We have tested the effects of annexin 1 (ANXA1) and its N-terminal peptide Ac2-26 on polymorphonuclear leukocyte (PMN) recruitment under flow. Differential effects of the full-length protein and its peptide were observed; ANXA1 inhibited firm adhesion of human PMNs, while Ac2-26 significantly attenuated capture and rolling without effect on firm adhesion. Analysis of the effects of ANXA1 and Ac2-26 on PMN adhesion molecule expression supported the flow chamber results, with Ac2-26 but not ANXA1 causing l-selectin and PSGL-1 shedding. ANXA1 and its peptide act via the FPR family of receptors. This was corroborated using HEK-293 cells transfected with FPR or FPRL-1/ALX (the 2 members of this family expressed by human PMNs). While Ac2-26 bound both FPR and FPRL-1/ALX, ANXA1 bound FPRL-1/ALX only. ANXA1 and Ac2-26 acted as genuine agonists; Ac2-26 binding led to ERK activation in both FPR- and FPRL-1/ALX-transfected cells, while ANXA1 caused ERK activation only in cells transfected with FPRL-1/ALX. Finally, blockade of FPRL-1/ALX with a neutralizing monoclonal antibody was found to abrogate the effects of ANXA1 in the flow chamber but was without effect on Ac2-26-mediated inhibition of rolling. These findings demonstrate for the first time distinct mechanisms of action for ANXA1 and its N-terminal peptide Ac2-26.
Introduction
The process of leukocyte extravasation is crucial to host survival, such that following application of an inflammatory or infectious stimulus, a coordinated series of cellular and vascular responses is set in train: in this scenario, the blood-borne polymorphonuclear leukocyte (PMN) represents the first line of defense in innate immunity as it is the first cell type to rapidly extravasate.1 PMN trafficking is tightly regulated, such that proinflammatory and anti-inflammatory mediators operate in concert to promote and control the spatiotemporal aspects of PMN extravasation.2 The full dynamics of this process have yet to be addressed, but it is now well accepted that besides adhesion molecules, cytokines, and chemokines, acting together to direct leukocyte subsets out of the bloodstream, there are counterregulatory mediators that act as stop signals to inhibit PMN extravasation.3
Annexin 1 (ANXA1), a 37-kDa protein, originally described as a mediator of glucocorticoid actions, is one of these counterregulatory mediators.4,5 Several pharmacologic investigations have reported that ANXA1 inhibits PMN extravasation in models of acute6 and chronic inflammation,7 as well as in models of systemic inflammation.8 These in vivo investigations, coupled with studies of ANXA1 behavior against human neutrophil activation, have led us to propose the following model: in resting PMNs, ANXA1 is largely localized within the cytosol,9 but it can be rapidly mobilized on the cell surface when the PMN adheres to endothelial monolayers.10 Once on the neutrophil plasma membrane, ANXA1 acts in an autocrine/paracrine fashion to reduce cell extravasation.11 This model is corroborated by studies with passive immunoneutralization strategies12 as well as by more recent investigations with ANXA1-null PMNs.13,14 In this context, it is worth noting that the antimigratory effects of ANXA1 have been reproduced by peptides derived from the N-terminal region, both in vivo15,16 as well as in neutrophil/endothelium interactions in static systems in vitro.17,18
A new interest in the field has been generated by the observation that ANXA1 and its N-terminal peptides bind to a specific class of G-protein-coupled 7-transmembrane receptors, the formyl-peptide receptors (FPRs).19 In the mouse, this family comprises several putative members, but only 3 receptor types have been identified in humans.20 Human FPR is the classic receptor for the chemoattractant formyl-Met-Leu-Phe, whereas the related receptor (∼ 70% similarity at the nucleotide level) is termed FPRL-1/ALX, indicating that it belongs to the FPR family and that the endogenous anti-inflammatory lipid lipoxin A4 activates it.20,21 Of interest, human FPRL-1/ALX-like 1 binds also aspirin-triggered lipoxin A422 and serum amyloid protein A.20 The third member of the family is FPRL-2, not expressed on neutrophils but on monocytes, which has recently been shown to bind an endogenous peptide derived from heme-binding protein.23 Using transfected cell systems, ANXA1-derived peptides have been shown to activate human FPR,24 FPRL-1/ALX,25,26 and FPRL-226 under different circumstances. However, binding data for the full-length protein have been reported only for FPRL-1/ALX,25 whereas its ability to bind to FPR and FPRL-2 is still a matter of speculation.
The present study was undertaken to determine, for the first time, the effect of ANXA1 and its N-terminal bioactive peptide Ac2-26 on neutrophil-endothelium interactions in vitro under defined flow conditions. Analysis of their efficacy in this system has been complemented by an investigation of their binding and activation of human FPR and FPRL-1/ALX, the only 2 receptors of the family present on human PMNs,20 using transfected cell systems. The data obtained indicate a dichotomy of behavior between these 2 agents and may initiate a revision of the “classical” model of the way the ANXA1 system controls PMN trafficking, the major arm of innate immunity.
