Major histocompatibility complex (MHC) molecules carrying selected peptides will bind specifically to their cognate T-cell receptor on individual clones of reactive T cells. Fluorescently labeled, tetrameric MHC-peptide complexes have been widely used to detect and quantitate antigen-specific T-cell populations via flow cytometry. We hypothesized that such MHC-peptide tetramers could also be used to selectively deplete unique reactive T-cell populations, while leaving the remaining T-cell repertoire and immune response intact. In this report, we successfully demonstrate that a tetramer-based depletion of T cells can be achieved in a murine model of allogeneic bone marrow transplantation. Depletion of a specific alloreactive population of donor splenocytes (< 0.5% of CD8+ T cells) prior to transplantation significantly decreased morbidity and mortality from graft-versus-host disease. There was no early regrowth of the antigen-specific T cells in the recipient and in vivo T-cell proliferation was greatly reduced as well. Survival was increased more than 3-fold over controls, yet the inherent antitumor activity of the transplant was retained. This method also provides the proof-of-concept for similar strategies to selectively remove other unwanted T-cell clones, which could result in novel therapies for certain autoimmune disorders, T-cell malignancies, and solid organ graft rejection.
Introduction
T cells mediate a specific adaptive immune response by recognition of targets via their T-cell receptor (TCR). Antigen specificity is achieved because the unique TCR of each T-cell clone interacts with a specific peptide antigen presented in the context of a cell surface major histocompatibility complex (MHC) molecule. The specificity of this interaction has allowed the preparation of soluble, recombinant, multimerized MHC molecules (MHC tetramers) carrying selected peptides that will bind specifically to their cognate TCR on individual clones of reactive T cells. Fluorescently labeled MHC tetramers have been widely used to detect and quantitate antigen-specific T-cell populations via flow cytometry.1,2
Bone marrow transplantation (BMT) is currently used successfully in the treatment of many diseases, including leukemia, lymphoma, myelodysplasia, aplastic anemia, and severe combined immunodeficiency.3 During allogeneic BMT, donor T cells play a critical role in bone marrow engraftment, T-cell reconstitution (through homeostatic expansion), and the elimination of the residual tumor (ie, graft-versus-tumor [GVT]).4-6 However, these donor T cells also mediate graft-versus-host disease (GVHD),4 a systemic illness during which specific organs (ie, gut, skin, liver) are targeted by cytolytic T cells. GVHD is a major hurdle to a successful BMT, resulting in significant morbidity in most patients and early mortality in as many as 17% of recipients of an allograft from a matched unrelated donor.7 In a histocompatible matched allogeneic BMT setting, GVHD is mediated by differential expression of, or genetic polymorphisms in, minor histocompatability antigens (mHAgs) between donor and host.8 Current therapeutic approaches to GVHD include T-cell depletion of the allograft or a variety of nonspecific immunosuppressive strategies, which can cause prolonged immunosuppression, resulting in infection as well as relapse of neoplastic disease.9
In these studies, we propose that MHC-peptide tetramers can be used to selectively deplete antigen-specific T cells and their specific immunity. We chose as a model to test this hypothesis a murine model system of allogeneic BMT. We proposed to selectively deplete the small number of antigen-specific T-cell clones responsible for the deleterious GVHD by use of antigen-specific MHC tetramers prior to transplantation, thus allowing for adoptive transfer of the remaining CD4, CD8, natural killer (NK), and regulatory T-cell populations and their necessary immune functions. This, and similar approaches to antigen-specific T-cell depletion, might be applicable to clinical situations in which select T-cell clones can be implicated, such as T-cell malignancies, organ graft rejection, and several autoimmune diseases (such as type 1 diabetes).
Materials and methods
Cell lines and mice
F9 teratocarcinoma cells were obtained from ATCC (Manassas, VA); BCZ 108-2 T-cell hybridoma (LYL8 tetramer+) was kindly provided by Derry Roopenian (Jackson Laboratory, Bar Harbor, ME). Male BALB.B (H2b) and female C57BL/6 (B6, H2b) mice were obtained from the Jackson Laboratory. Mice were used for experiments between 8 and 14 weeks of age. All experiments with mice were performed in accordance with the guidelines of the American Association for the Advancement of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center (MSKCC).
BMT
BMTs were performed as previously described.10 BM cells were removed aseptically from femurs and tibias. Donor BM was T-cell depleted by incubation with anti-Thy-1.2 for 30 minutes at 4°C followed by incubation with low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, ON, Canada) for 1 hour at 37°C. Splenic T cells were obtained by purification over a nylon wool column followed by red blood cell removal with red blood cell lysis buffer (0.14 M NH4Cl, 17 mM Tris, pH 7.2) and in some cases were tetramer depleted by the described method. BM cells (5 × 106) and the indicated dose of CD3+ T cells (assessed by flow cytometry, routinely between 70% and 80%) were infused into the tail vein of lethally irradiated recipients. Prior to transplantation, recipients received on day -1 a lethal dose of 900 cGy to 950 cGy total body irradiation as a split dose, with 3 hours between doses.
