We found a population of nonlymphoid cells expressing both CD4 and CD8 in peripheral blood mononuclear cells (PBMCs) of human T-cell leukemia virus type-I pX transgenic rats with autoimmune diseases. These cells, which showed a monocytic phenotype, were also found in wild-type rats, and their number increased by adjuvant-assisted immunization. GM-CSF increased the number of these double-positive (DP) monocytes in PBMCs. Consistent with the idea that DP monocytes differentiate into DP macrophages at sites of inflammation, we found infiltration of DP macrophages at the site of myosin-induced myocarditis in wild-type rats; these cells exhibited a T-helper 1 (Th1)-type cytokine/chemokine profile and expressed high levels of Fas ligand, perforin, granzyme B, and NKR-P2 (rat orthologue of human NKG2D). Adoptive transfer of GFP-positive spleen cells confirmed hematogenous origin of DP macrophages. DP monocytes had a cytotoxic phenotype similar to DP macrophages, indicating that this phenotypic specialization occurred before entry into a tissue. In line with this, DP monocytes killed tumor cells in vitro. Combined evidence indicates that certain inflammatory stimuli that induce GM-CSF trigger the expansion of a population of DP monocytes with a cytotoxic phenotype and that these cells differentiate into macrophages at inflammatory sites. Interestingly, human PBMCs also contain DP monocytes.
Introduction
Despite their origin from a common bone marrow progenitor population, cells of the monocyte/macrophage lineage display considerable phenotypic and functional heterogeneity. Thus, macrophages residing in the liver and the lungs differ in their basal activity as well as their ability to respond to inflammatory mediators. Even within a single organ, macrophages are heterogeneous; macrophages localized in the centrilobular and periportal regions of the liver differ in size, the ability to produce superoxide anion, and phagocytic activities.1 In an inflammatory response, the early stages are dominated by macrophages showing inflammatory and tissue-destructive activities, and, in the late stages, macrophages with tissue-restructuring activities predominate.2 In tumor tissues, infiltrating macrophages tend to acquire a polarized M2 phenotype, promoting tumor growth and progression.3 Accumulated evidence indicates that such macrophage heterogeneity is largely attributable to microenvironmental signals including cytokines and microbial products. Although this suggests that macrophages do not have stable, lineage-defined subsets and that their functional phenotypes change in response to a microenvironment, definitions of macrophage subpopulations are important not only for understanding their role in host defense and disease pathogenesis but also for designing effective therapeutic interventions.
In our previous study, we made F1 rats by mating F344 transgenic rats expressing the human T-cell leukemia virus type-I (HTLV-I) pX gene4 to nontransgenic Wistar rats and found that they developed disorders, including atrophy of the thymus, lymphocytopenia, and inflammatory cell infiltration into multiple organs, as typically seen in chronic graft-versus-host disease (GVHD).5 In these rats (hereafter referred to as FW-pX rats), the HTLV-I pX transgene induced neonatal apoptosis of the thymic epithelial cells, resulting in lymphocytopenia accompanied by compensatory expansion of peripheral myeloid cells, production of autoreactive T cells, and subsequent development of chronic GVHD-like autoimmune diseases.
In the present study, we found that a population of monocytic cells expressing both CD4 and CD8 was expanded in the peripheral blood of FW-pX rats. Monocytes/macrophages constitutively express CD4 in humans and rats6,7 but not in mice. On the other hand, some myeloid cells including natural killer (NK) cells, mast cells, macrophages, and dendritic cells (DCs) express CD8.8-14 We therefore hypothesized that rat peripheral blood contains a population of monocytes expressing both CD4 and CD8 and that this population is expanded under certain activating conditions. To test this hypothesis, we used a rat model of myosin-induced myocarditis. Here we show that the number of CD4/CD8 double-positive (DP) monocytes is indeed increased by adjuvant-assisted immunization with myosin or by administration of adjuvants alone and that these cells express cytotoxic factors such as perforin and granzyme B and exhibit cytotoxicity against tumor cells in vitro. Approximately half of the macrophages that infiltrated the cardiac lesion expressed both CD4 and CD8; these DP macrophages, shown to be of hematogenous origin by adoptive transfer experiments, expressed Fas ligand (Fas L), perforin, and granzyme B at high levels. Thus, our present work demonstrates the existence of a distinct population of monocytes/macrophages characterized by coexpression of CD4 and CD8 and by a cytotoxic phenotype. Interestingly, human peripheral blood also contains DP monocytes.
Materials and methods
Rats
FW-pX rats were obtained by mating male F344 transgenic rats expressing the HTLV-I pX gene without any tissue specificity (line 38)4 to nontransgenic female Wistar rats. Offspring were screened for the pX transgene by genomic polymerase chain reaction (PCR) as described.4 FW-wild-type (FW-wt) rats were obtained by mating nontransgenic male F344 rats to female Wistar rats. HTLV-I pX transgenic rats (line 38) were maintained at the Institute of Animal Experimentation, Hokkaido University Graduate School of Medicine. Inbred F344 and closed-colony Wistar rats were purchased from SLC (Shizuoka, Japan) and Charles River (Kanagawa, Japan), respectively. The EGFP transgenic rats that ubiquitously expressed green fluorescent protein (GFP)15 were obtained from the YS Institute (Utsunomiya, Japan) and Tohoku University (Sendai, Japan). All experiments using rats were done according to the Guideline for the Care and Use of Laboratory Animals in Hokkaido University Graduate School of Medicine.
Human blood samples
Human blood samples were obtained from 12 healthy donors after informed consent and used for flow cytometry (FCM). None of the donors had medical histories of autoimmune diseases, recent infection, or neoplasms.
Antibodies
Murine monoclonal antibodies (Abs) used were anti-rat CD3 (IF4 for immunohistochemistry, Cedarlane, Hornby, ON, Canada; and G4.18 for FCM, Pharmingen, San Diego, CA), anti-rat CD4 (OX-35; Pharmingen), anti-rat CD8 α-chain hinge region (OX-8; Pharmingen), anti-rat CD8 α-chain immunoglobulin (Ig) V-like region (G28; Pharmingen), anti-rat CD8 β-chain (341; Pharmingen), anti-rat CD11b/c (OX-42; Pharmingen), anti-rat CD68 (ED-1; Serotec, Oxford, United Kingdom), anti-rat CD163 (ED-2; Serotec), anti-rat B cell (RLN-9D3; Serotec), anti-rat DC (OX-62; Cedarlane), and NKR-P1A (10/78; Pharmingen), as well as anti-human CD4 (M-T466; Miltenyi Biotec, Bergisch Gladbach, Germany), anti-human CD8 (HIT8a; Pharmingen), and anti-human CD14 (MφP9; Pharmingen). Polyclonal rabbit anti-Fas L (N-20) and goat anti-granzyme B (N-19) Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse IgG1 or IgG2 (CBL600P or CBL601P, respectively; Chemicon International, Temecula, CA) and rabbit or goat IgG (Sigma-Aldrich, St Louis, MO) served as controls.
Recombinant cytokines/chemokines
Recombinant cytokines/chemokines used were rat RANTES and GM-CSF (PEPROTECH EC, London, United Kingdom) and mouse IL-12 (R&D Systems, Minneapolis, MN) previously shown to function in rats.16 For in vivo administration, GM-CSF (1.0 μg per 1 mL PBS) was injected intravenously into 6-week-old Wistar rats. Peripheral blood was assayed 24 hours after injection.
FCM and MACS
Peripheral blood cells were stained by the direct method without removal of serum. After reaction with Abs, erythrocytes were depleted by treatment with ammonium chloride. Expression of cell surface molecules was analyzed using FACScan (Becton Dickinson, Franklin Lakes, NJ) with CellQuest software (Becton Dickinson). Magnetic-activated cell sorting (MACS) was done using Magnetic Cell Separator (Miltenyi Biotec) as described.17
Phagocytosis assay
Yellow-green carboxylate-modified 1.0 μm latex beads (Sigma-Aldrich) were mixed with rat peripheral blood (1.5 × 107 beads/300 μL blood). After incubation for 2 hours at 37°C, PE-conjugated anti-CD4 (OX-35) and PerCP-conjugated anti-CD8 (OX-8) Abs were added to the mixture, followed by depletion of erythrocytes using ammonium chloride. After 3 times wash with cold PBS, CD4+/CD8+ DP cells were gated to determine uptake of the fluorescence-labeled beads using FACScan.
