serine linker between ULBP2 and BB4, and a tandem myc- and his-tag epitope at the 3′ end of BB4. The his-tag was used for detection and purification of BB4 and ULBP2-BB4 from the supernatants of transfected 293T or Cos cells.
For expression of ULBP2 on the surface of 293T cells, the ULBP2 insert was cloned into the eukaryotic expression vector p3.1 (Invitrogen) following amplification with gene-specific primers (gagctagcatggcagcag and cgggatcctgccagggagga) using the NheI and BamHI sites (underlined).
Expression and purification of soluble BB4 and ULBP2-BB4
Transfection of 293T and Cos cells was performed with lipofectamine 2000 (Invitrogen). According to the manufacturer's instructions 0.81 μg DNA and 23 μL lipofectamine 2000 were used to transfect 0.5 × 105 2 × 105 cells in a 24-well cell-culture plate. Two days after transfection, cells were transferred into medium-size cell-culture flasks (85 cm2 Nunc; Nunc, Roskilde, Denmark) and grown in RPMI-1640 (PAA, Pasching, Germany) supplemented with 200 μg/mL Zeocin (Invitrogen). The recombinant proteins BB4 and ULBP2-BB4 were expressed simultaneously with enhanced green fluorescent protein (EGFP) because they were encoded by a bicistronic mRNA. Thus, after 1 to 2 weeks, single transfected clones could be detected by fluorescence microscopy and were established by subcultivation. The purification of his-tagged proteins from the supernatant was achieved by the Ni-NTA metal-affinity method as recommended by Qiagen, which was described previously.33 For a higher productivity transfected 293T cells were cultivated in CD293 medium (Invitrogen) instead of RPMI-1640, with supplements remaining unchanged. The size, homogenicity, and immunoreactivity (against the anti–penta-his monoclonal antibody; Qiagen) BB4 and ULBP2-BB4 were analyzed in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Cell lines
All cell lines, including the embryonic kidney–derived cell line 293T, the multiple myeloma lines U266 and RPMI-8226 (identical with Colo-677) were purchased from DSMZ (Braunschweig, Germany). The cells were cultivated in complex medium (RPMI-1640) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Invitrogen), 50 μg/mL penicillin, 50 μg/mL streptomycin, and 2 mm l-glutamine at 37°C in a 5% CO2 atmosphere.
Flow cytometry
To detect the expression of CD16, CD56, NKG2D, and CD138, the NK cells and tumor cells were incubated with the corresponding mouse antibodies (CD16, CD56, NKG2D, and CD138 [Becton Dickinson, Heidelberg, Germany]) using 10 μg/mL at 4°C. After 1 hour the cells were washed with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and 0.05% NaN3, and the bound antibodies were detected by incubating the cells with a fluorescein isothiocyanate (FITC)–labeled goat anti-mouse IgG at 4°C. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACSCalibur instrument (Becton Dickinson, Franklin Lakes, NJ). Background staining was estimated after incubation with the secondary FITC-labeled antibody alone.
The binding of ULBP2-BB4 or BB4 (10 μg/mL) was directly detected with a FITC-labeled anti–penta-his monoclonal antibody. Alternatively bound ULBP2-BB4 was detected with recombinant human NKG2D receptor (R&D Systems, Wiesbaden, Germany), that was visualized with an FITC-labeled anti-NKG2D antibody.
Enzyme-linked immunosorbent assay
To detect binding of ULBP2-BB4 to the NKG2D receptor, microtiter plates were coated with a recombinant NKG2D receptor-Fc fusion protein (R&D Systems). The wells were blocked with 5% BSA solution before ULBP2-BB4 or BB4 was added. After 1 hour the wells were washed 3 times with PBS containing 0.1% Tween 20 and 1 time with PBS and incubated with an anti–penta-his monoclonal antibody to detect ULBP2-BB4 molecules. We used an NKG2D-specific antibody (Becton Dickinson, Heidelberg, Germany) for a positive control. The plates were washed 3 times with PBS and bound antibodies were detected with an alkaline phosphatase–labeled antimouse antibody at 37°C. The excess probe was washed from the wells and the plate was developed with pNPP (p-nitrophenyl phosphate). The optical density at 405 to 650 nm was determined using a microtiter plate reader.
Primary NK cells and multiple myeloma cells
To generate NK cells we obtained isolated peripheral blood mononuclear cells (PBMCs) from healthy-donor buffy coats using Ficoll-Paques density gradient centrifugation with Lucosep columns from Greiner bio-one (Solingen, Germany). NK cells were isolated using the NK Cell Isolation Kit and VarioMACS for the depletion of non–NK cells (Miltenyi, Bergisch Gladbach, Germany). Separated polyclonal NK cells were cultivated in minimal essential medium (MEM) alpha supplemented with 50 μg/mL penicillin, 50 μg/mL streptomycin, 20% fetal calf serum and recombinant human IL-2 (10 U/mL) at 37°C with 5% CO2. The NK cells used for functional ULBP2-BB4 assays were prestimulated overnight with IL-2 (10 U/mL) and recombinant human IL-15 (100 ng/mL) to induce NKG2D receptor expression (IL-2 and IL-15 were purchased from R&D Systems).