Tumor induction and assessment of tumor death versus death from GVHD
Mice received 1 × 105 F9 cells intraperitoneally on day -1 of BMT after irradiation. Cause of death (tumor or GVHD) was determined by clinical evaluation and autopsy. Mice were checked daily for survival and evidence of GVHD or tumor growth. Additionally, each mouse was scored on a weekly basis for 5 clinical GVHD parameters (weight loss, activity, kyphosis, fur ruffling, and skin flaking), were scored 0 to 2 in each category, and killed once a score of 5 was attained.11 The tumor caused ascites and production of solid tumors (weighing up to 1 g) in the peritoneal cavity; additional metastases were located around the liver and in the spleen (confirmed with histopathology). The cause of death was determined based on the presence of signs and symptoms of GVHD and by postmortem autopsy. Death from tumor was defined as microscopic or macroscopic evidence of tumor at autopsy, as detailed in this paragraph.
Flow cytometry and tetramer depletion
Flow cytometric analysis of splenocytes was performed with fluorochrome-labeled monoclonal antibodies (mAbs; anti-CD8α, CALTAG, Burlingame, CA; anti-CD8α, anti-CD3ϵ, -NK1.1, -CD11b, GR-1, -CD19, and Vβ repertoire, Pharmingen, San Diego, CA), MHC-tetramers (Beckman Coulter, Fullerton, CA, and MSKCC, New York, NY) and 7-amino-actinomycin D (7-AAD; Becton Dickinson, San Jose, CA), acquired on a FACSCalibur (Becton Dickinson) or FC500 cytometer (Beckman Coulter), and analyzed with FlowJo software (Tree Star, Ashland, OR).
Splenocytes for tetramer depletion were purified over nylon wool, subjected to red blood cell lysis, and resuspended in phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA)/2 mM EDTA. Cells were incubated with phycoerythrin (PE)-labeled H2Kb tetramers (1 μg/106 cells-10 μg/106 cells), LTFNYRNL (LYL8), or nonspecific control SSIEFARL (HSV8), washed and incubated with anti-PE microbeads (Miltenyi Biotech, Auburn, CA). Cells were sorted with an autoMACS (Miltenyi Biotech) and the negative fraction (“tetramer depleted”) was collected.
Carboxyfluorescein succinimidyl ester (CFSE) labeling
Following tetramer depletion, cells were washed twice in PBS and resuspended in PBS at a concentration of 50 × 106 cells/mL. CFSE (Molecular Probes, Carlsbad, CA) was added to the cells (final concentration 2.5 μM) and incubated at 37°C for 20 minutes. The cells were spun without adding PBS, washed twice with PBS, and the cells were counted and resuspended at an appropriate concentration in PBS.
Immunization and IFNγ ELISPOT assay
BALB.B splenocytes for immunization were rbc lysed, irradiated (2000 cGy), washed, and resuspended in Dulbecco modified Eagle medium (DMEM). Cells (2 × 107) were injected intraveously into B6 mice. For interferon γ (IFNγ) enzyme-linked immunoSPOT (ELISPOT) assays, HA-Multiscreen plates (Millipore, Burlington, MA) were coated with 1 μg rat anti-mouse IFNγ antibody (clone AN18; Mabtech, Nacka Strand, Sweden) in PBS, incubated overnight at 4°C, washed with PBS, and blocked with RPMI/7.5% fetal calf serum (FCS) for 2 hours at 37°C. Purified CD8+ T cells were plated at 2 × 105 per well. Irradiated splenocytes from nonimmunized animals were used as antigen-presenting cells (APCs) and were plated at 2 × 104 cells per well. Test peptide (1 μg), positive control influenza matrix peptide, or irrelevant control peptides were added to the T-cell/APC mixture at a final volume of 100 μL/well to 200 μL/well. Control wells contain APCs with or without CD8+ cells. After incubation for 20 hours at 37°C, plates were extensively washed with PBS/0.05% Tween, and 0.1 μg/well biotinylated detection antibody against mouse IFNγ (clone R4-6A2; Mabtech) was added. Plates were incubated for an additional 2 hours at 37°C and spot development was performed as described.12 Spot numbers were automatically determined with the use of a computer-assisted video image analyzer with KS ELISPOT 4.0 software (Carl Zeiss Vision, Hamburg, Germany).
Mixed lymphocyte reaction (MLR)
B6 (H2b) T cells (1 × 105; depleted or nondepleted) were coincubated with 2 × 105 BALB.B (H2b) or BALB/c (H2d) irradiated splenocytes in quadruplicates in round-bottom 96-well plates. Cells were cultured in RPMI/10% FBS/50 μM 2-mercaptoethanol. On day 5, wells were pulsed with 500 nCi (18.5 kBq) 3H-thymidine for 20 hours. Plates were harvested with a Tomtec 96-well plate harvester (Tomtec, Hamden, CT) and read in Packard Top Count·NXT (Packard, Meriden, CT.
Statistics
For survival rates, groups were compared using log-rank statistics. For MLR, groups were compared using the rank sum statistic. For GVHD, mice were randomly assigned to treatment groups and were followed for changes in GVHD status over a period of 12 weeks. GVHD status was recorded weekly and a GVHD status of 5 indicates that the mouse had died. To compare the difference between groups with respect to GVHD status over time, a permutation test was used. The null hypothesis for this test is that the treatment had no differential effect on GVHD status. The statistic used to test this hypothesis was the sum of the absolute differences between the average GVHD score between groups over the k time points.