Immunization of rats with porcine heart myosin and induction of myocarditis
Killed tuberculosis germs were added to Freund incomplete adjuvant (Sigma-Aldrich) to reach the concentration of 100 mg/mL. Two milligrams of myosin from porcine heart (Sigma-Aldrich) were emulsified with an equal volume (200 μL) of the prepared adjuvant. The emulsion containing porcine myosin was inoculated into bilateral footpads of 3-week-old FW-wt rats (200 μL/site).
Histopathology and immunohistochemistry
Tissue samples were fixed in 10% phosphate-buffered formaldehyde and embedded in paraffin blocks. Each 4-μm section was stained with hematoxylin and eosin. For immunohistochemistry, an avidin-biotin immunoperoxidase kit (DAKO, Glostrup, Denmark) was used. After immunostaining, tissue sections were counterstained with Mayer hematoxylin (Merck, Darmstadt, Germany).
Isolation of macrophages from cardiac tissues with myocarditis
At 3 weeks after myosin immunization, the heart was extirpated and then perfused by PBS ex vivo. The cardiac tissues were cut into small pieces and digested by 0.16% collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ). After removal of tissue fragments, cell suspension was incubated in a plastic dish at 37°C. One hour later, adherent cells were harvested and used as tissue-infiltrating macrophages. The purity of ED-1-positive (CD68+) positive cells regarded as macrophages was 94% (data not shown).
Immunocytochemistry
Mononuclear cells separated from rat spleen using Histopaque-1083 (Sigma-Aldrich) or isolated from cardiac tissues were cultured in chamber slides (Nalge Nunc International, Roskilde, Denmark) for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes or 4% paraformaldehyde for 15 minutes at room temperature and then stained by the standard method (for details, see the legends of Figures 2 and 4, 5, 6). After washing with PBS, the slides were mounted in fluorescent mounting medium (DAKO). Immunofluorescence was detected using a confocal microscope (MRC-1024; BIO-RAD, Hercules, CA) or a fluorescence microscope (ECLIPSE E600; Nikon, Tokyo, Japan).
Image processing
Microscopic photographs were taken using the objective lens (40 ×/0.75 numeric aperture) in the DP70 system (Olympus, Tokyo, Japan). DP Controller software (Olympus) was used for image processing.
RT-PCR and quantitative real-time RT-PCR
Total RNAs were extracted from cells by RNeasy Mini Kit (QIAGEN, Alameda, CA) and then reverse transcribed using M-MLV reverse transcriptase (Invitrogen, Paisley, United Kingdom). PCR was performed using the cDNAs, 2 mM dNTP mix (GeneAmp dNTPMix; Applied Biosystems, Foster City, CA), Taq DNA polymerase kit (AmpliTaq Gold; Applied Biosystems), and primer sets for 28 cycles of 95°C 1 minute, 56°C 1 minute, and 72°C 1 minute. Quantitative real-time reverse transcription-PCR (RT-PCR) was performed using the cDNAs, QuantiTec SYBR Green PCR Kit (QIAGEN), and primer sets. Relative expression of target genes was analyzed using the ΔΔCT method.18 The expression level of the Gapdh (glyceraldehyde-3-phosphate dehydrogenase) gene was used as an internal control. PCR was conducted for 40 cycles using an ABI PRISM 7000 Sequence Detector System (Applied Biosystems) with 2-step reactions (95°C for 30 s, 60°C for 30 s) after initial denaturation of 95°C for 15 minutes. The primer sequences for PCR are listed in Table 1.
Transfer of GFP-positive spleen cells into nontransgenic recipients
The EGFP transgenic rats and nontransgenic Wistar rats (all rats were 4 weeks old) were immunized with porcine myosin as described under “Immunization of rats with porcine heart myosin and induction of myocarditis.” Mononuclear cells were isolated from the spleen of EGFP transgenic rats one week after immunization and transferred into Wistar rats that had been immunized with myosin 2 weeks before (1 × 107/rat intravenously). Five days later, the hearts of the recipients were extirpated, and tissue-infiltrating macrophages were isolated.
Cytotoxic assay
Six-week-old Wistar rats were immunized with adjuvants containing killed tuberculosis germs. One week later, mononuclear cells were separated from the spleen and incubated in plastic dishes for 20 minutes at 37°C. Resultant adherent cells were collected and then divided into CD8- and CD8+ cells using the MACS system. These cells were added to the culture of allogenic epithelial thymoma cells (originated from F344 rats carrying the HTLV-I pX transgene19 ) with effector-target (E/T) ratios of 30, 10, 1, and 0.1 (4 × 104 target cells per well in 24-well plates). After incubation for 18 hours, cytotoxicity was measured using the CytoTox 96 test kit (Promega, Madison, WI).
Statistical analysis
Data were represented as mean ± standard deviation (SD). Statistical significance between any 2 groups was determined by 2-tailed Student t test. P values less than .05 were considered to be significant.
Results
Expansion of CD4+/CD8+ cells in the peripheral blood of FW-pX rats with chronic GVHD-like autoimmune diseases
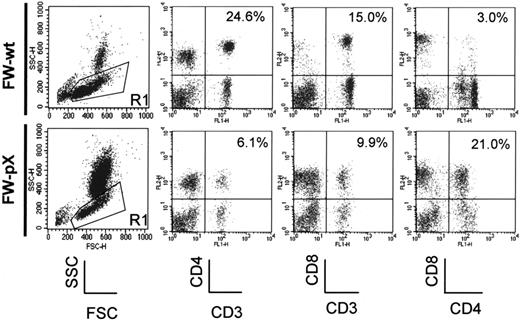
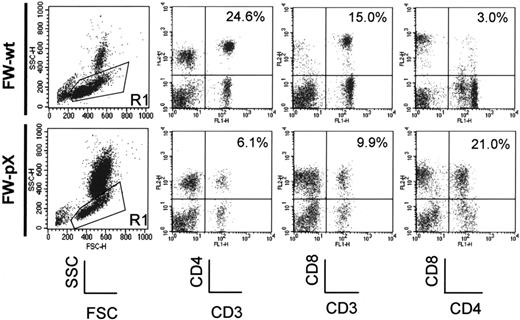
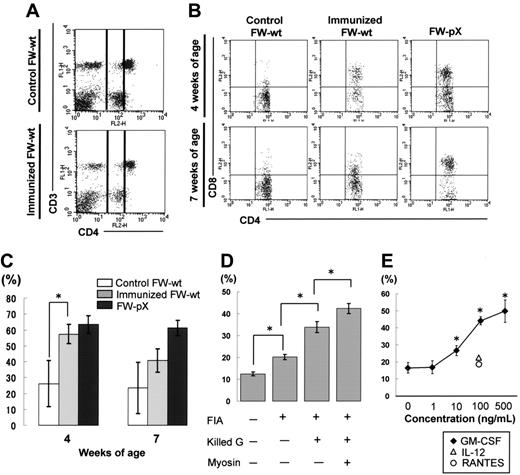
In FW-pX rats, the HTLV-I pX transgene induced atrophy of the thymus, resulting in lymphocytopenia, production of autoreactive T cells, and subsequent development of chronic GVHD-like autoimmune diseases.5 These rats also displayed a compensatory increase in the number of peripheral myeloid cells. To characterize immunophenotypic alterations in their peripheral blood mononuclear cells (PBMCs), we performed 2-color FCM analysis using PBMCs isolated from 6-week-old FW-pX and age-matched control FW-wt rats (Figure 1). The percentages of CD4+/CD8- and CD4-/CD8+ T cells (6.1% and 9.9%, respectively) in FW-pX rats were reduced in comparison with those of FW-wt rats (24.6% and 15.0%, respectively). The reduction of CD4+ T cells was more pronounced than that of CD8+ T cells. On the other hand, CD4+/CD8+ cells were few in FW-wt rats (3.0%) but markedly increased in number in FW-pX rats (21.0%). In FW-wt rats, CD4+ cells were made up of CD4medium and CD4high populations. By contrast, the majority of CD4+/CD8+ cells in FW-pX rats expressed CD4 at a medium level. Jefferies et al6 reported that rat CD4medium and CD4high populations represented monocytes and T cells, respectively, whereas Nascimbeni et al20 showed that some CD4+/CD8+ T cells expressed CD4 at a medium level. Thus, we decided to examine whether CD4+/CD8+ cells in FW-pX rats were monocytes or T cells.
