Abstract
Cell adhesion molecules are critical in monocyte (MN) recruitment in immune-mediated and hematologic diseases. We investigated the novel role of recombinant human migration inhibitory factor (rhMIF) in up-regulating vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) and their signaling pathways in human MNs. rhMIF-induced expression of VCAM-1 and ICAM-1 was significantly higher compared with nonstimulated MNs. rhMIF induced MN VCAM-1 and ICAM-1 expression in a concentration-dependent manner (P < .05). Antisense oligodeoxynucleotides (ODNs) and inhibitors of Src, PI3K, p38, and NFκB significantly reduced rhMIF-induced MN VCAM-1 and ICAM-1 expression (P < .05). However, Erk1/2 and Jak2 were not involved. Silencing RNA directed against MIF, and inhibitors of Src, PI3K, NFκB, anti–VCAM-1, and anti–ICAM-1 significantly inhibited rhMIF-induced adhesion of HL-60 cells to human dermal microvascular endothelial cells (HMVECs) or an endothelial cell line, HMEC-1, in cell adhesion assays, suggesting the functional significance of MIF-induced adhesion molecules (P < .05). rhMIF also activated MN phospho-Src, -Akt, and -NFκB in a time-dependent manner. rhMIF induced VCAM-1 and ICAM-1 up-regulation in 12 hours via Src, PI3K, and NFκB as shown by Western blotting and immunofluorescence. MIF and MIF-dependent signaling pathways may be a potential target for treating diseases characterized by up-regulation of cell adhesion molecules.
Introduction
Migration inhibitory factor (MIF) is a pleotropic cytokine which plays a pivotal role in inflammatory and immune-mediated diseases such as rheumatoid arthritis (RA) and atherosclerosis. MIF is secreted by T lymphocytes and macrophages on lipopolysaccharide (LPS) exposure and induces secretion of tumor necrosis factor-α (TNF-α) by mouse macrophages.1,2
In RA, MIF is highly expressed in macrophages, endothelial cells, synovial tissue (ST) fibroblasts, serum, and synovial fluids.1,2 MIF stimulates macrophage release of proinflammatory cytokines such as TNF-α, interleukin 1 β (IL-1β), IL-6, and IL-8.3,4 MIF up-regulates IL-1β, matrix metalloproteinases (MMPs) MMP-1, MMP-3, MMP-9, and MMP-13 in RA ST fibroblasts.5,6 In rodent arthritis models, administration of anti-MIF antibody ameliorates arthritis with profound inhibition of clinical and histologic features of disease.7-9 Anti-MIF treatment ameliorates acute encephalomyelitis and experimental autoimmune myocarditis in mice.10,11 These studies show a key role of MIF in the pathogenesis of immunologic and inflammatory diseases.
We have shown that MIF is a potent angiogenic factor.12 Anti-MIF inhibits tumor growth and tumor-associated angiogenesis, and MIF is a required factor for tumor-initiated endothelial cell proliferation and tumor neovascularization.13,14 Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) in soluble forms (sVCAM-1 and sICAM-1, respectively) are potent angiogenic mediators, and RA synovial fluid-induced angiogenesis is blocked by anti–VCAM-1.15,16 MIF is found in human vascular endothelial cells, which have been considered to play a pivotal role in systemic inflammatory and immunologic diseases by producing cytokines and growth factors.17
Adhesion of inflammatory cells to vascular endothelium is the initial step in leukocyte recruitment and is mediated by a number of cell adhesion molecules such as ICAM-1, VCAM-1, E-, P-, and L-selectin, as well as integrins. MIF up-regulates ICAM-1 on endothelial cells.18 Rat kidney VCAM-1 and ICAM-1 expression are decreased by anti-MIF treatment, blocking the development of glomerulonephritis.19 Similarly, anti-MIF prevents VCAM-1 up-regulation on endothelial cells and improves acute encephalomyelitis in mice.11
Cell adhesion molecules mediate and amplify the inflammatory response by allowing the ingress of leukocytes into diseased tissues.20-22 VCAM-1 and ICAM-1 may be used as a reliable measure of the extent of atherosclerotic progression, and focal expression of adhesion molecules is consistently found in atherosclerotic plaques in humans.22-24 The most compelling data for the necessity of adhesion molecules in the development of atherosclerotic plaques came from a report indicating that mice deficient in adhesion molecules are protected against atherosclerosis when fed an atherogenic diet.25 Those studies support the role of adhesion molecules in immune-mediated diseases.