Results
GVHD model and tetramer specificity
We tested our hypothesis in a well-characterized murine MHC matched BMT model: C57BL/6 (B6) → BALB.B. These 2 strains are MHC compatible (H2b) and are disparate at more than 29 mHAgs.13 Friedman et al14 and Jones et al15 have explored the therapeutic potential of transplanting selective CD4+ Vβ family members in this model and have had success in altering the graft-versus-host response. Several studies suggest that an immunodominant mHAg, the H60 antigen that is expressed in BALB.B mice, is the primary target of donor B6 CD8+ T cells mediating GVHD after transplant.13,16,17 These studies, however, do not prove that H60 is necessary or sufficient for the generation of GVHD in this BMT model. The octamer peptide, LTFNYRNL (LYL8), is the naturally processed epitope of H60 that is presented by the MHC class I molecule and recognized by B6 CD8+ T cells.16,17
We investigated the incidence and severity of GVHD after lethally irradiated recipient mice (B6 or BALB.B) were transplanted with donor allogeneic (BALB.B or B6) T-cell-depleted (TCD) BM cells with or without the addition of splenic T cells.13,16,18 As expected, all BALB.B recipients of B6 TCD-BM plus splenic T cells rapidly died of severe acute GVHD, whereas recipients of B6 TCD-BM only exhibited 100% survival, showing that GVHD is T-cell mediated and that complete T-cell depletion eliminates GVHD. In contrast, B6 recipients did not develop GVHD upon receipt of BALB.B TCD-BM with or without the addition of BALB.B T cells. These data might be correlative and due to differences in the immune response status between B6 and BALB.B T cells. Therefore, we wanted further evidence that H60 was capable of eliciting a B6 T-cell response.
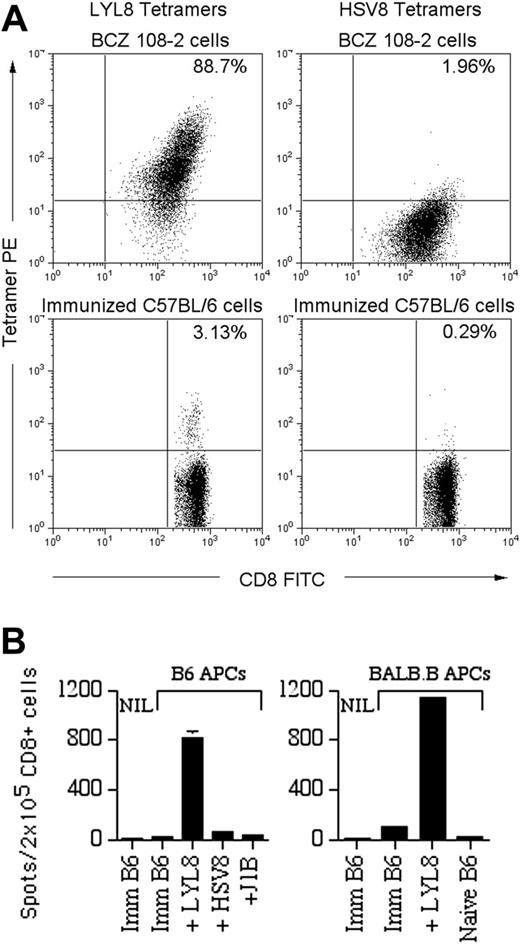
The B6 anti-BALB.B reaction was confirmed as T-cell mediated and BALB.B-mHAg specific using a series of immunologic assays. First, B6 anti-BALB.B T cells specific for the LYL8 H60 epitope were generated in vivo in B6 mice that were immunized with BALB.B splenocytes, as documented with PE-conjugated LYL8-H2Kb MHC tetramers (Figure 1A). Mixed lymphocyte reactions (MLRs) showed that immunized B6 T cells proliferated at a significantly greater rate when in the presence of irradiated allogeneic BALB.B splenocytes (P < .05 for effector-stimulator ratios between 0.2 and 2) as compared with syngeneic B6 splenocytes. Moreover, IFNγ ELISPOT assays,19,20 used to determine the specificity and level of B6 T-cell activation, confirmed that the LYL8 epitope stimulates B6 T cells (Figure 1B). In the presence of syngeneic and allogeneic APCs pulsed with LYL8 peptide, immunized B6 CD8+ T cells greatly increase their secretion of IFNγ, whereas control peptides including H2Kb binding peptide (HSV8) and H2Kd binding peptide (J1B), did not elicit a response. These data confirmed H60 as an important alloantigen in the strain combination B6 → BALB.B.
Tetramer-based depletion of antigen-specific T cells
This system thus provided us with an appropriate model in which to ask whether we could develop a method of selective depletion of the T cells responsible for GVHD, that is, the T-cell clones reactive with the H60 mHAg. To this end, we took advantage of the PE tag on the LYL8 MHC tetramer that was used to identify positive cells via flow cytometry. In order to remove the H60-reactive precursor T-cell clones from splenocytes of naive B6 mice, freshly processed donor cells were incubated with PE-conjugated tetramers. Based on a series of experiments varying the time (15-60 minutes), temperature (4°C, 22°C, and 37°C) and tetramer concentration (0.1 μg/106 cells-20 μg/106 cells), we found that a 30-minute incubation at room temperature gave us maximal staining above background. Therefore, cell depletions were conducted for 30 minutes at 22°C, using a tetramer concentration for each lot as determined by flow cytometry prior to each depletion. Subsequent reaction with anti-PE antibody conjugated to magnetic beads followed by the most stringent autoMACS magnetic separation depletion protocol (Deplete 025) was used to selectively remove the rare H60-specific T-cell clones from the entire population. This technique was capable of depleting the reactive clones with a mean efficiency of 89% (range, 78%-100%) in repeated experiments (n = 6).