Materials and methods
ANXA1 and other ligands
Human ANXA1 cDNA was cloned into the expression vector pGEX-4T-327 and the protein expressed and purified (Scientific Proteins, Witterswil, Switzerland). ANXA1 was expressed as a fusion protein with major basic protein linked to the N-terminal and subsequently cleaved with TEV protease. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis showed that the recombinant ANXA1 was more than 98% pure, with an endotoxin contamination less than 40 U/mg as measured by the Limulus amebocyte chromogenic assay. Peptide Ac2-26 (acetyl-AMVSEFLKQAWIENEEQEYVVQTVK; Mr 3050) was synthesised by the Advance Biotechnology Centre (Imperial College School of Medicine, London, United Kingdom) using solid-phase stepwise synthesis. Purity was more than 90% as assessed by high-performance liquid chromatography (HPLC) and capillary electrophoresis (data supplied by the manufacturer). The nonspecific FPR family antagonist Boc2 (N-t-butyloxycarbonyl-Phe-DLeu-Phe-DLeu-Phe) was obtained from ICN Pharmaceuticals (Basingstoke, United Kingdom), whereas peptide WRWWWW was from Calbiochem (Darmstadt, Germany).
Flow chamber assay
Confluent human umbilical vein endothelial cell (HUVEC; Glycotech, Gaithersburg, MD) monolayers (up to passage 4) were stimulated with TNF-α (10 ng/mL; Sigma-Aldrich, Poole, United Kingdom) for 4 hours. Experiments with healthy volunteers were approved by the local research ethics committee (P/00/029 ELCHA). Informed consent was provided according to the Declaration of Helsinki. Blood was collected into 3.2% sodium citrate and diluted 1:1 in RPMI 1640 (Sigma-Aldrich) before separation through a double-density gradient as described.28 After PMN isolation and washing, contaminating erythrocytes were removed by hypotonic lysis. PMNs were diluted to 1 × 106/mL in Dulbecco phosphate-buffered saline (DPBS) supplemented with Ca2+ and Mg2+, and incubated with or without ANXA1 or peptide Ac2-26 prior to flow for 10 minutes at 37°C. In some experiments, PMNs were preincubated for 1 hour at 37°C with a human anti-FPRL-1 neutralizing antibody (5 μg/mL; clone 6C7-3; a kind gift from Dr Duncan Henderson, Astra Zeneca, Loughborough, United Kingdom) for 10 minutes with either mouse anti-human l-selectin (CD62L, 20 μg/mL; clone Dreg-56; BD Pharmingen, San Diego, CA), mouse anti-human PSGL-1 (CD162, 10 μg/mL; clone PL1; Serotec, Abingdon, United Kingdom) neutralizing monoclonal antibodies (mAbs) or Boc2 (10 μM) prior to addition of ANXA1 or peptide. The flow chamber was placed under a Nikon Eclipse TE3000 microscope with × 100 magnification (Nikon, Melville, NY), and PMNs (1 × 106/mL) were perfused over the endothelial monolayers at a constant rate of 1 dyn/cm2 using a syringe pump (Harvard Apparatus, South Natick, MA). After 8 minutes of perfusion, 6 random fields were recorded for 10 seconds each using a JVC TK-C1360B digital color video camera (JVC, Tokyo, Japan), ready for off-line analysis.
Video sequences were transferred to a computer and loaded into ImagePro-Plus software (Media Cybernetics, Wokingham, United Kingdom). PMNs were manually tagged and their movements on the endothelium monitored. The total number of interacting cells was quantified, as initial cell capture, and further classified as either rolling or firmly adherent (cells that remained stationary for the 10-second observation period) as described in the literature.29
Adhesion molecule levels
Peripheral blood PMNs, isolated as for flow chamber assay, were plated at a density of 2 × 105 cells per well in 96-well plates and incubated with or without peptide Ac2-26, ANXA1, or platelet-activating factor (PAF; C16 form: C26H54NO7P; Sigma-Aldrich, Poole, United Kingdom) for 30 minutes at 37°C. Cells were incubated with purified mAbs: mouse anti-human l-selectin (2 μg/mL; clone FMC46; Serotec), mouse anti-human CD11b (5 μg/mL; clone ICRF44; Serotec), or mouse anti-human phycoerythrin (PE)-conjugated PSGL-1 (10 μg/mL; clone KPL-1; BD Pharmingen) for 1 hour on ice, prior to staining with fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse IgG (1:200; Serotec). Isotype and unstained controls were also prepared for accurate calibration of the fluorescence-activated cell sorter (FACS) machine. Flow cytometry was performed using a FACScan II analyzer (Becton Dickinson, Cowley, United Kingdom) with air-cooled 100-mW argon laser tuned to 488 nm connected to an Apple Macintosh G3 computer (Cupertino, CA) running CellQuest II (Becton Dickinson, Franklin Lakes, NJ). l-Selectin and CD11b expression was recorded as units of fluorescence where the median fluorescence intensity for 10 000 cells was measured in the FL1 green channel (548 nm). In the case of the anti-PSGL-1 antibody the red FL2 channel was used (590 nm).