Cell adhesion molecules may be up-regulated via different protein tyrosine kinases such as Src, phosphatidylinositol 3 kinase (PI3K), Erk1/2, Jak2, Stat3, and NFκB.26-29 We and others have shown that vascular endothelial growth factor and sE-selectin mediate angiogenesis via Src kinases.30,31 Src family kinases mediate the multistep process of leukocyte adhesion, migration, and accumulation at inflammatory sites by the interaction of a number of adhesion molecules on leukocytes and endothelial cells.32-35 NFκB is activated by different cytokines such as TNF-α, IL-1β, and IL-18, and it rapidly translocates to nucleus to regulate gene expression.26,36,37 It is a downstream target of Src, PI3K, p38, and Jak2 and a key transcription factor regulating inflammation.28,38,39
In this study, we demonstrate a direct effect of rhMIF in up-regulating VCAM-1 and ICAM-1 in human peripheral blood (PB) monocytes (MNs) using cell-surface enzyme-linked immunosorbent assays (ELISAs). We report the role of Src, PI3K, p38, and NFκB in up-regulating these adhesion molecules. We also confirm our results by using sense and antisense oligodeoxynucleotides (ODNs) of signaling intermediates. MIF activates phosphorylation of Src, Akt, and NFκB, as shown by Western blotting and immunofluorescence. We demonstrate that MIF-induced adhesion of HL-60 cells to HMEC-1 cells is dependent on Src, PI3K, and NFκB. In contrast, Erk1/2 is not involved. We suggest that MIF has proinflammatory effects on MN recruitment via up-regulating adhesion molecules. Our results suggest MIF and its signaling pathways may be potential targets in MN-dependent inflammatory diseases such as RA and atherosclerosis.
Materials and methods
Cell-surface ELISAs for adhesion molecule expression
The study protocol was approved by the University of Michigan Medical School Institutional Review Board. Human MNs were isolated from PB of healthy volunteers provided with informed consent using AccuPrep (Accurate Chemical & Scientific, Westbury, NY).40 MNs were collected at the interface, washed (× 2) with phosphate-buffered saline (PBS), and resuspended in Hanks balanced salt solution (HBSS) with calcium and magnesium at 2.5 × 106 cells/mL. Mononuclear cells (1 × 107 cells) were gently layered over 8 mL isolation material (1.65 mL 10 × HBSS in 10 mL sterile Percoll, pH 7.0; Amersham Biosciences, Piscataway, NJ). After centrifugation at 600g for 30 minutes at room temperature, MNs at the interface were collected. Cell viability was determined by trypan blue exclusion and was greater than 98%, and purity was greater than 80%. MNs (1 × 105/well) were seeded in sterile 96-well plates (BD Falcon, Bedford, MA) in RPMI 1640 with 5% fetal bovine serum (FBS) for 2 hours at 37°C. After 2 hours, MNs were incubated with serum-free RPMI for 6 to 8 hours to allow cells to achieve quiescence. MNs were stimulated with rhMIF (50 nM; R&D Systems, Minneapolis, MN) for various time periods or with different concentrations of rhMIF. Cells were fixed with 3.7% formalin in PBS at 37°C for 15 minutes. The plates were carefully washed with PBS + 0.5% Tween 20 (1 ×). MNs were blocked in blocking buffer having 1% BSA and 30% goat serum in PBS for 15 minutes. Mouse anti–human VCAM-1, ICAM-1, and anti–E-selectin (R&D Systems) were added to the blocking buffer at 10 μg/mL, and the plates were incubated at 37°C for 2 hours. Cells were washed twice with PBS + 0.5% Tween 20. Polyclonal goat anti–mouse IgG peroxidase conjugate (Sigma Aldrich, St Louis, MO) diluted (1:1000) in blocking buffer was applied, and plates were incubated at 37°C for 1 hour. Cells were then washed twice, and 200 μL/well 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma Aldrich) was added, and H2SO4 (1N) was used to stop the reaction after 15 minutes. The plates were read by an ELISA reader (Bio-Rad, Hercules, CA) at 450 nM.