In vivo evidence of a T-cell-mediated B6 anti-LYL8 response. (A) Top panels: 1 × 106 BCZ 108-2 cells (positive control for LYL8 tetramers) were coincubated with fluorescein isothiocyanate (FITC)-anti-CD8α and PE-LYL8 (left panel) or HSV8 (right panel) H2Kb tetramers. Dot plots of the cells are shown as acquired. Bottom panels: Splenocytes from immunized B6 mice were stained in the same fashion as the BCZ 108-2 cells. Prior to acquisition, 7-AAD was added to each sample to allow gating on live cells. Dot plots gated on live CD8+ cells are shown; the percentage of tetramer-positive CD8+ cells is shown in the top right quadrant. (B) IFNγ ELISPOT assay on CD8+ splenocytes from immunized B6 (imm B6) mice or naive mice with APCs from naive B6 mice (left) or BALB.B mice (right). APCs, listed along the x-axis, were pulsed with nothing (“imm B6”), H2Kb peptides LYL8 or HSV8 (nonspecific control), or H2Kd peptide J1B (irrelevant control). NIL indicates negative controls without APCs. Shown are the data from one representative experiment of 2 experiments total. Bars indicate the mean number of spots plus or minus the standard error of the mean (SEM).
In vivo evidence of a T-cell-mediated B6 anti-LYL8 response. (A) Top panels: 1 × 106 BCZ 108-2 cells (positive control for LYL8 tetramers) were coincubated with fluorescein isothiocyanate (FITC)-anti-CD8α and PE-LYL8 (left panel) or HSV8 (right panel) H2Kb tetramers. Dot plots of the cells are shown as acquired. Bottom panels: Splenocytes from immunized B6 mice were stained in the same fashion as the BCZ 108-2 cells. Prior to acquisition, 7-AAD was added to each sample to allow gating on live cells. Dot plots gated on live CD8+ cells are shown; the percentage of tetramer-positive CD8+ cells is shown in the top right quadrant. (B) IFNγ ELISPOT assay on CD8+ splenocytes from immunized B6 (imm B6) mice or naive mice with APCs from naive B6 mice (left) or BALB.B mice (right). APCs, listed along the x-axis, were pulsed with nothing (“imm B6”), H2Kb peptides LYL8 or HSV8 (nonspecific control), or H2Kd peptide J1B (irrelevant control). NIL indicates negative controls without APCs. Shown are the data from one representative experiment of 2 experiments total. Bars indicate the mean number of spots plus or minus the standard error of the mean (SEM).
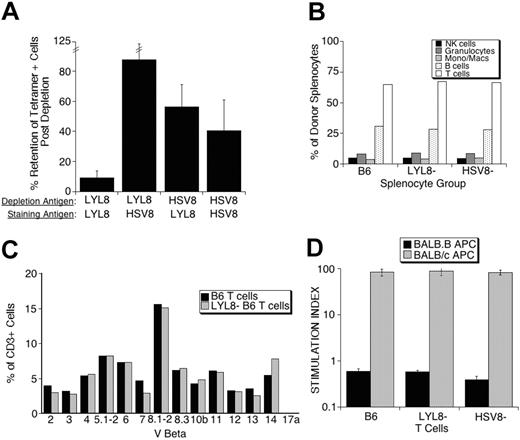
The specificity of these depletions is demonstrated by analyzing the depleted populations for retained reactivity with the tetramer used for depletion and a control tetramer. In 4 independent tetramer-depletion experiments, the LYL8 tetramer depleted 91% of the basal LYL8 reactivity, whereas these same cells retained more than 87% of their nonspecific binding. Interestingly, control (HSV8) tetramer-depleted cells retained more than 56% of their basal LYL8 reactivity, while retaining only 40% of the initial reactivity activity against the nonspecific HSV8 tetramer used for the depletion (Figure 2A).
In vitro and in vivo effects of tetramer depletion
Further analysis of the specific LYL8-depleted splenocytes, control-depleted splenocytes, and nondepleted splenocytes by flow cytometry showed no differences in the number of T cells, B cells, NK cells, monocytes, and granulocytes (Figure 2B) or in the Vβ repertoire of the T cells (Figure 2C) between samples. To determine whether the depleted populations had retained their reactivity against antigens other than LYL8, we assessed their proliferative response in a third-party MLR. All 3 populations of splenocytes had retained third-party reactivity as shown by robust proliferation in the presence of BALB/c APCs (MHC mismatched) as compared with no proliferation in the presence BALB.B APCs (MHC matched; Figure 2D).
In vitro analysis of donor splenocyte depletion. (A) Following tetramer depletion, pre- and postdepletion fractions were stained with LYL8 and HSV8 tetramers. The percent retention of tetramer-positive cells after depletion was determined as follows: 100 × (% postdepletion / % predepletion). These data are from 4 independent experiments and represent the mean plus or minus SEM. (B) B6 splenocytes from pre- and postdepletion fractions were incubated with anti-CD3, CD20, CD11b, GR-1, and NK1.1 fluorescently labeled antibodies. Percentage is based on positive gating on single parameter histograms. (C) B6 splenocytes from pre- and postdepletion fractions were coincubated with FITC-labeled antibodies against the members of the Vβ family represented on the x-axis and PE-anti-CD3 antibodies. Percentage is based on positive gating on single parameter histograms of CD3+ cells. These data are representative of 2 repeated experiments. (D) B6 splenocytes from pre- and postdepletion fractions were incubated in MLRs with either mHAg (BALB.B) or MHC (BALB/c) mismatched irradiated splenocytes. Each bar is representative of the mean of quadruplicates plus or minus SEM.