Receptor cloning
Cloning and stable expression of the human FPR and FPRL-1/ALX was performed using standard techniques. The cDNAs encoding the open reading frame of both receptors were obtained by polymerase chain reaction (PCR) amplification of U937 cell cDNA library. PCR was performed using specific oligonucleotide primers introducing a HindIII restriction site immediately 5′ to the initial ATG site and a NotI site 3′ to the stop codon of the receptor DNA. The oligonucleotide sequences used were as follows: FPR, forward primer, 5′-GCG CAA GCT TAT GGA GAC AAA TTC CTC TCT CCC C, reverse primer, 5′-GCG CGG CCG CTC ACT TTG CCT GTAACT CCA CCT CTG C; FPRL-1/ALX, forward primer, 5′-GCG CAA GCT TAT GGA AAC CAA CTT CTC CAC TCC TC, reverse primer, 5′-GCG CGG CCG CTC ACA TTG CCT GTA ACT CAG TCT CTG C). PCR products were digested, gel-purified, and ligated into pRc/CMV expression vector (Invitrogen, Paisley, United Kingdom), by sticky end ligation. Verification of the correct receptor construct was confirmed by sequencing. FPR and FPRL-1/ALX constructs were used to stably transfect HEK-293 cells using Fugene 6 transfection reagent (Roche, Lewes, United Kingdom) according to manufacturer's instructions. In brief, 2 μg plasmid DNA was used to transfect HEK-293 cells (2 × 105 cells/well in 6-well plates) cultured in supplemented Eagle minimum essential medium (EMEM). The medium was changed approximately 12 hours later and cells left for a further 36 hours prior to addition of 800 μg/mL neomycin (Invitrogen) to select for transfectants, which were subsequently maintained in 400 μg/mL neomycin.
Receptor binding assay
Binding experiments were conducted as previously described.25 Peptide Ac2-26 was iodinated on Tyr21 with a specific activity of 2000 Ci (7.4 × 1013 Bq)/mmol ([125I-Tyr]-Ac2-26; Amersham Biosciences, Little Chalfont, United Kingdom). Receptor competition binding studies were performed on transfected HEK-293 cells. Aliquots (1 × 106 cells/mL) of either FPR-HEK- or FPRL-1/ALX-HEK-transfected cells were incubated with increasing concentrations of cold Ac2-26 (0.03-50 μM) and a fixed concentration (50 nM) of [125I-Tyr]-Ac2-26 for 1 hour in DPBS at 4°C. The bound and unbound tracer were separated by filtration through Whatman GF/C glass microfibre filters (Kent, United Kingdom) using a vacuum manifold, prior to γ-counting. Identical competition experiments were performed with recombinant ANXA1.
Assessment of ERK activation
Activation of ERK was assessed by Western blotting using specific antibodies to detect both phosphorylated and total ERK (Cell Signalling Technology, Hitchin, United Kingdom). HEK-293 cells, either mock transfected or stably transfected clones, were seeded at 1 × 106 cells/well in 6-well plates in supplemented Eagle modified essential medium for approximately 18 hours, prior to addition of peptide Ac2-26 or ANXA1 in serum-free medium. In separate experiments, the effect of pertussis toxin (2 hours before treatment; 1 μg/mL; Sigma-Aldrich) was also examined. In all cases, cell activation was terminated by ice incubation, medium aspiration, and immediate addition of boiling lysis buffer (50 mM Tris-HCl [pH 6.8], 1% NP-40, 2% SDS, 10% glycerol). Total protein was determined by the bicin choninic acid (BCA) method (Perbio Bioscience, Cramlington, United Kingdom) followed by addition of 100 mM DTT and 0.1% bromophenol blue and heat denaturation prior to SDS-PAGE on 10% gels. Separated proteins were electrotransferred to nitrocellulose membranes (Hybond-C; Amersham Biosciences) presoaked in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol) at 100 mA per blot for 1 hour. Membranes were blocked with 5% nonfat milk in Tris-buffered saline, pH 7.4 (50 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween-20) for 1 hour, followed by overnight incubation at 4°C in the relevant primary antibody (1:1000) and finally detection for 1 hour with an antirabbit secondary antibody HRP-conjugate (1:2000; DakoCytomation, Ely, United Kingdom). Immunoreactive protein bands were detected by enhanced chemiluminescence (ECL; Amersham Biosciences).
Data handling and statistical analysis
Flow chamber experiments and Western blotting were repeated at least 3 times. Flow cytometry and receptor binding experiments were performed in duplicate or triplicate and repeated at least 3 times. Within each set of experiments, where applicable, different blood donors were used for each repetition. Data are reported as mean ± SEM and statistical differences were determined by analysis of variance followed by the Dunnett test.
Results
Effect of annexin 1 and peptide Ac2-26 on neutrophil-endothelium interaction under flow
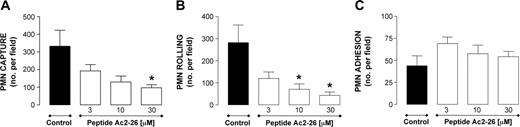
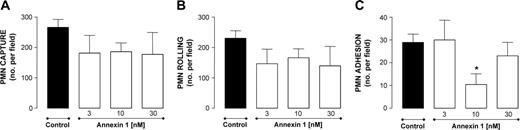
Leukocyte incubation with peptide Ac2-26 produced a concentration-dependent inhibition of PMN capture (Figure 1A) and rolling (Figure 1B). In contrast, the ANXA1-derived peptide did not modify the extent of PMN adhesion (Figure 1C). A different result was obtained when the full-length protein was tested. This time, no inhibition of PMN capture and rolling was measured (Figure 2A-B), while marked and significant attenuation of PMN adhesion was obtained (-60%, n = 6, P < .05).