For the generation of malignant plasma cells we obtained a sample of 20 mL heparinized peripheral blood from patient no. 2 (Figure 6) and a sample of 15 mL bone marrow aspirate from the other patients in the occasion of diagnostic procedures. Peripheral myeloma cells (patient no. 2; Figure 6) and bone marrow myeloma cells were obtained by Ficoll-Paques density gradient centrifugation and resuspended in RPMI with penicillin (50 μg/mL), streptomycin (50 μg/mL), and 10% fetal calf serum. The amount of myeloma cells varied between 20% and 40% of the purified mononuclear cells. Highly purified populations of MM cells were obtained after incubation of the patients' mononuclear cells in the presence of 10 ng/mL IL-6 (R&D Systems) for at least 2 days, which induced rapid proliferation of the tumor cells that presented about 95% to 100% of the cell population. The amount of CD138-positive malignant plasma cells in the population was determined using fluorescence-activated cell-sorting (FACS) analysis and light microscopy. Cytotoxicity tests were performed with samples that had at least 95% tumor cells.
Cytotoxicity assay
The cytotoxicity was estimated in a standard 2-hour europium release assay in a 96-well microtiter plate in a total volume of 200 μL in the presence or absence of ULBP2-BB4 (10 μg/mL) and BB4 (5 μg/mL). For blocking experiments the NK effector cells or the target cells were preincubated for 30 minutes at room temperature with the anti-NKG2D antibody (clone 1D11; Becton Dickinson, Heidelberg, Germany) or recombinant human NKG2D receptor (R&D Systems; used with CD16-negative NK cells), respectively. The effector cells were mixed with 5 × 103 tumor target cells that were labeled with europium chloride (Fluka, Buchs, Switzerland) using varying ratios. Supernatants were assayed for europium release in a gamma counter. Spontanous release was determined by incubation of the target cells in the absence of effectors and maximal release was obtained by target cell lysis using 1% Triton X-100. The spontanous release did not exceed 25% of the maximum release. The percentage of specific lysis was calculated as 100 × ([experimental release - spontanous release]/[maximum release - spontanous release]). All measurements were done in triplicates.
IFN-γ enzyme-linked immunosorbent assay
Primary NK cells or peripheral blood lymphocytes (PBLs) from healthy donors were incubated with IL-2 (10 U/mL) or IL-2 (10 U/mL) plus IL-15 (100 ng/mL) overnight and cultured in the presence or absence of immobilized ULBP2-BB4 or BB4 for 48 hours. The supernatant was used for a human IFN-γ detection ELISA Kit (R&D Systems) as recommended by the manufacturer. Absorbance of the plates was measured after 1 hour of reaction in an enzyme-linked immunosorbent assay (ELISA) reader in parallel with the measurement of the IFN-γ standard. In competition experiments NK cells were preincubated for 30 minutes at room temperature with the anti-NKG2D antibody (clone 1D11).
Xenograft model
RPMI-8226 cells (1 × 107) resuspended in 200 μL PBS were injected subcutaneously into 72 CD1 nude mice (Charles River, Sulzfeld, Germany) to establish tumors. PBLs containing about 10% NK cells were isolated from healthy donors by Ficoll-Paques density gradient centrifugation with Lucosep columns. Two days after tumor cell inoculation mice were treated with unstimulated human PBLs intravenously (5 × 106), alone or in combination with ULBP2-BB4. Tumor development was measured periodically and the tumor volume was determined using the formula (length × width × height) ÷ 2.
Statistics
The results of the cytotoxicity assays are indicated as means ± standard deviation. Significance levels were estimated using the student t test and P values of .5 or less were significant. The calculation was performed with the GraphPadPrism software (San Diego, CA).
Results
Overexpression of ULBP2 in 293T cells stimulates NK cytolytic activity
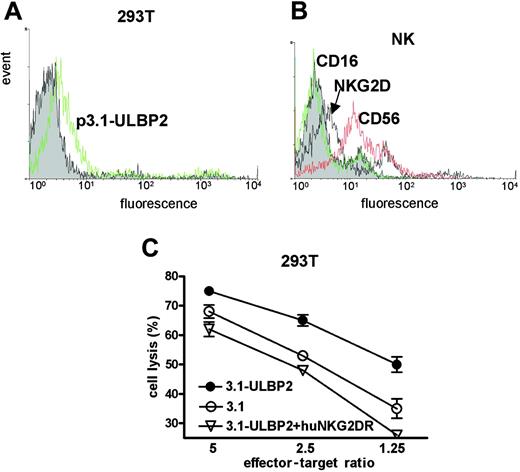
To determine whether overexpression of the NKG2D ligand ULBP2 in tumor cell lines attributes to an enhanced NK cell stimulation we transiently transfected the human embryonic kidney cell line 293T with an expression vector encoding ULBP2 (p3.1-ULBP2). The FACS analysis using phycoerythrin (PE)–labeled recombinant human NKG2D receptor showed that ULBP2 expression on the tumor cell surface was significantly enhanced after transfection (Figure 1A).
Effector NK cells were obtained from donor buffy coats by magnetic cell sorting. The presence of NK cell markers such as CD56 and CD16 and the expression of the NKG2D receptor was monitored (Figure 1B). The cytotoxicity experiments revealed that both mock-transfected (p3.1) and ULBP2-transfected (p3.1-ULBP2) cells were killed by NK cells (Figure 1C), indicating that NK-mediated lysis of 293T cells was not exclusively dependent on ULBP2. The enhanced ULBP2 expression, however, increased the susceptibility of 293T cells to cell lysis, especially when the effector-target ratio was low. The addition of soluble human NKG2D receptor blocked the ULBP2-based sensitization of the target cells, confirming that the increase in cell lysis depends on the ULBP2/NKG2D interaction. In addition to a complete inhibition of stimulated cell lysis, we also observed partial inhibition of the basal lysis rate, suggesting an endogeneous expression of NKG2D ligands on 293T cells.