In vitro analysis of donor splenocyte depletion. (A) Following tetramer depletion, pre- and postdepletion fractions were stained with LYL8 and HSV8 tetramers. The percent retention of tetramer-positive cells after depletion was determined as follows: 100 × (% postdepletion / % predepletion). These data are from 4 independent experiments and represent the mean plus or minus SEM. (B) B6 splenocytes from pre- and postdepletion fractions were incubated with anti-CD3, CD20, CD11b, GR-1, and NK1.1 fluorescently labeled antibodies. Percentage is based on positive gating on single parameter histograms. (C) B6 splenocytes from pre- and postdepletion fractions were coincubated with FITC-labeled antibodies against the members of the Vβ family represented on the x-axis and PE-anti-CD3 antibodies. Percentage is based on positive gating on single parameter histograms of CD3+ cells. These data are representative of 2 repeated experiments. (D) B6 splenocytes from pre- and postdepletion fractions were incubated in MLRs with either mHAg (BALB.B) or MHC (BALB/c) mismatched irradiated splenocytes. Each bar is representative of the mean of quadruplicates plus or minus SEM.
Having demonstrated that there are no discernable differences in cell populations in the grafts regardless of depletion and that each graft retained equal third-party reactivity, we were interested in assessing how the specific depletion would affect in vivo expansion of donor T cells after BM transplantation. To test this, we performed 2 BM transplantations and harvested spleens shortly thereafter to assess regrowth of LYL8-tetramer positive cells and in vivo proliferation of donor T cells.
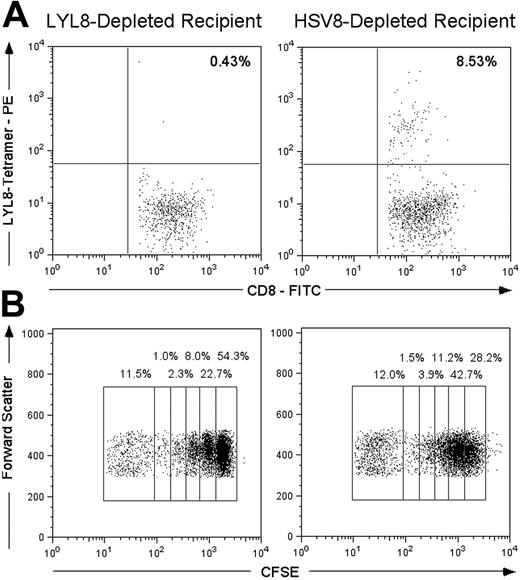
Data supporting that the specific tetramer depletion of LYL8 reactive cells was capable of preventing clonal expansion of LYL8 reactive T cells was generated by performing transplantations on mice with 5 × 106 LYL8- or control-depleted CD3+ T cells and analyzing their splenocytes with LYL8 tetramers 6 days after BMT. Mice that received control-depleted T cells (n = 3) had an average of 8.53% ± 1.93% of their CD8+ T cells stain LYL8 tetramer positive (Figure 3A). In contrast, those mice that received LYL8-depleted T cells (n = 3) had on average 0.46% ± .23% of their CD8+ T cells stain with an LYL8 tetramer. This demonstrates that tetramer depletion prior to transplantation results in the absence of tetramer-positive cells during the early and critical stages of T-cell expansion in the development of GVHD after BMT.
In a second transplantation, we labeled donor T cells with carboxyfluorescein succinimidyl ester (CFSE) after tetramer depletion and prior to transplantation to assess alloreactive proliferation in vivo as previously described.5 With each cell division, half of the CFSE fluorescence is distributed to each daughter cell, allowing for the quantitative tracking of successive generation of cells by flow cytometry. We infused 12 × 106 CD3+ CFSE-labeled T cells per mouse (n = 2 per group) and harvested their spleens 3 days after transplantation. The CD8+ splenocytes were analyzed for the dilution of the CFSE dye. We demonstrated that removing 0.11% of CD8+ T cells with LYL8-tetramer depletion in this transplantation resulted in a nearly 50% reduction of CD8+ T-cell proliferation as compared with those mice that received control-depleted T cells: 54.3% of cells underwent no cell division compared with 28.2% (Figure 3B).
Selective depletion of GVHD
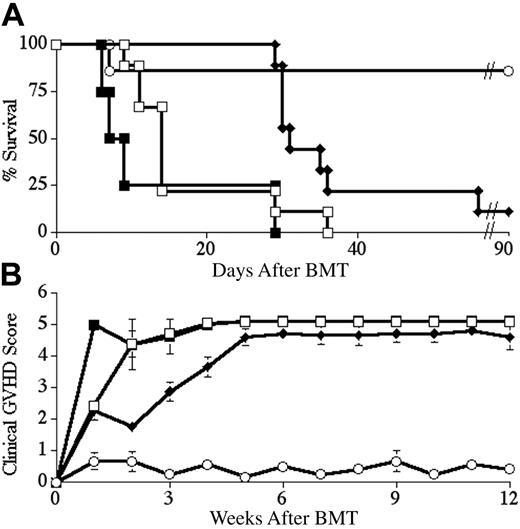
Next, we asked whether ex vivo LYL8 tetramer depletion of naive B6 splenocytes could specifically alter the B6 GVHD immune response in vivo, yet leave the recipient immunocompetent and able to recognize the tumor graft. We performed a series of BM transplantations in which we tested these different aspects of the immune response. The first objective was to eliminate or significantly delay the onset of GVHD in BALB.B recipients of B6 TCD-BM and T cells using tetramer depletion. Lethally irradiated BALB.B mice underwent transplantation with B6 TCD-BM cells along with 2 × 106 CD3+ B6 splenocytes. In different groups, splenocytes were depleted with either LYL8 tetramers or control tetramers (HSV8), or not depleted at all. In 2 reproducible BMTs, BALB.B recipients of LYL8-depleted T cells showed a significant increase in median survival as compared with mice receiving whole T cells (P < .01) or control-depleted T cells (P < .001). At a donor T-cell dose of 2 × 106, median survival for the 3 groups was 31 days, 14 days, and 8 days after BM transplantation respectively; with one long-term survivor in the LYL8-depleted T-cell group (Figure 4A). In these BM transplantations, BALB.B recipients of LYL8-depleted T cells also showed a significant decrease in the degree of GVHD over time as compared with mice receiving whole T cells (P < .01) or control-depleted T cells (P < .01), as determined by weekly evaluation of clinical GVHD scoring of the mice (Figure 4B).