Effect of peptide Ac2-26 on PMN interaction with HUVECs under flow. PMNs were incubated with peptide Ac2-26 for 10 minutes prior to flow over TNF-α-stimulated (10 ng/mL, 4 hours) HUVECs. PMN-endothelium interactions were quantified off-line. (A) Number of PMNs captured. (B) Number of rolling PMNs. (C) Number of firmly adherent PMNs. Data are presented as mean ± SEM of 3 independent experiments. *P < .05 versus control group.
Effect of peptide Ac2-26 on PMN interaction with HUVECs under flow. PMNs were incubated with peptide Ac2-26 for 10 minutes prior to flow over TNF-α-stimulated (10 ng/mL, 4 hours) HUVECs. PMN-endothelium interactions were quantified off-line. (A) Number of PMNs captured. (B) Number of rolling PMNs. (C) Number of firmly adherent PMNs. Data are presented as mean ± SEM of 3 independent experiments. *P < .05 versus control group.
To gain some mechanistic information on the adhesion molecule(s) responsible for PMN rolling in this experimental setting, the effect of specific neutralizing antibodies was tested. Table 1 reports the inhibition of PMN capture and rolling produced by PMN cell incubation with an anti-l-selectin monoclonal antibody. A similar attenuation was observed with an anti-PSGL-1 mAb.
Effect of annexin 1 and peptide Ac2-26 on neutrophil adhesion molecule expression
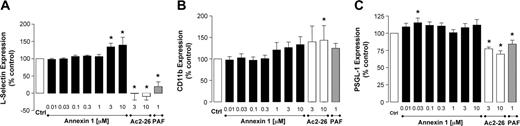
Experiments were then performed to determine whether the observed effects of ANXA1 and Ac2-26 observed in the flow chamber were due to a modulation of PMN adhesion molecule expression. To this end, a large concentration range of ANXA1 was tested with the finding that significant increases in l-selectin expression occurred only at the highest concentrations tested (3 and 10 μM; Figure 3A). This was different from the effect produced from peptide Ac2-26, which provoked marked shedding of l-selectin, an effect replicated by PMN incubation with an active concentration of PAF (Figure 3A). Similar differences were also observed with respect to CD11b and PSGL-1 (CD162) levels, with ANXA1 being essentially inactive and peptide Ac2-26 causing modifications in line with those produced by PAF (Figure 3B-C).
Since ANXA1 and its peptide have been reported to activate members of the FPR family, and only 2 of these receptors are present on PMNs, we next determined the binding and activation characteristics for either the full-length protein or the short N-terminal peptide on cells engineered to overexpress each single receptor type.
Binding and activation of FPR and FPRL-1/ALX by annexin 1 and peptide Ac2-26
Homoligand competition binding experiments demonstrated that peptide Ac2-26 competed with [125I-Tyr]-Ac2-26 at both the FPR and FPRL-1/ALX receptors with EC50 values of 1.4 and 1.8 μM, respectively, and Hill coefficients less than 1 (Figure 4A-B). These values were comparable with previously published data24,25 ; similarly, displacement curves from human PMNs were in line with those obtained previously.25 In a representative experiment, peptide Ac2-26 displaced [125I-Tyr]-Ac2-26 binding with the following values: 5%, 20%, 50%, 80%, and 85% for the concentrations of 1 nM, 10 nM, 100 nM, 1 μM, and 10 μM, respectively.
Cell incubation with ANXA1 displaced the tracer only at the FPRL-1/ALX receptor, with a calculated mean EC50 value of 0.15 μM and a Hill coefficient close to 1, providing an indication of the kinetics of binding (Figure 4D). In fact, no displacement of tracer was observed in HEK-293 cells transfected with the FPR receptor (approximate EC50 value > 1600 μM; Figure 4C). This result suggests an inherent selectivity of the full-length molecule toward FPRL-1/ALX that is not seen with the short N-terminal peptide.
Effect of ANXA1 on PMN interaction with HUVECs under flow. PMNs were incubated with ANXA1 for 10 minutes prior to flow over TNF-α-stimulated (10 ng/mL, 4 hours) HUVECs. PMN-endothelium interactions were quantified off-line. (A) Number of PMNs captured. (B) Number of rolling PMNs. (C) Number of firmly adherent PMNs. Data are presented as mean ± SEM of 3 to 5 independent experiments. *P < .05 versus control group.
Effect of ANXA1 on PMN interaction with HUVECs under flow. PMNs were incubated with ANXA1 for 10 minutes prior to flow over TNF-α-stimulated (10 ng/mL, 4 hours) HUVECs. PMN-endothelium interactions were quantified off-line. (A) Number of PMNs captured. (B) Number of rolling PMNs. (C) Number of firmly adherent PMNs. Data are presented as mean ± SEM of 3 to 5 independent experiments. *P < .05 versus control group.