Cloning, expression, and purification of recombinant ULBP2-BB4
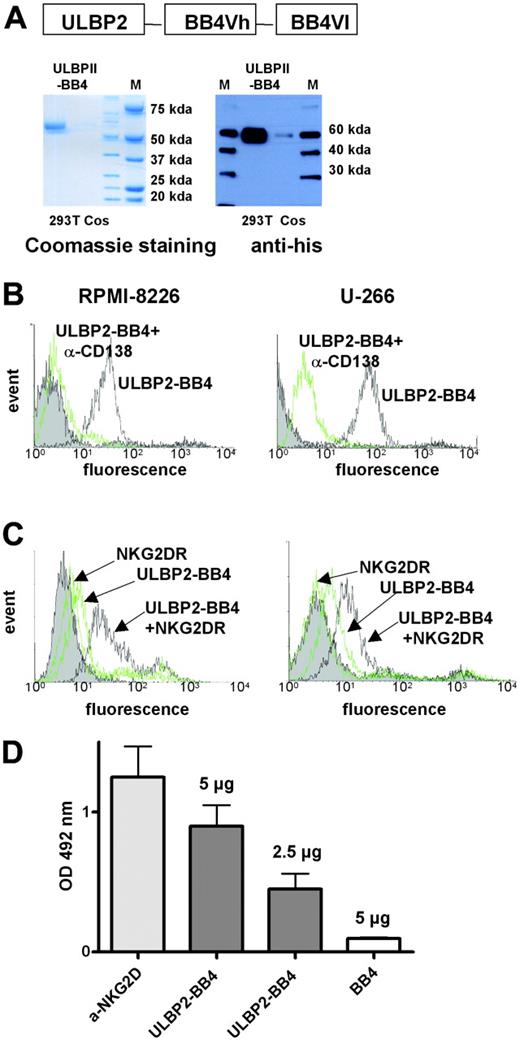
The ability of membrane-bound ULBP2 to stimulate NK-mediated lysis prompted us to design a bispecific protein consisting of ULBP2 and a tumor cell-binding antibody. The ULBP2 cDNA was cloned in front of the BB4 single chain that is derived from a monoclonal antibody raised against CD138 (syndecan1).27,29 CD138 represents an excellent target for immunotherapeutic approaches because it is selectively overexpressed on malignant plasma cells.27,29 The fusion protein with an expected molecular weight of 58 kDa is efficiently expressed in 293T cells, but is hardly expressed in Cos cells. After purification, ULBP2-BB4 was easily detectable with Coomassie or immunostaining using an anti-his antibody against the c-terminal 6xhistidine tag (Figure 2A).
ULBP2 overexpression enhances NK cell–mediated cell lysis of 293T tumor cells. (A) Soluble FITC-labeled NKG2D receptor was used in flow cytometry analysis to detect ULBP2 on 293T cells that were transfected either with the expression vector p3.1 (filled) or with p3.1-ULBP2 (open). (B) The binding of antibodies recognizing NKG2D (marked with an arrow), CD56, or CD16 on primary NK cells was visualized with FITC-coupled goat anti–mouse IgG. Note that NK cells used for the cytotoxicity experiments expressed NKG2D and CD56 and lack CD16 expression. (C) 293T cells transfected with p3.1 (○) or p3.1-ULBP2 (•) were incubated with purified NK cells in different ratios for 2 hours and NK cell–mediated lysis of the target cells was determined in a europium release assay. The target cells were incubated with 10 μg/mL soluble NKG2D receptor to block NKG2D dependent lysis of p3.1-ULBP2 transfected target cells (▿). Average NK lysis and SD (n = 3) are indicated. The lysis increase of p3.1-ULBP2 overexpressing cells is significant in all effector-target ratios (P = .04) and was estimated with the paired t test using GraphPadPrism software.
ULBP2 overexpression enhances NK cell–mediated cell lysis of 293T tumor cells. (A) Soluble FITC-labeled NKG2D receptor was used in flow cytometry analysis to detect ULBP2 on 293T cells that were transfected either with the expression vector p3.1 (filled) or with p3.1-ULBP2 (open). (B) The binding of antibodies recognizing NKG2D (marked with an arrow), CD56, or CD16 on primary NK cells was visualized with FITC-coupled goat anti–mouse IgG. Note that NK cells used for the cytotoxicity experiments expressed NKG2D and CD56 and lack CD16 expression. (C) 293T cells transfected with p3.1 (○) or p3.1-ULBP2 (•) were incubated with purified NK cells in different ratios for 2 hours and NK cell–mediated lysis of the target cells was determined in a europium release assay. The target cells were incubated with 10 μg/mL soluble NKG2D receptor to block NKG2D dependent lysis of p3.1-ULBP2 transfected target cells (▿). Average NK lysis and SD (n = 3) are indicated. The lysis increase of p3.1-ULBP2 overexpressing cells is significant in all effector-target ratios (P = .04) and was estimated with the paired t test using GraphPadPrism software.
The binding affinity and specificity of the parental antibody for CD138-expressing MM cell lines (RPMI-8226 and U-266) was preserved in the BB4 single chain (Figure 2B). The binding of the ULBP2-BB4 fusion protein to the tumor cells could be competed with an anti-CD138 antibody, which proofed the specific recognition of the CD138 antigen. We next tested whether ULBP2-BB4 was capable of binding simultaneously both NK cells via NKG2D and tumor cells via CD138 (Figure 2C). As the binding of ULBP2-BB4 to RPMI-8226 and U-266 cells could be detected with soluble NKG2D NKG2DR), we concluded that the construct was able to cross-link NK cells and the MM target antigen. No specific cell binding was detectable in control experiments using ULBP2-BB4 or soluble NKG2D alone. To verify the binding of ULBP2-BB4 to the NKG2D receptor in an independent assay we performed an ELISA using NKG2DR-coated plates (Figure 2D). A dose-dependent binding of ULBP2-BB4, but no unspecific binding of the BB4 single chain was measured. These results indicate that the bispecific construct ULBP2-BB4 can target CD138+ tumor cells to NK-mediated cell lysis via NKG2DR engagement.