CD4+/CD8+ cells in the peripheral blood of FW-pX rats are monocytes
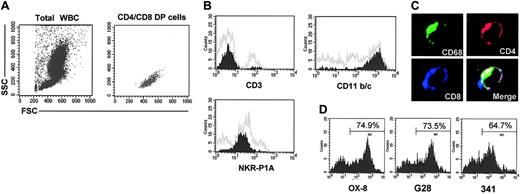
Peripheral blood was obtained from 6-week-old FW-pX rats. At first, we gated CD4+/CD8+ DP cells and confirmed that these cells were mononuclear but not aggregated cells (Figure 2A). Since rat monocytes could not be separated from lymphocytes or NK cells according to the light scatter pattern alone, we examined the expression of surface markers specific for each type of cell. Histograms were obtained by gating of CD4+/CD8+ cells (Figure 2B). Most CD4+/CD8+ cells in FW-pX rats were CD11b/chigh and NKR-P1Alow but did not express CD3. CD11b/c is expressed on monocytes, granulocytes, and macrophages, thereby known as a marker of myeloid cells.21 NKR-P1A is highly expressed on NK cells and some T cells but expressed on monocytes at a low level.22,23 We additionally found that the CD4+/CD8+ cells did not express OX-62, a marker for DCs24 (data not shown). These observations suggest that these DP cells have a monocytic but not T, NK, or DC phenotype. To further confirm this suggestion, adherent splenocytes from 6-week-old FW-pX rats were stained for OX-35 (anti-CD4), OX-8 (anti-CD8), and ED-1 (anti-CD68, as a marker for monocytes/macrophages25,26 ) and then observed using a confocal microscope. The 3-color merged image indicates that the DP cells also express CD68 (Figure 2C). It is known that human CD68 can be expressed in activated T and B cells at a low level.27,28 However, there was no CD68+ population that expressed CD3 or the B-cell marker RLN-9D3 in our rat model. We therefore designated these CD4+/CD8+ cells as DP monocytes. In addition, we noted that CD4 and CD8 were distributed not only on the cell surface but also in the cytoplasm of DP monocytes. These findings are consistent with the previous observation that CD4 is also expressed in the cytoplasm of human monocytes.29
Expansion of CD4+/CD8+ cells in the peripheral blood of FW-pX rats. The top and bottom panels show the results of FCM analyses of peripheral blood from 6-week-old FW-wt (F1 generation of wild-type F344 and Wistar) and FW-pX (F1 generation of HTLV-I pX transgenic F344 and wild-type Wistar) rats, respectively. Peripheral blood cells were stained with FITC-conjugated anti-CD3 (G4.18), FITC- or PE-conjugated anti-CD4 (OX-35), and PE-conjugated anti-CD8 (OX-8) Abs, followed by depletion of erythrocytes. At first, the cells were divided based on the forward (FSC) and side scatter (SSC) patterns. Then, PBMCs in region 1 (R1) were gated to analyze the expression of CD3, CD4, and CD8. In both groups, at least 3 rats were examined. Representative data are shown. The numbers in each panel represent the percentage of CD4+ T cells, CD8+ T cells, and CD4+/CD8+ cells, respectively.
Expansion of CD4+/CD8+ cells in the peripheral blood of FW-pX rats. The top and bottom panels show the results of FCM analyses of peripheral blood from 6-week-old FW-wt (F1 generation of wild-type F344 and Wistar) and FW-pX (F1 generation of HTLV-I pX transgenic F344 and wild-type Wistar) rats, respectively. Peripheral blood cells were stained with FITC-conjugated anti-CD3 (G4.18), FITC- or PE-conjugated anti-CD4 (OX-35), and PE-conjugated anti-CD8 (OX-8) Abs, followed by depletion of erythrocytes. At first, the cells were divided based on the forward (FSC) and side scatter (SSC) patterns. Then, PBMCs in region 1 (R1) were gated to analyze the expression of CD3, CD4, and CD8. In both groups, at least 3 rats were examined. Representative data are shown. The numbers in each panel represent the percentage of CD4+ T cells, CD8+ T cells, and CD4+/CD8+ cells, respectively.
Characterization of CD4+/CD8+ cells in the peripheral blood of FW-pX rats. Peripheral blood from 6-week-old FW-pX rats was used. In each experiment, at least 3 rats were used. Representative data are shown. (A) Peripheral blood cells were stained with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 (OX-8) Abs, followed by depletion of erythrocytes. CD4/CD8 DP cells were gated to confirm that these cells were mononuclear cells. WBCs indicates white blood cells. (B) Peripheral blood cells were stained with FITC- or PE-conjugated anti-CD4 (OX-35), PerCP-conjugated anti-CD8 (OX-8), and FITC- or PE-conjugated anti-CD3 (G4.18), CD11b/c (OX-42), or NKR-P1A Ab (10/78), followed by depletion of erythrocytes. Painted histograms represent the expression of CD3, CD11b/c, and NKR-P1A on CD4+/CD8+ cells. Gray histograms represent the expression of these molecules on total PBMCs. (C) Mononuclear cells separated from the spleen of FW-pX rats were cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes and then stained for CD68 (ED-1, green), CD4 (OX-35, red), and CD8 (OX-8, blue). The merged image shows the cell stained with 3 colors (total magnification: × 600). (D) Peripheral blood cells were stained with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 Abs for the α-chain hinge region (OX-8), α-chain Ig V-like region (G28), or β-chain (341) followed by depletion of erythrocytes. Histograms represent reactivity with the anti-CD8 Abs gated on CD4medium cells.
Characterization of CD4+/CD8+ cells in the peripheral blood of FW-pX rats. Peripheral blood from 6-week-old FW-pX rats was used. In each experiment, at least 3 rats were used. Representative data are shown. (A) Peripheral blood cells were stained with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 (OX-8) Abs, followed by depletion of erythrocytes. CD4/CD8 DP cells were gated to confirm that these cells were mononuclear cells. WBCs indicates white blood cells. (B) Peripheral blood cells were stained with FITC- or PE-conjugated anti-CD4 (OX-35), PerCP-conjugated anti-CD8 (OX-8), and FITC- or PE-conjugated anti-CD3 (G4.18), CD11b/c (OX-42), or NKR-P1A Ab (10/78), followed by depletion of erythrocytes. Painted histograms represent the expression of CD3, CD11b/c, and NKR-P1A on CD4+/CD8+ cells. Gray histograms represent the expression of these molecules on total PBMCs. (C) Mononuclear cells separated from the spleen of FW-pX rats were cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes and then stained for CD68 (ED-1, green), CD4 (OX-35, red), and CD8 (OX-8, blue). The merged image shows the cell stained with 3 colors (total magnification: × 600). (D) Peripheral blood cells were stained with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 Abs for the α-chain hinge region (OX-8), α-chain Ig V-like region (G28), or β-chain (341) followed by depletion of erythrocytes. Histograms represent reactivity with the anti-CD8 Abs gated on CD4medium cells.