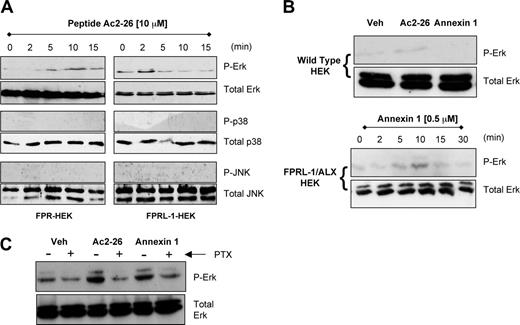
Next we sought to test whether receptor binding was associated with downstream activation of the major MAP kinase pathways ERK, p38, and JNK. Incubation of peptide Ac2-26 (10 μM) for up to 15 minutes with FPR- or FPRL-1/ALX-transfected cells resulted in a time-dependent activation of ERK1/2, with no concomitant changes in the phosphorylation status of p38 or JNK (Figure 5A). ERK phosphorylation occurred at early time points, within the first 2 to 10 minutes of incubation. Addition of recombinant ANXA1 (0.5 μM) to FPRL-1/ALX-HEK cells produced a similar time-dependent activation of ERK1/2 that was not seen in wild-type HEK cells (Figure 5B). Furthermore, mock-transfected cells did not respond to peptide Ac2-26 treatment at any time point examined (eg, see Figure 5B for 10-minute data), confirming that ERK phosphorylation was a receptor-dependent event and not simply a response to nonspecific interactions with plasma membrane. The G-protein dependency of this receptor signaling was investigated by pretreating the cells with pertussis toxin (at the 10-minute time point), which attenuated the effects of peptide Ac2-26 and ANXA1 on ERK activation in FPRL-1/ALX-HEK cells (Figure 5C), suggesting the involvement of a Gi-coupled mechanism. The same was true for the peptide Ac2-26 effect in FPR-HEK cells (data not shown).
Effect of a neutralizing anti-FPRL-1 mAb
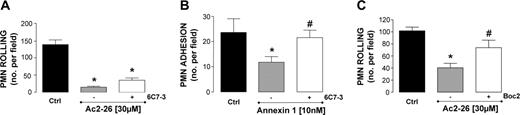
To functionally relate the results produced with transfected HEK cells, we tested the effect of a neutralizing anti-FPRL-1/ALX mAb (6C7-3).30 PMN incubation with an active concentration of the antibody (as tested by flow cytometry on both PMNs and monocytes; data not shown) did not block the effect of peptide Ac2-26 on neutrophil capture (not shown) or PMN rolling (Figure 6A). In contrast, mAb 6C7-3 abrogated the antiadhesive activity of ANXA1 (Figure 6B). Finally, cell incubation with compound Boc2, a pan-receptor antagonist of the FPR family, prevented the inhibitory action of peptide Ac2-26 on PMN rolling (Figure 6C). Similar conclusions were also reached from the static assays, as peptide Ac2-26-induced l-selectin shedding was sensitive to Boc2 but not to selective blockade of FPRL-1/ALX functions. In this set of experiments, PMN incubation with peptide Ac2-26 (10 μM) produced 67% ± 4% reduction in l-selectin expression, abrogated to 9 ± 5% when Boc2 (10 μM) was added (P < .05; n = 3 experiments). In contrast, addition of the putative FPRL-1/ALX selective synthetic antagonist WRWWWW (10 μM; see Karlsson et al31 ) did not alter peptide Ac2-26 effect (75% ± 5% reduction in l-selectin levels; not significant).
Discussion
Whereas the effects of ANXA1 and its N-terminal peptide on PMN trafficking have been tested in a variety of experimental animal models of inflammation, little has been done to investigate their effect on human PMN activation. In the present study, we have monitored for the first time their effect on PMN-endothelium interaction under defined flow conditions, using an in vitro human system that resembles physiologic events occurring in inflammation. Analysis of the properties of ANXA1 and peptide Ac2-26 in this experimental system has led to the unexpected observation that they might be acting through distinct receptors, thereby initiating responses in human PMNs using different mechanisms.
The inhibitory role of ANXA1 on the process of leukocyte trafficking has predominantly been studied using a pharmacologic approach in the experimental animal. Thus, systemic treatment with human recombinant ANXA1 or peptide Ac2-26 dampens PMN recruitment in different vascular beds and in response to several distinct stimuli,7,27,32,33 the only exception being the lung.34 These studies have led to a working model in which exogenous and endogenous ANXA1 acts in an autocrine/paracrine fashion to down-regulate PMN extravasation, and the N-terminal-derived peptide Ac2-26 is a genuine pharmacophore of the protein.11 Much less investigated, though, are the putative effects of these 2 agents on human neutrophil functions. In an initial study, we investigated the effect of peptide Ac2-26 and reported its ability to suppress PMN adhesion to endothelial monolayers.17 Another group extended these observations, with similar results obtained with the full-length protein as well as a nonapeptide derived from the core region of ANXA1.18 However, both these studies used static assays of PMN adhesion, whereas it is now clear that PMN interaction with the endothelium under flow conditions is not only of more relevance for modeling initial inflammatory events29,35 but is also crucial to produce appropriate cell activation leading to firm adhesion and emigration.36,37
Effect of peptide Ac2-26 and ANXA1 on PMN adhesion molecule expression. PMNs were incubated with either ANXA1, peptide Ac2-26, or PAF (1 μM) for 30 minutes at 37°C. Cell surface levels of l-selectin (A), CD11b (B), and PSGL-1 (C) were analyzed by flow cytometry. Data are presented as mean percent of control fluorescence ± SEM of 3 independent experiments. *P < .05 versus control group.