ULBP2-BB4 binds to NK cells and CD138-expressing tumor cells simultaneously via NKG2D receptor and CD138 antigen. (A) ULBP2-BB4 was expressed in eucaryotic cell lines (293T and Cos) and purified with affinity chromatography using a 6× his tag. The eluted protein was separated on SDS-PAGE for Coomassie staining (left blot) and Western blotting with an anti-his–specific antibody (right blot). (B) Two CD138-positive cell lines were incubated with ULBP2-BB4 and labeled with an anti-his–FITC antibody for flow cytometry. The strong binding of ULBP2-BB4 (solid black line) could be competed after preincubation with a CD138-specific antibody (green line). (C) The binding of ULBP2-BB4 to RPMI-8226 and U-266 cells was visualized using soluble NKG2D receptor (NKG2DR) that was detected with an FITC-labeled anti-NKG2D antibody (dark line). No specific binding was seen using ULBP2-BB4 or soluble NKG2D receptor alone, both detected with the anti-NKG2D–FITC antibody (faint lines). (D) ELISA to measure the binding of ULBP2-BB4 (5 μg or 2.5 μg) to immobilized NKG2D receptor using an enzyme-linked anti-his antibody. An anti-NKG2D antibody (light gray bar) and the recombinant soluble single-chain BB4 that contained a 6× his tag (white bar) were used for the positive and the negative control, respectively. The mean OD492 nm ± SD (n=3) is indicated.
ULBP2-BB4 binds to NK cells and CD138-expressing tumor cells simultaneously via NKG2D receptor and CD138 antigen. (A) ULBP2-BB4 was expressed in eucaryotic cell lines (293T and Cos) and purified with affinity chromatography using a 6× his tag. The eluted protein was separated on SDS-PAGE for Coomassie staining (left blot) and Western blotting with an anti-his–specific antibody (right blot). (B) Two CD138-positive cell lines were incubated with ULBP2-BB4 and labeled with an anti-his–FITC antibody for flow cytometry. The strong binding of ULBP2-BB4 (solid black line) could be competed after preincubation with a CD138-specific antibody (green line). (C) The binding of ULBP2-BB4 to RPMI-8226 and U-266 cells was visualized using soluble NKG2D receptor (NKG2DR) that was detected with an FITC-labeled anti-NKG2D antibody (dark line). No specific binding was seen using ULBP2-BB4 or soluble NKG2D receptor alone, both detected with the anti-NKG2D–FITC antibody (faint lines). (D) ELISA to measure the binding of ULBP2-BB4 (5 μg or 2.5 μg) to immobilized NKG2D receptor using an enzyme-linked anti-his antibody. An anti-NKG2D antibody (light gray bar) and the recombinant soluble single-chain BB4 that contained a 6× his tag (white bar) were used for the positive and the negative control, respectively. The mean OD492 nm ± SD (n=3) is indicated.
ULBP2-BB4 activates NK cells: cytotoxicity and induction of IFN-γ secretion
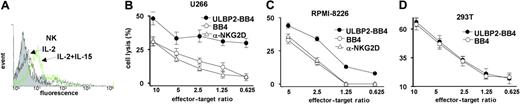
To investigate whether soluble ULBP2-BB4 stimulates NK-cell cytotoxicity we monitored lysis of CD138-expressing tumor cells mediated by primary NK cells isolated from healthy donors. The basal expression of the NKG2D receptor was induced by overnight incubation with IL-15 (100 ng/mL), which is shown for 1 representative NK cell population (Figure 3A).
The MM lines U266 and RPMI-8226 (both CD138+; Figure 3B-C) and the CD138 control cell line 293T (Figure 3D) were incubated with NK cells in the presence or absence of ULBP2-BB4. The fusion protein enhanced the NK-mediated cell lysis of U-266 and RPMI-8226 cells, whereas the lysis of 293T cells remained unaffected. The addition of the BB4 single chain, which binds specifically to the CD138 antigen but lacks the ULBP2 moiety, did not increase basal cell lysis (not shown). The increase of cell lysis was suppressed when the effector cells were preincubated with a blocking anti-NKG2D antibody.