The majority of CD8 molecules are heterodimers composed of α- and β-chains. On the other hand, a subset of T cells and most NK cells are known to express CD8 as homodimers of α-chains.30 Hirji et al13 showed that rat alveolar and peritoneal macrophages express CD8 as αβ heterodimers but these CD8 molecules do not react with the anti-CD8 α-chain Ig V-like region Ab (G28). Since the anti-CD8 α-chain hinge region Ab (OX-8) can recognize macrophage CD8, these authors suggested that the Ig V-like region of the CD8 α-chain was masked or modified on rat alveolar and peritoneal macrophages. To analyze the subunit organization of CD8 molecules expressed on the DP monocytes, we performed 2-color FCM analysis using the anti-CD4 (OX-35) and 3 kinds of anti-CD8 Abs, including OX-8, G28, and anti-β-chain Abs (341). Histograms were obtained by gating of CD4medium cells in PBMCs isolated from 6-week-old FW-pX rats (Figure 2D). The percentage of cells reactive with OX-8 (74.9%) was comparable to that stained with G28 (73.5%). The CD8 β-chain was expressed in 64.7% of CD4medium cells. Thus, CD8 molecules expressed on the surface of DP monocytes in FW-pX rats are heterodimers composed of the β-chain and the α-chain with the conserved Ig V-like region.
Induction of DP monocytes in nontransgenic FW-wt rats
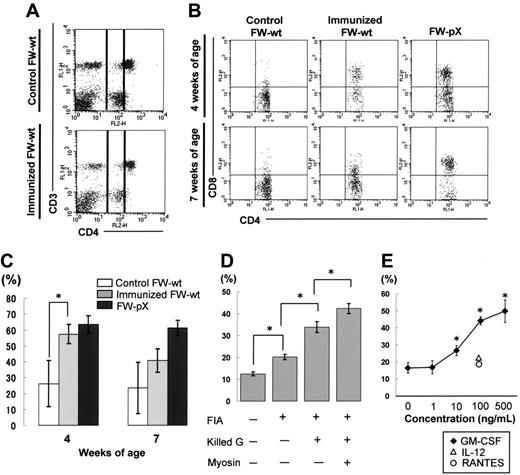
To examine whether DP monocytes are induced exclusively in FW-pX rats carrying the HTLV-I pX gene or also in other inflammatory situations unrelated to the transgene, we inoculated porcine myosin into the footpads of 3-week-old FW-wt rats along with the adjuvant containing killed tuberculosis germs. It is known that CD4+ T cells down-regulate their surface CD4 under certain activating conditions.31 However, we observed no significant down-regulation of CD4 molecules in T cells of our myosin-immunized rats (Figure 3A). The percentages of CD3+ T cells in CD4medium cells were 5.9% and 5.5% in the FW-wt rats with and without immunization, respectively. Thus, we concluded that CD4medium cells were monocytes. One week after immunization, the percentage of CD8+ population in CD4medium cells reached 57.3% ± 6.1% in myosin-immunized FW-wt rats, which was comparable to the proportion observed in 4-week-old FW-pX rats (63.5% ± 6.5%; Figure 3B-C). Four weeks after immunization, the percentage of CD8+ population in CD4medium cells declined to 40.6% ± 7.3% in myosin-immunized FW-wt rats, whereas that in 7-week-old FW-pX rats was maintained at a high level (61.4% ± 4.8%). In 4- and 7-week-old FW-wt rats without immunization, the size of CD8+ population in CD4medium cells was smaller (26.1% ± 14.6% and 23.5% ± 16.3%, respectively). These findings indicate that DP monocytes can be induced in rats without any influence of the pX transgene and suggest that they may be induced in an acute inflammatory phase.
Induction of CD4/CD8 DP monocytes in nontransgenic rats. (A) Myocarditis was induced in FW-wt rats by immunization with porcine cardiac myosin and the adjuvant containing killed tuberculosis germs at 3 weeks of age (immunized FW-wt). Peripheral blood was obtained from the FW-wt rats one week after immunization and age-matched controls and then PBMCs in region 1 (R1) were gated as in Figure 1. In both types of rats, the majority of CD4medium cells did not express CD3, thus they were considered to be monocytes. In each group, at least 3 rats were examined. Representative data are shown. (B) The expression of CD8 on peripheral monocytes (practically CD4medium cells) was examined 1 week and 4 weeks after immunization (4 and 7 weeks of age, respectively). Data were compared with those of nonimmunized FW-wt (control FW-wt) and FW-pX rats. In each group, at least 3 rats were examined. Representative data are shown. (C) The percentage of CD8+ cells in monocytes 1 week and 4 weeks after immunization (4 and 7 weeks of age, respectively) is shown as mean ± SD. (D) Inbred F344 rats (3 weeks of age) were immunized with various combinations of components used for the induction of myosin-induced myocarditis. The percentage of CD8+ cells in monocytes was examined 1 week after immunization. In each experiment, at least 3 rats were used. Data are represented as mean ± SD. FIA indicates Freund incomplete adjuvant; Killed G, killed tuberculosis germs. (E) PB-MCs from F344 rats (3 weeks old) were incubated with IL-12, RANTES, or GM-CSF under indicated concentrations. We chose these cytokines because they are known to be induced by BCG.31-33 Twenty-four hours later, the percentage of CD8+ cells in monocytes (practically CD4medium cells) was examined. Data are represented as mean ± SD of repeated experiments done in triplicate. *P < .05.
Induction of CD4/CD8 DP monocytes in nontransgenic rats. (A) Myocarditis was induced in FW-wt rats by immunization with porcine cardiac myosin and the adjuvant containing killed tuberculosis germs at 3 weeks of age (immunized FW-wt). Peripheral blood was obtained from the FW-wt rats one week after immunization and age-matched controls and then PBMCs in region 1 (R1) were gated as in Figure 1. In both types of rats, the majority of CD4medium cells did not express CD3, thus they were considered to be monocytes. In each group, at least 3 rats were examined. Representative data are shown. (B) The expression of CD8 on peripheral monocytes (practically CD4medium cells) was examined 1 week and 4 weeks after immunization (4 and 7 weeks of age, respectively). Data were compared with those of nonimmunized FW-wt (control FW-wt) and FW-pX rats. In each group, at least 3 rats were examined. Representative data are shown. (C) The percentage of CD8+ cells in monocytes 1 week and 4 weeks after immunization (4 and 7 weeks of age, respectively) is shown as mean ± SD. (D) Inbred F344 rats (3 weeks of age) were immunized with various combinations of components used for the induction of myosin-induced myocarditis. The percentage of CD8+ cells in monocytes was examined 1 week after immunization. In each experiment, at least 3 rats were used. Data are represented as mean ± SD. FIA indicates Freund incomplete adjuvant; Killed G, killed tuberculosis germs. (E) PB-MCs from F344 rats (3 weeks old) were incubated with IL-12, RANTES, or GM-CSF under indicated concentrations. We chose these cytokines because they are known to be induced by BCG.31-33 Twenty-four hours later, the percentage of CD8+ cells in monocytes (practically CD4medium cells) was examined. Data are represented as mean ± SD of repeated experiments done in triplicate. *P < .05.
To investigate the induction mechanisms of DP monocytes, we next immunized inbred F344 rats with various combinations of individual components used for initial immunization. When rats were immunized with the adjuvant containing killed tuberculosis germs, the percentage of CD8+ population in CD4medium cells was increased in the largest scale, though significant differences were observed by addition of any of the components (incomplete adjuvant only, an increase of 7.7%; adjuvant plus killed tuberculosis germs, an increase of 21.4%; myosin plus adjuvant plus killed tuberculosis germs, an increase of 29.9%; Figure 3D). This suggested that immunization with tuberculosis germs might be critical for the induction of DP monocytes. To test this hypothesis, we examined whether the cytokines and chemokines known to be induced by the recombinant Mycobacterium bovis bacillus Calmette-Guerin (BCG)32-34 can induce DP monocytes in vitro. PBMCs from F344 rats were stimulated with IL-12, RANTES, or GM-CSF for 24 hours and then the percentage of DP monocytes was measured. Among these cytokines/chemokines, only GM-CSF could induce DP monocytes in a dose-dependent manner (Figure 3E). We also observed that the percentage of DP monocytes (CD4medium CD8+ population in PBMCs) was increased from 7.8% to 11.0% by in vivo administration of GM-CSF. However, stimulation of bone marrow cells by GM-CSF failed to induce any significant expansion of CD4+/CD8+ cells (data not shown).