Effect of peptide Ac2-26 and ANXA1 on PMN adhesion molecule expression. PMNs were incubated with either ANXA1, peptide Ac2-26, or PAF (1 μM) for 30 minutes at 37°C. Cell surface levels of l-selectin (A), CD11b (B), and PSGL-1 (C) were analyzed by flow cytometry. Data are presented as mean percent of control fluorescence ± SEM of 3 independent experiments. *P < .05 versus control group.
The seminal study of Walther et al24 used N-terminal-derived peptides Ac2-26 and Ac9-25 to report their interaction with human FPR; the novelty here was that the peptides acted as PMN activators in vitro and transfected cell systems indicated activation of human FPR, though there was no binding data. The increase in PMN intracellular calcium fluxes produced by the peptides down-regulated subsequent cellular responses to other activators,24 and was congruent with the detachment effects earlier reported in the inflamed microcirculation of the mouse,27,33 supporting the idea that controlled activation of the adherent PMN promotes its “release and go” signal when the microenvironment is not yet prepared for the actual transendothelial migration. In the absence of evidence for a direct interaction between full-length ANXA1 or its peptides and the FPR family, no firm conclusions could be reached about the subtypes used. The only exception was our previous study in which the binding characteristics of ANXA1 and peptide Ac2-26 to FPRL-1, also known as the lipoxin receptor,38 were studied.25 To rectify this omission, we tested the efficacy of peptide Ac2-26 and ANXA1 on PMN adhesive interactions in the flow chamber assay, with the further aim of relating potential effects to specific receptor mechanisms.
Analysis of competition binding in FPR- and FPRL-1/ALX-transfected HEK-293 cells. Homoligand (peptide Ac2-26) and heteroligand (ANXA1) competition binding curves in FPR- and FPRL-1/ALX-transfected HEK cells using 50 nM [125I-Tyr]-Ac2-26 as tracer. (A,B) Displacement of tracer by unlabeled Ac2-26 at FPR and FPRL-1/ALX receptor, respectively. (C) Lack of competitive binding by ANXA1 at FPR-HEK cells. (D) Displacement of tracer by ANXA1 in FPRL-1/ALX-HEK cells. Data are presented as mean ± SEM of 4 independent experiments.
Analysis of competition binding in FPR- and FPRL-1/ALX-transfected HEK-293 cells. Homoligand (peptide Ac2-26) and heteroligand (ANXA1) competition binding curves in FPR- and FPRL-1/ALX-transfected HEK cells using 50 nM [125I-Tyr]-Ac2-26 as tracer. (A,B) Displacement of tracer by unlabeled Ac2-26 at FPR and FPRL-1/ALX receptor, respectively. (C) Lack of competitive binding by ANXA1 at FPR-HEK cells. (D) Displacement of tracer by ANXA1 in FPRL-1/ALX-HEK cells. Data are presented as mean ± SEM of 4 independent experiments.
Peptide Ac2-26 produced clear inhibitory effects on PMN capture and rolling on TNF-activated HUVECs. This effect was concentration dependent and linked to promotion of l-selectin shedding; in fact, under these conditions PMN rolling was dependent on this adhesion molecule. More modest effects were measured for PSGL-1, another glycoprotein able to sustain PMN rolling.39 As reported in the literature,40 changes in PSGL-1 levels were detected only using purified PMNs; peptide Ac2-26 provoked a significant shedding that was similar to that observed with PAF but lower than for l-selectin.
From these collective experiments, we concluded that peptide Ac2-26, at these concentrations, acts as a genuine PMN activator. Much less clear was the effect of full-length ANXA1. PMN incubation with low, but not high, concentrations of ANXA1 did not affect PMN rolling but markedly reduced cell adhesion as measured under flow. In contrast to peptide Ac2-26, ANXA1 did not cause l-selectin or PSGL-1 shedding but at higher concentrations ANXA1 even caused up-regulation of l-selectin, a phenomenon seen with the selective FPRL-1/ALX lipid ligand lipoxin A4.41 The lack of effect of ANXA1 on PMN l-selectin expression was somewhat unexpected, since data from our and other laboratories have previously reported it,42,43 and shown that the l-selectin shedding activity of ANXA1 on purified PMNs was mediated by the N-terminal region.42 It is possible that using purified PMNs versus whole blood might affect the ability of ANXA1 to promote l-selectin shedding.