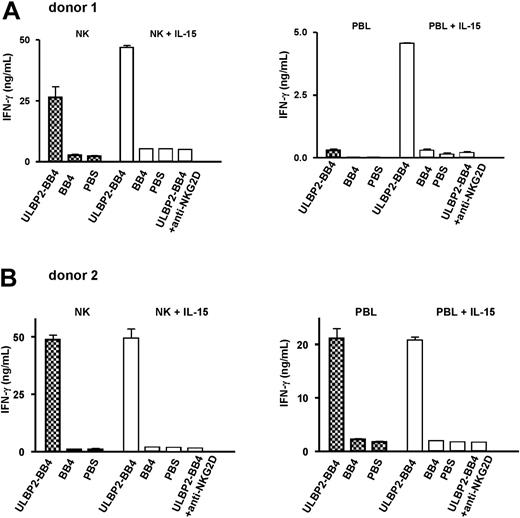
No enhancement of NK cell-mediated cell death of CD138 cells was detected in this 2-hour cytotoxicity assay. To analyze the activation potential of soluble ULBP2-BB4 in the absence of target cells, we incubated PBLs and isolated NK cells for 48 hours with ULBP2-BB4. NK cell activation was measured by IFN-γ secretion. The bispecific protein was either added in its soluble form or coated on the culture wells. In accordance with the cell lysis assay, only minor effects were measured using soluble ULBP2-BB4 (data not shown). In contrast, a strong IFN-γ secretion was induced when NK cells or PBLs were exposed to immobilized ULBP2-BB4, but not when exposed to BB4 or PBS alone (Figure 4A-B). The ULBP2-BB4–dependent activation was reproducible using NK cells from 2 different donors, while the quantity of IFN-γ secretion in response to ULBP2-BB4 differed among donor cells (compare donors 1 and 2, Figure 4A and 4B). This was probably due to the differential expression level of the NKG2D receptor on the NK cells used, which was induced using IL-15. The stimulation of PBLs and NK cells with IL-15 resulted in a stronger basal IFN-γ secretion as well as in a higher stimulation with ULBP2-BB4 especially for donor 1 (right part of each panel, Figure 4). Specificity of the activation for the interaction of ULBP2-BB4 with the NKG2D receptor was shown by inhibition of IFN-γ secretion through a blocking anti-NKG2D antibody (right column in each panel, Figure 4). The total amount of IFN-γ was higher when isolated NK cells were used instead of PBLs, suggesting that the T-cell contribution to the IFN-γ secretion was rather small. Thus, ULBP2-BB4 was able to activate NK cells when immobilized on the culture vials imitating a membrane-bound molecule. Since soluble ULBP2-BB4 failed to induce such a IFN-γ secretion, we do not expect nonspecific NK cell activation.
ULBP2-BB4 increases the suspectibility of MM cell lines to NK cell–mediated cytotoxicity. (A) NK cells from healthy donors were incubated overnight with IL-2 (10 U/mL; black line, marked with an arrow) or IL-2 + IL-15 (100 ng/mL; green line, marked with an arrow), and the expression of the NKG2D receptor was analyzed with a mouse anti-NKG2D antibody and a secondary FITC-coupled goat anti–mouse IgG. Note that NKG2D receptor expression was induced using IL-2/IL-15, whereas IL-2 alone revealed only minor effects. One representative polyclonal NK cell population is shown. (B-D) The IL-2/IL-15–stimulated NK cells were used at the indicated effector-target ratios for killing assays with U-266 (B), RPMI-8226 (C), and 293T (D) tumor cells in the presence of ULBP2-BB4 (5 μg/mL; •) or the single-chain BB4 (2.5 μg/mL; ○). For blocking experiments (▵) the NK cells were preincubated with 10 μg/mL anti-NKG2D antibody. Measurements were performed in triplicates and 1 of at least 2 independent experiments is indicated. The increase of cell lysis through ULBP2-BB4 was significant at all effector-target ratios for U-266 and RPMI-8226 cells, whereas no increase was observed for 293T cells. Error bars indicate SD (n=3).
ULBP2-BB4 increases the suspectibility of MM cell lines to NK cell–mediated cytotoxicity. (A) NK cells from healthy donors were incubated overnight with IL-2 (10 U/mL; black line, marked with an arrow) or IL-2 + IL-15 (100 ng/mL; green line, marked with an arrow), and the expression of the NKG2D receptor was analyzed with a mouse anti-NKG2D antibody and a secondary FITC-coupled goat anti–mouse IgG. Note that NKG2D receptor expression was induced using IL-2/IL-15, whereas IL-2 alone revealed only minor effects. One representative polyclonal NK cell population is shown. (B-D) The IL-2/IL-15–stimulated NK cells were used at the indicated effector-target ratios for killing assays with U-266 (B), RPMI-8226 (C), and 293T (D) tumor cells in the presence of ULBP2-BB4 (5 μg/mL; •) or the single-chain BB4 (2.5 μg/mL; ○). For blocking experiments (▵) the NK cells were preincubated with 10 μg/mL anti-NKG2D antibody. Measurements were performed in triplicates and 1 of at least 2 independent experiments is indicated. The increase of cell lysis through ULBP2-BB4 was significant at all effector-target ratios for U-266 and RPMI-8226 cells, whereas no increase was observed for 293T cells. Error bars indicate SD (n=3).
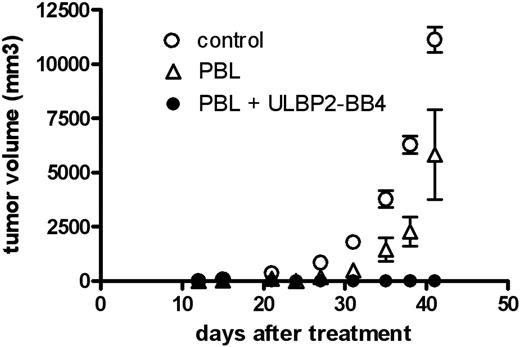
Treatment of xenotransplanted RPMI-8226 tumors in nude mice by a combination of human PBLs and ULBP2-BB4
To assess the antitumor effect of the recombinant protein against CD138+ malignancies we used a multiple myeloma xenograft model in nude mice (Figure 5). Tumors were established by a single injection of RPMI-8226 cells growing to an average volume of 1 cm3 within 15 days and an incidence of 100%. Two days after tumor cell inoculation, mice were treated with unstimulated human PBLs intravenously, alone or in combination with ULBP2-BB4. All animals in the control group (no PBL, no ULBP2-BB4) developed tumors larger than 1 cm3 within 15 days. Treatment with PBLs caused a tumor regression for 10 to 12 days. Thereafter, the tumor progression was comparable to that in untreated animals. Although the tumor growth was slightly attenuated after administration of PBLs in all animals (5 of 5), the differences in the control group were not significant (P = .14). In contrast, in animals treated with PBLs and ULBP2-BB4 (5 of 5) no tumors developed at all.