Infiltration of CD4+/CD8+ cells at the site of myocarditis
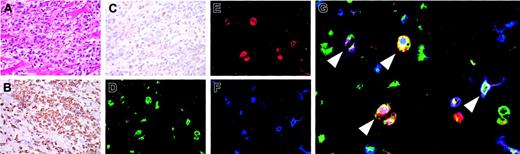
In myosin-immunized FW-wt rats, myocarditis occurred 3 to 4 weeks after immunization (Figure 4A). At the site of inflammation, macrophages (reactive with ED-1, Figure 4B) infiltrated more abundantly than CD3+ T cells (Figure 4C). To determine if infiltrating cells contained DP macrophages, adherent cells were isolated from collagenase-digested cardiac tissues and subjected to immunocytochemical analysis. Half of macrophages (reactive with ED-1) expressed both CD4 and CD8 (Figure 4D-G). These DP macrophages were not reactive with ED-2 (a marker for tissue-resident macrophages25 ) or OX-62 (a marker for DCs; data not shown). Thus, infiltrating DP macrophages are neither tissue-resident macrophages nor DCs but are derived from the blood.
Infiltration of DP macrophages at the site of myocarditis. Myocarditis was induced in FW-wt rats by immunization with porcine myosin and the adjuvant containing killed tuberculosis germs at 3 weeks of age. Three weeks later, the heart was extirpated and used for histologic and immunocytochemical examinations. Experiments were repeated twice. Representative results are shown. (A) Hematoxylin and eosin staining. (B-C) Immunohistochemical staining for CD68 (ED-1) and CD3 (IF4), respectively. The cardiac tissues were cut into small pieces and digested with 0.16% collagenase type II. After removal of tissue fragments, cell suspension was incubated in a plastic dish at 37°C. One hour later, adherent cells were harvested and immunofluorescent triple staining was done for CD68 (ED-1, green; D), CD4 (OX-35, red; E), and CD8 (OX-8, blue; F). (G) The merged image. Arrowheads indicate DP macrophages also stained for CD68. Total magnification: × 40 (A-C), × 80 (D-F), and × 200 (G).
Infiltration of DP macrophages at the site of myocarditis. Myocarditis was induced in FW-wt rats by immunization with porcine myosin and the adjuvant containing killed tuberculosis germs at 3 weeks of age. Three weeks later, the heart was extirpated and used for histologic and immunocytochemical examinations. Experiments were repeated twice. Representative results are shown. (A) Hematoxylin and eosin staining. (B-C) Immunohistochemical staining for CD68 (ED-1) and CD3 (IF4), respectively. The cardiac tissues were cut into small pieces and digested with 0.16% collagenase type II. After removal of tissue fragments, cell suspension was incubated in a plastic dish at 37°C. One hour later, adherent cells were harvested and immunofluorescent triple staining was done for CD68 (ED-1, green; D), CD4 (OX-35, red; E), and CD8 (OX-8, blue; F). (G) The merged image. Arrowheads indicate DP macrophages also stained for CD68. Total magnification: × 40 (A-C), × 80 (D-F), and × 200 (G).
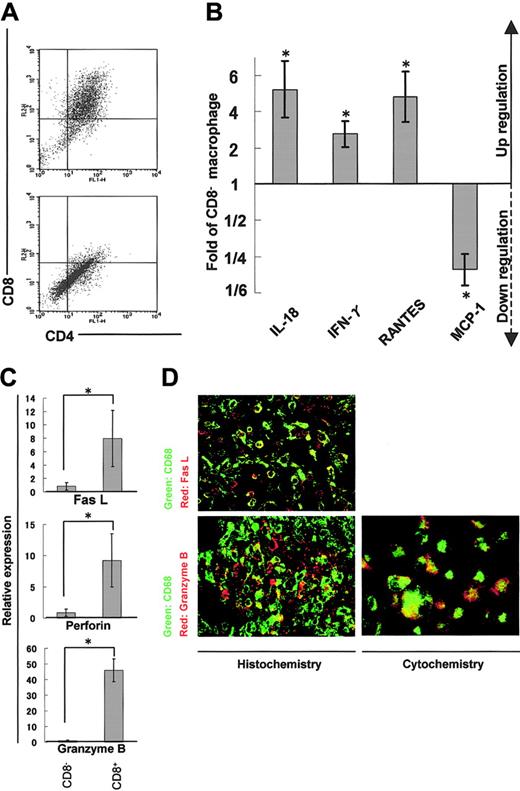
Cytokine/chemokine profiles and the cytotoxic phenotype of CD4/CD8 DP macrophages
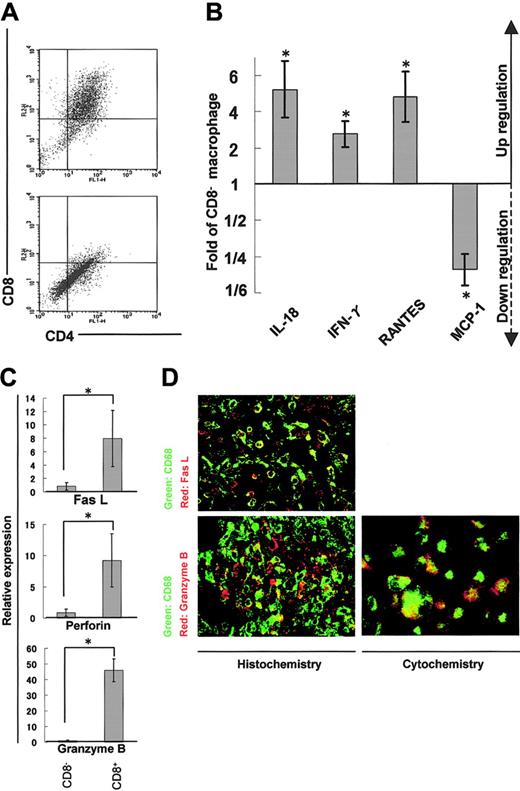
We isolated adherent cells from rat cardiac tissues affected with myocarditis and separated DP macrophages from other, practically CD8- macrophages (hereafter referred to as CD8- macrophages) using the MACS system (Figure 5A). We then compared expression profiles of cytokines, chemokines, and cytotoxic factors by quantitative real-time RT-PCR in DP and CD8- macrophages. DP macrophages showed higher expression of IL-18 (5.2-fold), IFN-γ (2.8-fold), and RANTES (4.8-fold) in comparison with CD8- macrophages (Figure 5B). By contrast, the expression of MCP-1 was lower in DP macrophages than in CD8- macrophages (1/4.7-fold). There was no significant difference in the expression of IL-4, IL-12, monocyte-derived chemokine (MDC), or TGF-β, or TNF-α between DP and CD8- macrophages (data not shown). When we focused on cytotoxic factors, DP macrophages showed significantly higher expression of Fas L (8.0-fold), perforin (9.2-fold), and granzyme B (45.9-fold) compared with CD8- macrophages (Figure 5C). Expression of iNOS did not show a significant difference between DP and CD8- macrophages (data not shown). When we examined the expression of Fas L and granzyme B in tissue-infiltrating macrophages by immunohistochemistry, colocalization of these molecules and ED-1 (CD68) was observed (Figure 5D left panels). To rule out the possibility that ED-1-positive (CD68+) cells are overlaid with scattered, secreted granzyme B, we examined the expression of granzyme B in macrophages isolated from the cardiac tissue (Figure 5D right panel). These experiments confirmed that part of ED-1-positive (CD68+) macrophages did express Fas L and granzyme B.