Human PMNs express only 2 members of the FPR family, FPR and FPRL-1/ALX.20 Both receptors can be blocked by Boc-derivatives, so reversal of peptide Ac2-26 inhibition of PMN rolling and l-selectin shedding by Boc2 excludes the interference of other recently postulated mechanisms31 but is in line with previous studies.24,42 The differences in cell behavior between peptide Ac2-26 and ANXA1 could be explained on the basis of differential FPR and FPRL-1/ALX activation. To this end, stably transfected HEK cells expressing either FPR or FPRL-1/ALX (as determined by flow cytometry with selective mAbs) were used showing that peptide Ac2-26 bound both receptors. The approximate affinity constants calculated for peptide Ac2-26 were in line with those produced by other studies with transfected cells or PMNs.25,44 The same applies to the approximate EC50 calculated in the radioligand displacement experiments. In contrast, ANXA1 did not bind human FPR, but competed for FPRL-1/ALX with a similar profile to our previous study.25 Peptide Ac2-26 and ANXA1 acted as genuine agonists since their addition provoked rapid and transient ERK phosphorylation, as reported for other ligands including serum amyloid A.45 It was of interest that the ERK response was selective and detected with peptide Ac2-26 at both FPR and FPRL-1/ALX: (1) in neither case have other mitogen-activated kinases been phosphorylated; (2) the response was not evident in mock-transfected cells; (3) it was also genuinely linked to activation of Gi associated with these receptors.23,46,47 Thus, early intracellular phosphorylation events seem common to receptor activation by ANXA1 and bioactive peptide preceding their anti-inflammatory and antimigratory actions on the PMN interacting with the endothelium. It is also possible that this early ERK response might lead to subsequent activation of phosphatases and inhibition of proinflammatory signals, a mechanism possibly behind aspirin-triggered lipoxin A4 inhibition of leukocyte-specific protein-1 phosphorylation.48 Indeed, activation of this receptor by lipoxin A4 inactivated the phosphorylation status of cell-specific receptors as well as of postreceptor transduction pathways.49-51
FPR- and FPRL-1/ALX-mediated early activation of ERK. (A) Representative Western blots illustrating the stimulatory effects of 10 μM peptide Ac2-26 on the short-term (2-15 minutes) activation of ERK in FPR- and FPRL-1/ALX-transfected HEK cells. The lack of effect of Ac2-26 on p38 and JNK activation is also shown. (B) As in panel A, but also using 0.5 μM ANXA1, showing 10-minute data with FPRL-1/ALX-HEK- and empty plasmid (mock)-transfected HEK cells. (C) FPRL-1/ALX-HEK cells were incubated with pertussis toxin (1 μg/mL) for 2 hours prior to 10-minute addition of 10 μM peptide Ac2-26 or 0.5 μM ANXA1. Blots are representative of at least 3 separate experiments.
FPR- and FPRL-1/ALX-mediated early activation of ERK. (A) Representative Western blots illustrating the stimulatory effects of 10 μM peptide Ac2-26 on the short-term (2-15 minutes) activation of ERK in FPR- and FPRL-1/ALX-transfected HEK cells. The lack of effect of Ac2-26 on p38 and JNK activation is also shown. (B) As in panel A, but also using 0.5 μM ANXA1, showing 10-minute data with FPRL-1/ALX-HEK- and empty plasmid (mock)-transfected HEK cells. (C) FPRL-1/ALX-HEK cells were incubated with pertussis toxin (1 μg/mL) for 2 hours prior to 10-minute addition of 10 μM peptide Ac2-26 or 0.5 μM ANXA1. Blots are representative of at least 3 separate experiments.
In the final part of the study, we sought to provide a functional connection between the data produced with transfected HEK cells and those obtained using the flow chamber. To do so, we relied on a neutralizing anti-FPRL-1/ALX mAb30 in combination with Boc derivatives, since the commercially available anti-FPR clone does not neutralize. Whereas peptide Ac2-26 inhibition of PMN rolling was not affected by the anti-FPRL-1/ALX mAb, the antibody abrogated the antiadhesive effect of ANXA1. These data were supported by experiments using a synthetic selective antagonist to FPRL-1/ALX, peptide WRWWWW.31 The picture that emerges is that ANXA1 selectively interacts with FPRL-1/ALX, causing phosphorylation of ERK in a Gi-dependent fashion and inhibition of PMN adhesion. Identification of other intermediate signaling steps requires further investigation, though the complexity of the ERK pathways in different cellular systems is starting to be appreciated.52 An apparent discrepancy that emerges from these experiments lies in the active concentration of ANXA1 (10 nM) and its EC50 for the tracer from FPRL-1/ALX (∼ 150 nM); it is likely that use of peptide Ac2-26 as a tracer does not favor a precise assessment of the affinity of the full-length protein for the receptor. It is unfortunate that ANXA1 cannot be reproducibly iodinated without causing protein unfolding and cleavage,53 but in the few studies where ANXA1 binding has been studied, with direct or indirect protocols, affinity constants in the range of 1 to 10 nM were calculated.54,55 ANXA1 selectivity for FPRL-1/ALX mirrors the effect of other natural ligands, including serum amyloid A and lipoxin A4 and aspirin-triggered lipoxin A4,45,56 and opens the intriguing and stimulating question of how distinct ligands can produce different modes of receptor activation; investigation of the plasticity of 7-transmembrane G-protein-coupled receptors57 will possibly provide an explanation.
Impact of a neutralizing anti-FPRL-1/ALX mAb on ANXA1 effects PMN interaction with HUVECs under flow. (A) PMNs were incubated with a neutralizing anti-FPRL-1/ALX mAb (6C7-3, 5 μg/mL) for 1 hour at 37°C prior to addition of 30 μM peptide Ac2-26. (B) PMNs were incubated with a neutralizing anti-FPRL-1/ALX mAb (6C7-3, 5 μg/mL) for 1 hour at 37°C prior to addition of ANXA1 (10 nM). (C) PMNs were incubated with Boc2 (10 μM) for 10 minutes prior to addition of Ac2-26 (30 μM). In all cases, the number of rolling or adherent PMNs was quantified off-line. Data are presented as mean ± SEM of 3 to 5 independent experiments. *P < .05 versus respective control group. #P < .05 versus appropriate ANXA1 or peptide Ac2-26 group.