IFN-γ release by NK cells is induced through immobilized ULBP2-BB4 in a NKG2D-dependent way. Purified NK cells and PBLs of 2 healthy donors were incubated with IL-2 (10 U/mL, left part of each panel, patterned bars) or IL-2 plus IL-15 (100 ng/mL, right part of each panel, white bars) overnight and stimulated through immobilized ULBP2-BB4, immobilized BB4, or PBS for 48 hours. The IFN-γ concentration of the supernatant was estimated using ELISA and is indicated in nanograms per milliliter. In blocking experiments, an anti-NKG2D antibody (10 μg/mL) was added to the effector cells stimulated with IL-2/IL-15 and ULBP2-BB4 (right column in each panel, white bar. Two independent experiments, each measured in duplicate, were performed for each donor, and the mean ± SD is indicated.
IFN-γ release by NK cells is induced through immobilized ULBP2-BB4 in a NKG2D-dependent way. Purified NK cells and PBLs of 2 healthy donors were incubated with IL-2 (10 U/mL, left part of each panel, patterned bars) or IL-2 plus IL-15 (100 ng/mL, right part of each panel, white bars) overnight and stimulated through immobilized ULBP2-BB4, immobilized BB4, or PBS for 48 hours. The IFN-γ concentration of the supernatant was estimated using ELISA and is indicated in nanograms per milliliter. In blocking experiments, an anti-NKG2D antibody (10 μg/mL) was added to the effector cells stimulated with IL-2/IL-15 and ULBP2-BB4 (right column in each panel, white bar. Two independent experiments, each measured in duplicate, were performed for each donor, and the mean ± SD is indicated.
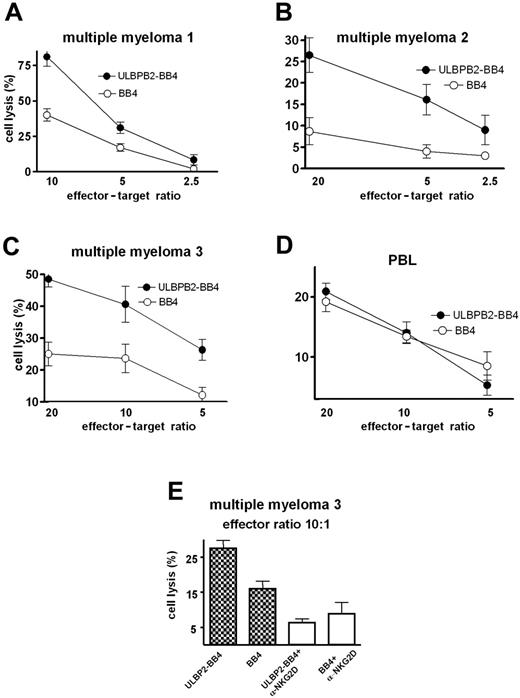
ULBP2-BB4 stimulates NK-mediated killing of primary malignant MM cells in a NKG2D-dependent manner
Subsequently, NK-mediated cell lysis assays were performed using primary malignant plasma cells from bone marrow samples (Figure6A,C) or PBLs (Figure 6B) obtained from patients with MM. The cell lysis of all patient samples was about 2-fold or more enhanced in the presence of ULBP2-BB4 at the effector-target ratio of 10 or 20, respectively (Figure 6A-C) compared with BB4 alone. Additional experiments were performed using cells from MM patient no. 3 with independent NK donor cells (Figure 6E) evaluating the ULBP2-BB4–mediated NK cell activation, which was significantly enhanced by the construct (P = .02). The presence of a blocking NKG2D antibody reduced the ULBP2-BB4 mediated cell lysis. Lysis rates of ULBP2-BB4 and BB4 samples were nearly identical (P = .49), demonstrating that the effects measured are NKG2D dependent. The lysis rate estimated in the presence of BB4 was also slightly decreased by the NKG2D antibody, suggesting that endogeneous ligands of NKG2D are expressed on MM cells. ULBP2-BB4–dependent enhancement of NK-mediated killing of PBLs from healthy donors was not observed (Figure 6D), reflecting the specificity of ULBP2-BB4 for CD138-overexpressing tumor cells.
Because these experiments were performed in the allogenic setting we assumed that the NK-mediated killing was enhanced by KIR incompatibility of effector and target cells. Subsequently, cytotoxicity assays were conducted in the autologous system using MM target cells and PBL effector cells in different effector-target ratios (Figure 7A). Interestingly, in this KIR-compatible setting no basal NK cell–mediated target lysis was observed in the absence of ULBP2-BB4. However, addition of ULBP2-BB4 resulted in a significant target cell killing, which demonstrates that NKG2D engagement is capable of activating NK cells even in the context of “self inhibition.” The second experiment with another patient (Figure 7B) was performed with 2 independent series (independent labeling) of the target cells and effector cells in a ratio of 10:1. Again, there was no basal cell lysis, but a reproducible ULBP2-BB4–dependent stimulation was measured. This is promising for a potential clinical application. It is also remarkable and encouraging that the ULBP2-BB4 action is observed using PBLs instead of purified NK cells as effector cells (Figure 7). It cannot be excluded that T-cell activation is also involved in target cell killing, as the NKG2D receptor serves as a costimulatory molecule on T cells.18
ULBP2-BB4 reveals antitumor activity in a xenograft MM model. Effect of PBL + ULBP2-BB4 (•), PBLs (▵) and no treatment (○) on the tumor growth of subcutaneous RPMI-8226 tumors in nude mice is shown. The mean tumor volume ± SD (n=5) is shown. Statistical analysis of the tumor volumes measured was done with the paired t test using GraphPadPrism software. The differences between PBLs and the control are not significant (P = .14; n = 5).