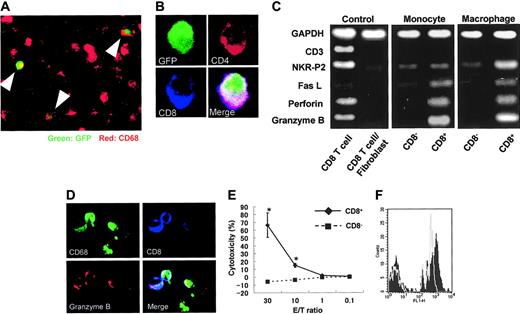
DP monocytes are precursors of DP macrophages
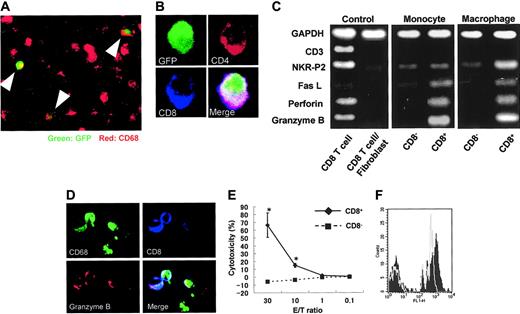
To determine if DP monocytes in the blood migrated into sites of inflammation and differentiated to DP macrophages, transfer of GFP-positive spleen cells was made into nontransgenic rats immunized with myosin. GFP-positive macrophages were found in cardiac tissues with myocarditis (Figure 6A), and some GFP-positive macrophages expressed both CD4 and CD8 (Figure 6B). Furthermore, when the profiles of DP monocytes and DP macrophages were compared by RT-PCR, the expression patterns of Fas L, perforin, and granzyme B were similar (Figure 6C). On the other hand, the expression of NKR-P2 (rat orthologue of human NKG2D35 ), known to play an important role in killing by NK cells and cytotoxic T lymphocytes (CTLs),36,37 was higher only in DP macrophages. These findings suggest that DP monocytes are precursors of tissue-infiltrating DP macrophages with a cytotoxic phenotype. In our experiments, evaluation of contaminated CD8+ CTLs was critical. As previously described, the purity of macrophages in the cells recovered from the cardiac tissues with myocarditis was 94% (see “Isolation of macrophages from cardiac tissues, with myocarditis”). We therefore used a mixed cDNA sample (a mixture of CD8+ T cells and fibroblasts in a ratio of 1:9) as a negative control. In this negative control, the expression of CD3, NKR-P2, Fas L, perforin, and granzyme B was hardly detectable, whereas the expression level of the Gapdh gene was comparable to that of other samples. Although these experiments do not provide information as to the identity of CD4+/CD8+ DP cells or assure the absence of contamination of T cells in the samples, they indicate that NKR-P2, Fas L, perforin, and granzyme B are produced by DP monocytes/macrophages. Moreover, immunocytochemistry demonstrated the existence of granzyme B-containing granules in CD8+ CD68+ adherent splenocytes regarded as DP monocytes (Figure 6D).
Expression profiles of cytokines/chemokines and cytotoxic factors in DP macrophages. Infiltrating macrophages were isolated from the cardiac tissues by collagenase digestion followed by adhesion to the plastic dish. DP macrophages were separated from other macrophages by MACS based on the presence or absence of CD8. Prior to the MACS sorting, we confirmed by light microscopy that macrophages detached from the plastic dish were in a single-cell suspension (data not shown). (A) Macrophages collected from the cardiac tissues were reacted with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 (OX-8) Abs. MACS was conducted using anti-PE microbeads. The cells selected positively and negatively are shown in the top and bottom panels, respectively. Experiments were repeated at least twice, and representative results are shown. (B) The expression of cytokines/chemokines (IL-18, IFN-γ, RANTES, and MCP-1) in DP macrophages was analyzed by quantitative real-time RT-PCR. The data were compared with those of CD8- macrophages. Results are represented as a fold (mean ± SD of repeated experiments done in triplicate) against control macrophages. (C) The expression patterns of cytotoxic factors (Fas L, perforin, and granzyme B) in DP macrophages (right columns) were compared with those in CD8- macrophages (left columns). Data are represented as mean ± SD of repeated experiments done in triplicate. (D) Immunofluorescent double staining for CD68 (ED-1, green) and Fas L (N-20, red), or CD68 (ED-1, green) and granzyme B (N-19, red) in the cardiac tissue section (left panels). Infiltrating macrophages isolated from the tissues were stained for CD68 (ED-1, green) and granzyme B (N-19, red; right panel). Total magnification: × 80. *P < .05.
Expression profiles of cytokines/chemokines and cytotoxic factors in DP macrophages. Infiltrating macrophages were isolated from the cardiac tissues by collagenase digestion followed by adhesion to the plastic dish. DP macrophages were separated from other macrophages by MACS based on the presence or absence of CD8. Prior to the MACS sorting, we confirmed by light microscopy that macrophages detached from the plastic dish were in a single-cell suspension (data not shown). (A) Macrophages collected from the cardiac tissues were reacted with FITC-conjugated anti-CD4 (OX-35) and PE-conjugated anti-CD8 (OX-8) Abs. MACS was conducted using anti-PE microbeads. The cells selected positively and negatively are shown in the top and bottom panels, respectively. Experiments were repeated at least twice, and representative results are shown. (B) The expression of cytokines/chemokines (IL-18, IFN-γ, RANTES, and MCP-1) in DP macrophages was analyzed by quantitative real-time RT-PCR. The data were compared with those of CD8- macrophages. Results are represented as a fold (mean ± SD of repeated experiments done in triplicate) against control macrophages. (C) The expression patterns of cytotoxic factors (Fas L, perforin, and granzyme B) in DP macrophages (right columns) were compared with those in CD8- macrophages (left columns). Data are represented as mean ± SD of repeated experiments done in triplicate. (D) Immunofluorescent double staining for CD68 (ED-1, green) and Fas L (N-20, red), or CD68 (ED-1, green) and granzyme B (N-19, red) in the cardiac tissue section (left panels). Infiltrating macrophages isolated from the tissues were stained for CD68 (ED-1, green) and granzyme B (N-19, red; right panel). Total magnification: × 80. *P < .05.