In vivo analysis of donor splenocyte depletion. (A) Lethally irradiated BALB.B mice underwent transplantation on day 0 with B6 TCD-BM cells and 5 × 106 tetramer-depleted (LYL8 or HSV8) CD3+ B6 T cells. On day 6, host mice were killed and their splenocytes were coincubated with FITC-anti-CD8α and PE-LYL8 H2Kb tetramers. Prior to acquisition, 7-AAD was added to each sample to allow gating on live cells. Dot plots gated on live CD8+ lymphocytes are shown; the percentage of LYL8 tetramer-positive CD8+ cells is shown in the top right quadrant and is an average of 3 mice per condition. (B) Lethally irradiated BALB.B mice underwent transplantation on day 0 with B6 TCD-BM cells and 12 × 106 CFSE-labeled, tetramer-depleted (LYL8 or HSV8) CD3+ B6 T cells. On day 3, host mice were killed and their splenocytes were incubated with PECy7-anti-CD8α. Shown are dot plots gated on CD8+ lymphocytes; the percentage of cells within each population is shown above each region and represents an average of 4 samples per condition (2 independent samples from 2 mice per condition).
In vivo analysis of donor splenocyte depletion. (A) Lethally irradiated BALB.B mice underwent transplantation on day 0 with B6 TCD-BM cells and 5 × 106 tetramer-depleted (LYL8 or HSV8) CD3+ B6 T cells. On day 6, host mice were killed and their splenocytes were coincubated with FITC-anti-CD8α and PE-LYL8 H2Kb tetramers. Prior to acquisition, 7-AAD was added to each sample to allow gating on live cells. Dot plots gated on live CD8+ lymphocytes are shown; the percentage of LYL8 tetramer-positive CD8+ cells is shown in the top right quadrant and is an average of 3 mice per condition. (B) Lethally irradiated BALB.B mice underwent transplantation on day 0 with B6 TCD-BM cells and 12 × 106 CFSE-labeled, tetramer-depleted (LYL8 or HSV8) CD3+ B6 T cells. On day 3, host mice were killed and their splenocytes were incubated with PECy7-anti-CD8α. Shown are dot plots gated on CD8+ lymphocytes; the percentage of cells within each population is shown above each region and represents an average of 4 samples per condition (2 independent samples from 2 mice per condition).
These data demonstrated the ability to selectively abrogate the GVHD immune response by depletion with specific tetramers. However, these data did not show specificity, that is, whether the antitumor effects were retained, which can be considered as one surrogate marker for retention of immune function. A reduction of the antitumor effect along with GVHD would suggest that this approach would not be useful in this setting. Therefore, we sought to demonstrate this second key aspect of the approach by introducing an F9 teratocarcinoma. This tumor, which has previously been shown to be lethal to BALB.B mice that did not receive transplants, was infused into mice undergoing BM transplantation.21,22
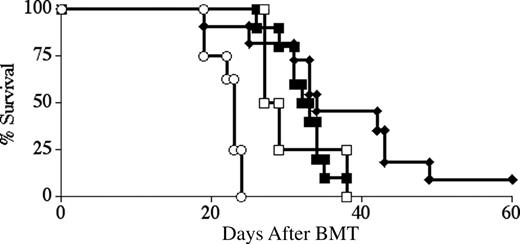
LYL8 depletion significantly increases survival and decreases the degree of GVHD over time. Lethally irradiated BALB.B mice underwent transplantation on day 0 with 5 × 106 B6 TCD-BM cells and 2 × 106 CD3+ B6 T cells. Survival was followed for 90 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). (A) ○ indicates no T cells (n = 7); □, nondepleted (n = 9); ♦, LYL8-depleted (n = 9); ▪, HSV8 control-depleted (n = 4). Depletion was 85.6% efficient in these transplants. ♦ versus ▪, P < .001; ♦ versus □, P < .01. (B) Clinical GVHD scoring for the recipient mice of the combined transplants plotted in panel A. ♦ versus ▪, P < .01; ♦ versus □, P < .01.
LYL8 depletion significantly increases survival and decreases the degree of GVHD over time. Lethally irradiated BALB.B mice underwent transplantation on day 0 with 5 × 106 B6 TCD-BM cells and 2 × 106 CD3+ B6 T cells. Survival was followed for 90 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). (A) ○ indicates no T cells (n = 7); □, nondepleted (n = 9); ♦, LYL8-depleted (n = 9); ▪, HSV8 control-depleted (n = 4). Depletion was 85.6% efficient in these transplants. ♦ versus ▪, P < .001; ♦ versus □, P < .01. (B) Clinical GVHD scoring for the recipient mice of the combined transplants plotted in panel A. ♦ versus ▪, P < .01; ♦ versus □, P < .01.