ULBP2-BB4 reveals antitumor activity in a xenograft MM model. Effect of PBL + ULBP2-BB4 (•), PBLs (▵) and no treatment (○) on the tumor growth of subcutaneous RPMI-8226 tumors in nude mice is shown. The mean tumor volume ± SD (n=5) is shown. Statistical analysis of the tumor volumes measured was done with the paired t test using GraphPadPrism software. The differences between PBLs and the control are not significant (P = .14; n = 5).
NK cell–mediated cell lysis of primary MM cells is enhanced through ULBP2-BB4. (A-D) Primary malignant plasma cell samples from 3 patients (A-C) or peripheral blood lymphocytes from a healthy donor (D) were used as target cells for an europium release assay with purified NK cells in different effector-target ratios in the presence of ULBP2-BB4 or BB4. (E) The killing of malignant plasma cells of patient no. 3 was determined (effector-target ratio, 10:1) with or without blocking of the NKG2D receptor using an α-NKG2D antibody. Note that the NK cells used in panels A-E are derived from independent donors. Lysis rates were measured in triplicate, and the means with standard deviation are indicated.
NK cell–mediated cell lysis of primary MM cells is enhanced through ULBP2-BB4. (A-D) Primary malignant plasma cell samples from 3 patients (A-C) or peripheral blood lymphocytes from a healthy donor (D) were used as target cells for an europium release assay with purified NK cells in different effector-target ratios in the presence of ULBP2-BB4 or BB4. (E) The killing of malignant plasma cells of patient no. 3 was determined (effector-target ratio, 10:1) with or without blocking of the NKG2D receptor using an α-NKG2D antibody. Note that the NK cells used in panels A-E are derived from independent donors. Lysis rates were measured in triplicate, and the means with standard deviation are indicated.
Taken together, ULBP2-BB4 shows in vitro activity against CD138+ MM cell lines and against patient-derived MM cells in allogenic and autologous settings. In addition, this novel construct exhibits potent antitumor efficacy against CD138+ tumors in nude mice.
Discussion
The aim of the present study was to design a novel immunotherapeutic protein to target NK cells to malignant MM cells. The major findings were that ULBP2-BB4: (1) bound both NK cells and tumor cells; (2) triggered NK-mediated cell lysis of CD138+ malignant cell lines and primary MM cells in the allogenic and autologous setting; (3) activated IFN-γ secretion of NK cells exposed to immobilized protein; and (4) had potent antitumor activity in vivo and could overcome NK cell inhibition.
The ULBP2 part was targeted to NK cells via binding to the NKG2D receptor. NK cell activation by ULBP2-BB4 was shown to be mediated by NKG2D because it was inhibited by a blocking NKG2D-specific antibody. Thus, the biologic activity of ULBP2-BB4 was most likely mediated by the engagement of the NKG2D receptor. The activation of the NK cell cytotoxicity was interestingly also observed in autologous experiments, suggesting that the engagement of NKG2D via ULBP2-BB4 overcomes “self-inhibition” of NK cells.
The antibody single-chain fragment BB4 was cloned from a mouse monoclonal antibody and recognized the tumor antigen syndecan I (CD138). CD138 is frequently overexpressed on MM-derived cells compared with normal plasma cells,28 rendering this antigen an excellent target for immunotherapeutic approaches.34-37 Promising preclinical results were recently reported for a BB4 conjugate in which the binding moiety was linked to an antimicrotubule agent.38 With the rationale of targeting MM cells to NK cell–mediated cell lysis, we fused the BB4 scFv to the ligand of the triggering NK cell receptor. Our study suggests that ULBP2-BB4 may recruit and activate NK cells to the tumor cells, thus overcoming immune tolerance. We demonstrated that ULBP2-BB4 induces IFN-γ secretion from NK cells reflecting NK cell activation, provided that the protein is immobilized on the culture vials. No such NK cell activation could be observed using soluble ULBP2-BB4, making unspecific NK cell activation by ULBP2-BB4 rather unlikely. However, it has been described that soluble ULBPs activate signaling pathways in NK cells, including IFN-γ secretion, if they are produced as leucine-zipper proteins39 that form protein complexes. Moreover, the construct selectively enhanced the NK-dependent lysis of CD138+ tumor cells, and should therefore not induce systemic toxicity.
The in vivo antitumor activity of ULBP2-BB4 was demonstrated in a xenograft model of an aggressive MM line, since no relevant human models are available. Treatment of RPMI-8226 tumors with ULBP2-BB4 and PBLs could abrogate tumor growth, whereas control animals developed tumors of 2 cm3 within 1 month, indicating the substantial antitumor activity of this novel recombinant protein.
ULBP-BB4–dependent NK cell stimulation in the autologous setting. (A) Primary malignant plasma cell samples from were incubated with IL-6 (10 ng/mL) and used for an europium release assay with PBLs from the same patient in different effector-target ratios in the presence of BB4 or ULBP2-BB4. Lysis values were measured in triplicates, and the means ± SD are indicated. (B) Europium release assay using MM cells and PBLs from another patient with 2 independent labeled target cell samples. The absolute counts of the spontanous release (SR) and the release of cells in the presence of BB4 or ULBP2-BB4 are indicated as well as the percentage lysis of the maximum release (100 × [experimental release - spontanous release]/[maximum release - spontanous release]). The spontanous release of labeled primary tumor cells did not exceed 25% of the maximum release.