Origin of CD4/CD8 DP macrophages and function of CD4/CD8 DP monocytes. (A) GFP-positive spleen cells were transferred into GFP-negative recipients that had been immunized with porcine myosin. EGFP transgenic rats and nontransgenic Wistar rats (all rats were 4 weeks old) were immunized with myosin as described in “Immunization of rats with porcine heart myocin and induction of myocarditis.” Mononuclear cells were isolated from the spleen of EGFP transgenic rats 1 week after immunization and transferred into Wistar rats intravenously 2 weeks after immunization (1 × 107 cells per animal). Five days later, the hearts of recipients were extirpated, and then tissue-infiltrating macrophages were isolated and used. Experiments were repeated twice, and representative results are shown. Arrowheads indicate cells expressing both GFP and CD68 (ED-1, red). Total magnification: × 100. (B) The cells isolated from the cardiac tissues were cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes and then stained for CD4 (OX-35, red) and CD8 (OX-8, blue). The merged image shows that the cell expressing both CD4 and CD8 is also positive for GFP. Total magnification: × 600. (C) FW-wt rats were immunized with myosin and the adjuvant containing killed tuberculosis germs. Mononuclear cells separated from the spleen or cardiac tissues 1 week or 3 weeks after immunization, respectively, were cultured in plastic dishes at 37°C for 1 hour, and then the adherent cells were divided into CD8- and CD8+ populations, using the MACS system. Expression profiles of CD3, NKR-P2, Fas L, perforin, and granzyme B were compared by RT-PCR. The cDNA from CD8+ T cells served as a positive control. The negative control was the cDNA derived from the 1:9 mixture of CD8+ T cells and fibroblasts. (D) Six-week-old Wistar rats were immunized with adjuvants containing killed tuberculosis germs. One week later, mononuclear cells were separated from the spleen and then cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes, followed by staining for CD68 (ED-1, green), granzyme B (red), and CD8 (OX-8, blue). The merged image shows the cells stained with 3 colors. Total magnification: × 200. (E) Cytotoxic assay in vitro. Six-week-old Wistar rats were immunized with adjuvants containing killed tuberculosis germs. One week later, mononuclear cells were separated from the spleen and incubated in plastic dishes for 20 minutes at 37°C. Resultant adherent cells were collected and divided into CD8- and CD8+ cells using the MACS system. These cells were added to the culture of allogenic epithelial thymoma cells with E/T ratios of 30, 10, 1, and 0.1 (4 × 104 target cells per well in 24-well plates). After incubation for 18 hours, cytotoxicity was measured using the CytoTox 96 test kit. Data are represented as mean ± SD of experiments done in triplicate. *P < .05. (F) Phagocytosis assay. Yellow-green carboxylate-modified 1.0 μm latex beads were mixed with peripheral blood from Wistar rats that had been immunized with adjuvants containing killed tuberculosis germs one week before (1.5 × 107 beads/300 μL blood). After incubation for 2 hours at 37°C, PE-conjugated anti-CD4 (OX-35) and PerCP-conjugated anti-CD8 (OX-8) Abs were added to the mixture, followed by depletion of erythrocytes. After 3 times wash with cold PBS, CD4+/CD8+ cells were gated to determine uptake of the fluorescence-labeled beads using FACScan. Experiments were done in triplicate. Representative results are shown. The filled and gray histograms represent the profiles of CD4+/CD8+ and CD4+/CD8- monocytes, respectively.
Origin of CD4/CD8 DP macrophages and function of CD4/CD8 DP monocytes. (A) GFP-positive spleen cells were transferred into GFP-negative recipients that had been immunized with porcine myosin. EGFP transgenic rats and nontransgenic Wistar rats (all rats were 4 weeks old) were immunized with myosin as described in “Immunization of rats with porcine heart myocin and induction of myocarditis.” Mononuclear cells were isolated from the spleen of EGFP transgenic rats 1 week after immunization and transferred into Wistar rats intravenously 2 weeks after immunization (1 × 107 cells per animal). Five days later, the hearts of recipients were extirpated, and then tissue-infiltrating macrophages were isolated and used. Experiments were repeated twice, and representative results are shown. Arrowheads indicate cells expressing both GFP and CD68 (ED-1, red). Total magnification: × 100. (B) The cells isolated from the cardiac tissues were cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes and then stained for CD4 (OX-35, red) and CD8 (OX-8, blue). The merged image shows that the cell expressing both CD4 and CD8 is also positive for GFP. Total magnification: × 600. (C) FW-wt rats were immunized with myosin and the adjuvant containing killed tuberculosis germs. Mononuclear cells separated from the spleen or cardiac tissues 1 week or 3 weeks after immunization, respectively, were cultured in plastic dishes at 37°C for 1 hour, and then the adherent cells were divided into CD8- and CD8+ populations, using the MACS system. Expression profiles of CD3, NKR-P2, Fas L, perforin, and granzyme B were compared by RT-PCR. The cDNA from CD8+ T cells served as a positive control. The negative control was the cDNA derived from the 1:9 mixture of CD8+ T cells and fibroblasts. (D) Six-week-old Wistar rats were immunized with adjuvants containing killed tuberculosis germs. One week later, mononuclear cells were separated from the spleen and then cultured in chamber slides at 37°C for 1 hour. Resultant adherent cells were fixed using cold acetone for 5 minutes, followed by staining for CD68 (ED-1, green), granzyme B (red), and CD8 (OX-8, blue). The merged image shows the cells stained with 3 colors. Total magnification: × 200. (E) Cytotoxic assay in vitro. Six-week-old Wistar rats were immunized with adjuvants containing killed tuberculosis germs. One week later, mononuclear cells were separated from the spleen and incubated in plastic dishes for 20 minutes at 37°C. Resultant adherent cells were collected and divided into CD8- and CD8+ cells using the MACS system. These cells were added to the culture of allogenic epithelial thymoma cells with E/T ratios of 30, 10, 1, and 0.1 (4 × 104 target cells per well in 24-well plates). After incubation for 18 hours, cytotoxicity was measured using the CytoTox 96 test kit. Data are represented as mean ± SD of experiments done in triplicate. *P < .05. (F) Phagocytosis assay. Yellow-green carboxylate-modified 1.0 μm latex beads were mixed with peripheral blood from Wistar rats that had been immunized with adjuvants containing killed tuberculosis germs one week before (1.5 × 107 beads/300 μL blood). After incubation for 2 hours at 37°C, PE-conjugated anti-CD4 (OX-35) and PerCP-conjugated anti-CD8 (OX-8) Abs were added to the mixture, followed by depletion of erythrocytes. After 3 times wash with cold PBS, CD4+/CD8+ cells were gated to determine uptake of the fluorescence-labeled beads using FACScan. Experiments were done in triplicate. Representative results are shown. The filled and gray histograms represent the profiles of CD4+/CD8+ and CD4+/CD8- monocytes, respectively.
Cytotoxic function of DP monocytes
In order to evaluate the function of DP monocytes, we carried out in vitro cytotoxic assays against allogenic tumor cells. As a source of DP monocytes, we used CD8+ adherent splenocytes obtained from Wistar rats that had been immunized with adjuvants containing killed tuberculosis germs. These cells effectively killed epithelial thymoma cells originated from F344 rats carrying the HTLV-I pX transgene19 in a dose-dependent manner (Figure 6E). When the E/T ratio was 30, percent specific lysis was 70.8 ± 6.8. By contrast, CD8- monocytes hardly killed the tumor cells. These findings clearly indicate that DP monocytes possess a cytotoxic function and that these cells can kill tumor cells without major histocompatibility complex (MHC) restriction.
Phagocytic ability of DP monocytes
To determine the phagocytic ability of DP monocytes, yellow-green carboxylate-modified 1.0-μm latex beads were mixed with peripheral blood from Wistar rats that had been immunized with adjuvants containing killed tuberculosis germs one week before (1.5 × 107 beads/300 μL blood). After incubation for 2 hours at 37°C, uptake of fluorescence-labeled beads in DP monocytes was assayed using FACScan (Figure 6F). The black histogram indicates that the majority of DP monocytes (71.1% ± 4.4%) engulfed the beads during the experimental period. Phagocytic efficiency of DP monocytes was almost equivalent to that of CD4+/CD8- monocytes (67.4% ± 8.8%; Figure 6F gray histogram).
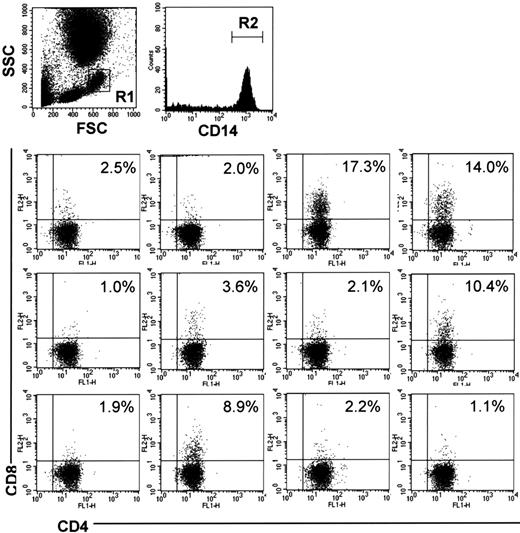
DP monocytes in the human peripheral blood
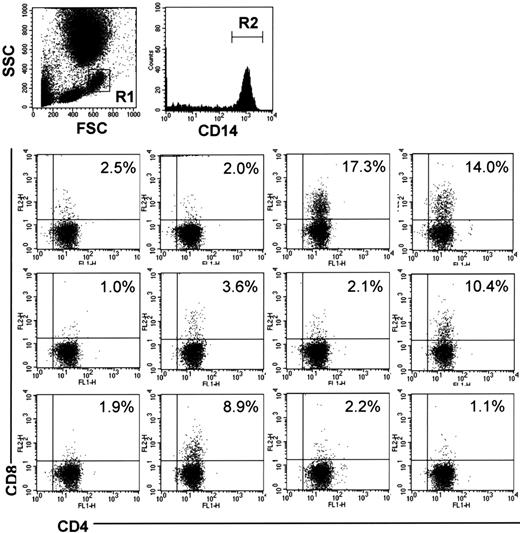
To examine whether humans also have DP monocytes, we analyzed PBMCs from 12 healthy volunteers by 3-color FCM. CD4+/CD8+ cells were identified in CD14+ monocytes in all samples examined (Figure 7). The percentage of CD4+/CD8+ cells in CD14+ monocytes showed considerable individual variations ranging from 17.3% to 1.0%.