BALB.B mice were lethally irradiated and inoculated intraperitoneally with F9 cells on day -1. On day 0 these mice received B6 TCD-BM cells along with CD3+ donor B6 splenocytes that were depleted with either LYL8 tetramers or control tetramers (HSV8), or that were not depleted at all. The initial T-cell dose chosen was 1 × 106 cells (lower than in the previous transplantations), partly in order to delay and decrease the incidence of early lethal GVHD but also to allow observation of tumor growth. In 2 reproducible BM transplantations, all mice receiving T cells displayed significant (P < .001) antitumor activity as compared with recipients of B6 TCD-BM only (Figure 5). However, all deaths in these 2 combined experiments were due to tumor growth.
LYL8 depletion does not alter antitumor activity. Lethally irradiated BALB.B mice underwent transplantation on day -1 with 1 × 105 F9 cells and on day 0 with 5 × 106 B6 TCD-BM cells and 1 × 106 CD3+ B6 T cells. Survival was followed for 60 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). ○ indicates no T cells (n = 8); □, nondepleted (n = 4); ♦, LYL8-depleted (n = 11); ▪, HSV8 control-depleted (n = 10). Depletion was 95.4% efficient in these transplants. ♦ versus ○, P < .001; ▪ versus ○, P < .001; □ versus ○, P = .003; ♦ versus ▪, P = not significant (ns); ♦ versus □, P = ns; ▪ versus □, P = ns.
LYL8 depletion does not alter antitumor activity. Lethally irradiated BALB.B mice underwent transplantation on day -1 with 1 × 105 F9 cells and on day 0 with 5 × 106 B6 TCD-BM cells and 1 × 106 CD3+ B6 T cells. Survival was followed for 60 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). ○ indicates no T cells (n = 8); □, nondepleted (n = 4); ♦, LYL8-depleted (n = 11); ▪, HSV8 control-depleted (n = 10). Depletion was 95.4% efficient in these transplants. ♦ versus ○, P < .001; ▪ versus ○, P < .001; □ versus ○, P = .003; ♦ versus ▪, P = not significant (ns); ♦ versus □, P = ns; ▪ versus □, P = ns.
In our next experiments we used a T-cell dose of 1.5 × 106 cells to assess whether an intermediate T-cell dose would allow for stronger antitumor activity, yet still delay the development of GVHD in recipients of LYL8-depleted T cells. In 2 reproducible experiments we found that recipients of LYL8 tetramer-depleted T cells exhibited 100% survival until day 28 after BMT, a significant increase in median survival compared with recipients of control-depleted or nondepleted T cells (39 days, P < .001), 33% long-term survival, and only 17% mortality from GVHD with retained antitumor activity (Figure 6). As predicted, LYL8-depleted T cells displayed similar potent antitumor activity as control tetramer-depleted T cells and nondepleted T cells, yet resulted in reduced GVHD.
These results suggest that antigen-specific T-cell depletion can be used to selectively alter immunity in this model. These results are not meant to imply that this is a relevant GVT model; however, it does reflect the findings that only specific T cells were removed and not nonspecific T cells.
Discussion
During T-cell development, a series of selection processes ensures that self-reactive T-cell clones are eliminated, preventing autoimmunity. However, in the context of autoimmune disease, T cells lose the ability to distinguish between “self” and “foreign” peptide-MHC complexes, resulting in the destruction of healthy tissue. Similarly, during solid organ transplantation and during BMT, T cells recognizing foreign antigens cause unwanted cytotoxicity, reducing the success of the procedures.
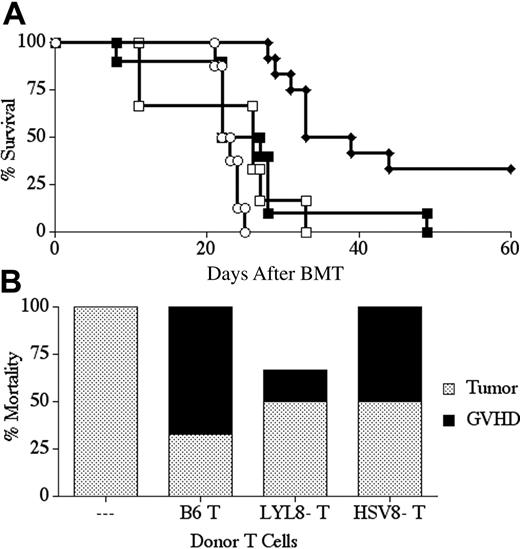
LYL8 depletion significantly decreases GVHD mortality, yet retains antitumor activity. Lethally irradiated BALB.B mice underwent transplantation on day -1 with 1 × 105 F9 cells and on day 0 with 5 × 106 B6 TCD-BM cells and 1.5 × 106 CD3+ B6 T cells. Survival was followed for 60 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). (A) ○ indicates no T cells (n = 8); □, nondepleted (n = 6); ♦, LYL8-depleted (n = 12); ▪, HSV8 control-depleted (n = 10). Depletion was 97.5% efficient in these transplants. ♦ versus ○, P < .001; ♦ versus □, P < .001; ♦ versus ▪, P < .001; ▪ versus ○, P = ns; □ versus ○, P = ns; ▪ versus □, P = ns. (B) Cause of death for recipient mice of the combined transplants plotted in panel A as determined by clinical evaluation and autopsy (gray bars indicates tumor; filled bars, GVHD).