ULBP-BB4–dependent NK cell stimulation in the autologous setting. (A) Primary malignant plasma cell samples from were incubated with IL-6 (10 ng/mL) and used for an europium release assay with PBLs from the same patient in different effector-target ratios in the presence of BB4 or ULBP2-BB4. Lysis values were measured in triplicates, and the means ± SD are indicated. (B) Europium release assay using MM cells and PBLs from another patient with 2 independent labeled target cell samples. The absolute counts of the spontanous release (SR) and the release of cells in the presence of BB4 or ULBP2-BB4 are indicated as well as the percentage lysis of the maximum release (100 × [experimental release - spontanous release]/[maximum release - spontanous release]). The spontanous release of labeled primary tumor cells did not exceed 25% of the maximum release.
In patients the NK cell recognition of primary myeloma cells is negatively regulated by MHC class I molecules, since NK cell susceptibility is enhanced after MHC class I masking.40 Interestingly, NKG2D ligands such as MICA, MICB, and ULBP molecules have a reduced expression on primary human myeloma cells lines derived from pleural effusion. This finding supports the hypothesis that tumor progression might be due to the escape of MM cells from NK cell surveillance by down-regulation of NKG2D ligands.40 Therefore, an immunotherapeutic approach targeting the NKG2D receptor seems to be promising. In this respect the novel ULBP2-BB4 construct counteracting tumor cell escape might be of clinical benefit. A decreased expression of NK receptor ligands is also frequently associated with leukemic transformation and is supposed to be reponsible for the resistance of blasts to killing by NK cells.41 Using 3 independent samples derived from patients with acute myeloid leukemia (AML), a significant increase of NK cell–mediated lysis was observed upon cytokine-dependent up-regulation of ULBP and NCR ligands on the AML blasts.41
The stimulation of NK cell activity has been reported upon overexpression of the murine NKG2D ligands Rae-1 and H60 in tumor cells.25 This study demonstrated that ectopic expression of NKG2D ligands leads to NK/T-cell–mediated tumor rejection and induces immunity to subsequent tumor challenge with the tumor cells, strongly suggesting an application of these ligands in the design of tumor vaccines. Promising results were also obtained when NKG2D ligands were overexpressed in a syngeneic mouse lymphoma model24 and in a glioma model.26 These data basically demonstrate that engagement of NKG2D can overcome inhibition of NK cell–mediated lysis due to MHC class 1 antigen expression. ULBP2-BB4 triggered NK cell cytotoxicity even in the autologous setting, and thus it seems possible that ULBP2-BB4 can circumvent NK cell suppression, which may allow the development of an efficient antitumor immune response. Although NK cells are defined as a component of the innate immune system, their participation in adaptive immune responses has become obvious in several studies.10,42 Most impressive is the cross-talk between dendritic cells and NK cells that can induce an effective tumor cell–specific immune response, including a long-term cytotoxic T-cell memory, without the contribution of CD4+ T helper cells.43 This immune response initiated by NK cell activity is dependent on IFN-γ secretion and the engagement of the NKG2D receptor, since blocking of the NKG2D ligands expressed on the tumor cells abrogates the antitumor immune response.43 The ability of NK cells to act like antigen-specific cells and to regulate T-cell activation in concert with the immune response further demonstrates the linkage of the innate and adaptive immune cells, probably via direct T-cell/NK cell interaction.44
While the clinical efficacy of ex vivo–expanded primary NK cells or NK cell lines is still rather limited,45 the in vivo modulation of NK cell function has raised increasing attention. An alternative to the use of a recombinant fusion protein such as ULBP2-BB4 might be an up-regulation of NKG2D ligands on tumor cells by agents such as retinoic acid or cytokines.45,46 Taken together, improving NK cell function in the treatment of patients is a promising approach. In this respect, the developed recombinant protein ULBP2-BB4 might contribute to optimize the NK cell attack against cancer cells, and thus warrants further evaluation.
Prepublished online as Blood First Edition Paper, October 6, 2005; DOI 10.1182/blood-2005-05-2177.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The excellent technical assistance of Gisela Schön and Anne Krüßmann is gratefully acknowledged.

![Figure 7. ULBP-BB4–dependent NK cell stimulation in the autologous setting. (A) Primary malignant plasma cell samples from were incubated with IL-6 (10 ng/mL) and used for an europium release assay with PBLs from the same patient in different effector-target ratios in the presence of BB4 or ULBP2-BB4. Lysis values were measured in triplicates, and the means ± SD are indicated. (B) Europium release assay using MM cells and PBLs from another patient with 2 independent labeled target cell samples. The absolute counts of the spontanous release (SR) and the release of cells in the presence of BB4 or ULBP2-BB4 are indicated as well as the percentage lysis of the maximum release (100 × [experimental release - spontanous release]/[maximum release - spontanous release]). The spontanous release of labeled primary tumor cells did not exceed 25% of the maximum release.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-05-2177/2/m_zh80050691700007.jpeg?Expires=1768052300&Signature=IqIWUI2fk7l~6w-lAl5VQrr8OnF7QCLgeh-M-kpv6BRGOMoIhaEUUa3vY8YRRPwUEB-Nz4vLq-5Vuk33DVnX6ZQDY2aPx5ZHPbWrmeT011HPe4rnyg895Bh2qv0gjs2qh8t9zptpL0MDukkRVei6vuCoc933qiBuodcSZRznQUUu4K~cNGIW2vT1jY~9esW7907jmiN956gHd0QJmVuED6ddQNYaeRPnt-DAYnYig9ds70HsSy2FfdnjvEzIp4ezdz8ncWp8G7LwAvOlqzyNogDULQt3pdplnRiU~uyRS3RYp8lrxpNpwHqqFirsZS7x5ZlJhkBfBGIV3MH6yssgcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