CD4/CD8 DP monocytes in human peripheral blood. Human peripheral blood was obtained from 12 healthy volunteers. The cells were stained with FITC-conjugated anti-CD4 (M-T466), PE-conjugated anti-CD8 (HIT8a), and PerCP-conjugated anti-CD14 (MφP9) Abs, followed by depletion of erythrocytes, and then monocytes in region 1 (R1) were gated. The bottom panels show expression of CD4 and CD8 on CD14+ cells in region 2 (R2). The percentage of CD4+/CD8+ cells in CD14+ monocytes is shown in each panel.
CD4/CD8 DP monocytes in human peripheral blood. Human peripheral blood was obtained from 12 healthy volunteers. The cells were stained with FITC-conjugated anti-CD4 (M-T466), PE-conjugated anti-CD8 (HIT8a), and PerCP-conjugated anti-CD14 (MφP9) Abs, followed by depletion of erythrocytes, and then monocytes in region 1 (R1) were gated. The bottom panels show expression of CD4 and CD8 on CD14+ cells in region 2 (R2). The percentage of CD4+/CD8+ cells in CD14+ monocytes is shown in each panel.
Discussion
In the present study, we have identified a population of monocytes/macrophages characterized by coexpression of CD4 and CD8. This population was originally identified in FW-pX rats carrying the HTLV-I pX transgene (Figures 1, 2) but later found to be present in wild-type rats (Figure 3). The number of DP monocytes showed a dramatic increase in rats with myosin-induced myocarditis (Figure 3), and the DP macrophages were predominant infiltrating cells at the cardiac lesion (Figure 4). The most notable feature characterizing this population of macrophages is that they express high levels of Fas L, perforin, granzyme B (Figure 5C-D), and NKR-P2 (Figure 6C). In particular, granzyme B was expressed at an extremely high level (45.9-fold) in DP macrophages compared with CD8- macrophages (Figure 5C). NKG2D is the receptor previously shown to be expressed on NK cells, CTLs, and activated macrophages.38 It binds to stress-inducible MHC class I molecules, MICA/B, and ULBP/RAET1 in humans and RAE-1 (retinoic acid early inducible-1) in mice.36,37 NK cells and CTLs bind to the target cells through NKG2D and destroy them through coordinated actions of perforin and cytotoxic factors such as granzyme B. Thus, the collective evidence clearly indicates that tissue-infiltrating DP macrophages exhibit a cytotoxic phenotype. They may therefore contribute to tissue damage by adhering to target cells via their NKR-P2 and secreting perforin and granzyme B.
Another notable feature of CD4/CD8 DP macrophages is that they express IL-18, IFN-γ, and RANTES at higher levels and MCP-1 at a lower level than CD8- macrophages (Figure 5B). IL-18, IFN-γ, and RANTES induce the T-helper 1 (Th1)-type immune response,33,39,40 whereas MCP-1 induces the Th2-type immune response.41 Although we were unable to detect Cd3 mRNA by RT-PCR in our macrophage samples (Figure 6C), contamination of a small number of T/NK cells cannot be ruled out. Therefore, we should keep in mind the possibility that the cytokine production profiles (Figure 5B) may have been affected by contaminating T/NK cells; this reservation applies especially to IFN-γ, a cytokine typically produced by T/NK cells. However, we can say that tissue-infiltrating DP macrophages are prone to induce IL-18 and Th1-type immune responses at the site of inflammation. Okura et al42 reported that Th1 cytokines were the major cytokines detected in the early phase of myosin-induced experimental myocarditis in rats. Our present study indicates that DP macrophages infiltrating in the cardiac lesion may enhance the Th1-type immune response observed in the early phase of myosin-induced myocarditis.
The number of DP monocytes showed a dramatic increase by immunization with myosin (Figure 3B-C). When we examined which component in the immunogen was critical for increasing the population of DP monocytes, we found that the killed tuberculosis germs were the most effective factors (Figure 3D). BCG containing killed tuberculosis germs works synergistically with IL-18 for induction of IFN-γ and GM-CSF and induces the Th1-type immune response.33 GM-CSF increased the number of DP monocytes in a dose-dependent manner in vitro (Figure 3E). These findings suggested that the secretion of GM-CSF induced by immunization with the killed tuberculosis germs triggered the expansion of DP monocytes in peripheral blood. The kinetics of expansion suggest that an increase in the number of DP monocytes occurs in the early phase of inflammation.
To examine whether CD4+/CD8+ cells are derived from DP monocytes in blood or are generated in situ from resident macrophages, we transferred GFP-positive spleen cells into GFP-negative recipients that had been immunized with myosin in advance (Figure 6A-B). This adoptive transfer experiment clearly indicates that tissue-infiltrating DP macrophages are of hematogenous origin. Consistent with this observation, DP macrophages in situ did not express ED-2 (a marker for tissue-resident macrophages) or OX-62 (a marker for DCs). Thus, overall data indicate that certain stimuli that induce the release of GM-CSF trigger the expansion of DP monocytes in peripheral blood and that these cells migrate to the site of inflammation and differentiate into macrophages displaying the Th1-type immune response and a cytotoxic phenotype. In line with these findings, DP monocytes could kill allogenic tumor cells in vitro (Figure 6E). This killing is unlikely to be mediated by the CTLs contaminated in the effector cells because CTLs can kill only MHC-matched targets. In addition, we demonstrated that DP monocytes were equipped with phagocytic activities comparable to those of CD4+/CD8- monocytes (Figure 6F).
Interestingly, human peripheral blood also contains CD14+ monocytes expressing both CD4 and CD8 (Figure 7). In this regard, it is notable that DP macrophage/dendritic cells, which express Fas L more abundantly than other macrophages, have been identified in the thyroid glands of patients with autoimmune thyroid diseases.43 Although the information available on their surface markers and cytokine profiles precludes us from drawing any conclusions, it is possible that they are derived from the DP monocytes identified in this study.
All volunteers who participated in this study were healthy donors. None of the donors apparently suffered from inflammatory, autoimmune, or neoplastic disorders. It is of great interest to examine whether a population of DP monocytes is increased in blood under infectious or other disease conditions. Studies along this line are ongoing using clinical samples. Whereas rat DP monocytes displayed cytotoxicity against allogenic tumor cells (Figure 6E), we have thus far been unable to demonstrate cytotoxic activities for human DP monocytes. This may be related to the fact that human DP monocytes were isolated from healthy volunteers, whereas rat DP monocytes were isolated from animals whose immune systems were activated by the transgene or artificial immunization. Studies are in progress to understand whether the rat and human DP monocytes/macrophages have any specialized roles in host defense against infection or cancer and in the pathogenesis of autoimmune disorders.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-06-2345.
Supported by grants from the Ministries of Education, Culture, Sports, Science, and Technology and of Health, Labor, and Welfare, of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ken-ichi Nakase, Chisato Sudo, and Masayo Tateyama for technical assistance.