LYL8 depletion significantly decreases GVHD mortality, yet retains antitumor activity. Lethally irradiated BALB.B mice underwent transplantation on day -1 with 1 × 105 F9 cells and on day 0 with 5 × 106 B6 TCD-BM cells and 1.5 × 106 CD3+ B6 T cells. Survival was followed for 60 days and is plotted as Kaplan-Meier curves (representative of 2 combined experiments for each condition). (A) ○ indicates no T cells (n = 8); □, nondepleted (n = 6); ♦, LYL8-depleted (n = 12); ▪, HSV8 control-depleted (n = 10). Depletion was 97.5% efficient in these transplants. ♦ versus ○, P < .001; ♦ versus □, P < .001; ♦ versus ▪, P < .001; ▪ versus ○, P = ns; □ versus ○, P = ns; ▪ versus □, P = ns. (B) Cause of death for recipient mice of the combined transplants plotted in panel A as determined by clinical evaluation and autopsy (gray bars indicates tumor; filled bars, GVHD).
In these studies, we demonstrated that the removal of less than 0.5% of CD8+ T cells resulted in no discernable differences in BMT cell populations (Figure 2B-C) or in vitro global T-cell activity (Figure 2D), but had a profound effect in vivo in an alloreactive environment. Taken together, the data demonstrating no early reappearance of LYL8-reactive T cells (Figure 3A), a significant decrease in the degree of GVHD over time (Figure 4B) and a significant increase in median survival (Figure 4A, 5, 6A) after specific depletion, shows that the removal of one specific set of alloreactive T cells is achievable, and in this model results in significantly ameliorated GVHD. We conclude that a strategy of tetramer-based depletion of clonal populations of reactive T-cell precursors is capable of selectively reducing a particular T-cell immune response, while sparing the greater immune repertoire; however, the clinical relevance is not clear.
In our GVHD model (Figure 4A), the LYL8-depleted recipients eventually succumbed to GVHD after a significant delay. This may be attributed to the residual H60 reactive clones in the graft, H60 reactive clones that had a low avidity for the tetramers, or to T cells reactive with other allogeneic mHAgs. It is likely that the full GVHD response in this model is dependent on several T cell specificities; therefore, depletion of the small number of immunodominant clones reactive with H60 is unlikely to fully abrogate GVHD. Malarkannan et al23 and Choi et al24 indicate that perhaps 3 additional BALB.B mHAgs—H28, H4, and H7—might also be target antigens of B6 alloreactive T cells.
Other murine models of GVHD have been studied with novel therapies as well, such as administration of a CD8 peptide mimetic to bind to the α3 region of the MHC class I molecule to prevent the costimulation necessary to mount an effective immune response25 or bulk depletion of alloreactive donor T cells after MLR with host cells using a variety of methods.26-28 These models have demonstrated effectiveness in delaying or diminishing GVHD pathology, yet only one of these reports28 demonstrates the retention of antitumor activity. The former approach should result in a ubiquitous blockade of all CD8 signaling and the latter approaches might result in the removal of alloreactive, tumor reactive, and beneficial virus reactive (Epstein-Barr virus [EBV], influenza) T cells. In order to retain specific antitumor activity it would be necessary that donor T cells be activated exclusively by host histocompatability antigens without contamination from tumor antigens, which is difficult to achieve.28 Furthermore, these bulk depletion studies were performed in an MHC-disparate model, whereas our studies were in an mHAg-disparate setting and did not require any culturing of donor cells prior to transplantation.
Riddell and colleagues have done extensive work on HY, identifying epitopes that appear to have distinct roles in GVHD and GVT.29 Other mHAg mediators of GVHD have been elucidated by Mutis et al8 and Goulmy and colleagues,30 who defined the roles of HA-1, -2, -4, and -5 as well as HY in HLA-identical BMTs and the development of GVHD. With our increasing knowledge and discovery of allogeneic transplant-associated mHAgs, the application of tetramer-mediated specific T-cell depletion could become a clinical strategy in the near future.
Antigen-selective T-cell depletion provides a pathway to the study and possible treatment of other pathologic processes involving reactive oligoclonal T-cell populations. This includes diabetes, solid organ allograft rejection, and autoimmune disorders. Moreover, if the peptide epitope of the unique TCR of neoplastic T-cell lymphomas and leukemias could be characterized, a similar approach for purging ex vivo could be attempted. The elucidation of a pattern of antigens important in these diseases will offer a variety of targets of interest to study. Recently, novel methods to determine T-cell antigen specificity and to screen for peptides of interest for immunotherapies have been developed.31,32 Studies such as these suggest additional avenues of promising investigation.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-07-2828.
Supported by grants from the National Institutes of Health (CA55 349 and CA23 766; D.A.S.) and (HL69 929 and HL72 412; M.R.M.v.d.B.). B.J.K. is the recipient of a predoctoral Howard Hughes Fellowship. D.A.S. is a Doris Duke Distinguished Clinical Scientist. M.R.M.v.d.B. is the recipient of a Damon Runyan Scholar Award of the Cancer Research Fund and awards from Golfers Against Cancer and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research.
D.A.S. and M.R.M.v.d.B. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Stephanie Muriglan for technical assistance; James Allison, Adam Boruchoff, Tao Dao, Onder Alpdogan, and Jaspreet Jaggi for helpful suggestions; and Derry Roopenian for helpful suggestions and careful review of this manuscript.