Impact of a neutralizing anti-FPRL-1/ALX mAb on ANXA1 effects PMN interaction with HUVECs under flow. (A) PMNs were incubated with a neutralizing anti-FPRL-1/ALX mAb (6C7-3, 5 μg/mL) for 1 hour at 37°C prior to addition of 30 μM peptide Ac2-26. (B) PMNs were incubated with a neutralizing anti-FPRL-1/ALX mAb (6C7-3, 5 μg/mL) for 1 hour at 37°C prior to addition of ANXA1 (10 nM). (C) PMNs were incubated with Boc2 (10 μM) for 10 minutes prior to addition of Ac2-26 (30 μM). In all cases, the number of rolling or adherent PMNs was quantified off-line. Data are presented as mean ± SEM of 3 to 5 independent experiments. *P < .05 versus respective control group. #P < .05 versus appropriate ANXA1 or peptide Ac2-26 group.
It is worth highlighting, however, that despite peptide Ac2-26 activating predominantly FPR in the present experimental conditions (2 cell systems under flow, with a 3-10 μM concentration range), this 25-aa long peptide exerts potent anti-inflammatory and tissue-protecting properties.33,58 In any case, peptide Ac2-26 appears to be a much more promiscuous ligand for members of the human FPR family: it activates24 and, as shown here, binds human FPR as well as FPRL-1/ALX25 ; in addition, it also activates FPRL-231 —this latter receptor perhaps being responsible for the effects reported on macrophages.59,60 Nevertheless, the present study is in line with other investigations that have indicated the susceptibility of peptide Ac2-26 effects on human PMNs to pan-FPR antagonists, as demonstrated with the Boc2 compound used here.24,25,42 It remains to be seen whether the effects measured with low micromolar concentrations of peptide Ac2-26 could be of any pathophysiologic relevance, since its proteolytic generation in inflamed tissue sites58,61 has often been postulated but yet to be demonstrated with the current analytic protocols. It is unlikely that high concentrations of 50 to 100 μM peptide Ac2-2631 could be reached in vivo. In any case, putative generation of peptide Ac2-26, or similar N-terminal peptides derived from ANXA1 at the site of inflammation, could control FPR activation on the PMN surface, perhaps modulating cell chemotaxis in the subendothelial matrix or affecting PMN lifespan at the site of inflammation.
To conclude, an analysis of the effects of peptide Ac2-26 and ANXA1 on human PMN-endothelium interaction under flow conditions has revealed differences. A study of the interaction of these species with the FPR subtypes expressed by this type of leukocyte revealed that both ANXA1 and its peptide produced receptor-mediated phosphorylation of ERK. These results indicate distinct sites of action for ANXA1 and derived N-terminal peptides, if the latter are at all produced during the process of PMN extravasation in innate immunity.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-08-3099.
Supported by a Senior Fellowship of the Arthritis Research Campaign UK (15755) to M.P. R.P.G.H. is supported by a PhD studentship (RAB03/PHD/07), and D.C. by a nonclinical fellowship (RAB03/F2), of the Research Advisory Board of St Bartholomew's and the Royal London Charitable Foundation (London, United Kingdom).
R.P.G.H. and A.M.K. contributed equally to the study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Kamala Patel (University of Calgary, Canada) for guidance with the flow chamber system. We also acknowledge the generous supply of anti-FPRL-1 mAb by Dr Duncan Henderson (AstraZeneca, Loughborough, United Kingdom). Finally, we thank Mr Jesmond Dalli and Ms Prescilla Sawmynaden for help with some of the binding experiments.

![Figure 4. Analysis of competition binding in FPR- and FPRL-1/ALX-transfected HEK-293 cells. Homoligand (peptide Ac2-26) and heteroligand (ANXA1) competition binding curves in FPR- and FPRL-1/ALX-transfected HEK cells using 50 nM [125I-Tyr]-Ac2-26 as tracer. (A,B) Displacement of tracer by unlabeled Ac2-26 at FPR and FPRL-1/ALX receptor, respectively. (C) Lack of competitive binding by ANXA1 at FPR-HEK cells. (D) Displacement of tracer by ANXA1 in FPRL-1/ALX-HEK cells. Data are presented as mean ± SEM of 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-08-3099/2/m_zh80050692020004.jpeg?Expires=1766143417&Signature=KGnxgnsGiDD-O-4q5pfvCBh9ysdOEHnrM3GjVdvVeWOls-~aiBzwKbzfmQtAmUqSl9FI85zOqxOnYYzG2LqWTGqH9okNVdf6qm5J~Gds~7BP0yNEPdFMpZzEEE10b5SOWacB0WN36i5V1-rWyiKAeslSy34krgJmCBRcIhiupxN9qnziWSWqk1mlWcxWGcXt3irP3pBwBO8YM54A8bn2tqZmUmyIeVScVGl0qy7cHDjsLuAycfGPQ5XoGg5qbCl03yENwN5-ixfQcPx~rE7bb1INDySB7o0uqSQHXUUpUFx74iLIJd4MmwUeCt5UClG7bS5NHx-G0uKD4ZjX9RtHVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)