To investigate the signaling mechanisms involved in rhMIF-induced adhesion molecule expression, we performed cell-surface ELISAs using chemical signaling inhibitors. MNs were isolated as described and 1 × 105 cells/well were seeded in 96-well plates in RPMI with 5% FBS. After 2 hours, MNs were incubated with serum-free RPMI. Cells were pretreated for 1 hour with 10 μM of each inhibitor except PTDC (100 μM): PD98059 (Erk1/2 inhibitor, PD), LY 294002 (PI3K inhibitor, LY), PP2 529573 (Src inhibitor, PP2), PDTC, and AG-490 (Jak2 inhibitor) before stimulating with rhMIF (50 nM). All inhibitors were from Calbiochem (San Diego, CA). TNF-α (1.15 nM; Upjohn, Kalamazoo, MI) served as a positive control. Cell-surface ELISAs were performed as described.
To confirm our data, MNs were transfected with Src, PI3K, NFκB, and Erk1/2 sense and antisense ODNs (2-5 μg) using LipofectAmine Plus reagent according to the protocol from Invitrogen (Carlsbad, CA).12,26-28,31,41 The sequences of the ODNs used in this study are as shown in Table 1. The corresponding sense ODN was used as a control for each antisense ODN. MNs were isolated as described and transfected for 24 hours. MNs were stimulated with rhMIF for 8 to 12 hours. The ODNs were synthesized and purified by the Northwestern University Biotechnology Laboratory and modified with phosphorothioate. Cell-surface ELISAs were performed as described.26,28
Cell adhesion assays
Ninety-six–well plates were placed under UV light for 30 minutes and coated with 0.02% sterile gelatin. HMEC-1 cells, an SV40 immortalized human dermal microvascular endothelial cell line, were plated into the 96-well plates at a concentration of 5 × 104 cells/well and incubated overnight at 37°C.27,28 Cells were stimulated with or without rhMIF (50 nM) or 1.15 nM TNF-α (positive control) for 8 hours at 37°C, 5% CO2. HL-60 cells (a human myelomonocytic cell line) were washed twice with PBS and adjusted to 5 × 106 cells/mL in serum-free RPMI 1640. HL-60 cells were incubated with calcAM (5 μM) fluorescent dye for 30 minutes at 37°C. HL-60 cells were then washed twice with RPMI 1640, to remove unincorporated dye and adjusted to 2.5 × 106 cells/mL. Labeled HL-60 cells (2.5 × 105/100 μL) were added to each well. Cells were incubated for 1 hour at 37°C and then washed very carefully 4 times with PBS. Fluorescence was determined using a fluorescence plate reader (Spectra-MAX Gemini; Molecular Devices, Sunnyvale, CA) set to 495 nm for excitation and 517 nm for emission. Adhesion was automatically expressed in relative fluorescence units. For better comparisons of the differentially treated groups and to avoid the use of relative fluorescence units, the adhesion of HL-60 cells to nonstimulated HMEC-1 cells was chosen as a reference. The adhesion index was, therefore, defined as the ratio of adhesion of HL-60 cells to stimulated HMEC-1 cells (in relative fluorescence units) to adhesion of HL-60 cells to unstimulated HMEC-1 cells (in relative fluorescence units).28 For experiments in which the adhesion to HMEC-1 cells was blocked by chemical signaling inhibitors and antibodies, cells were treated with inhibitors as well as mAbs to ICAM-1, VCAM-1, E-selectin (R&D Systems), or mouse isotype-matched control (2.5 μg/mL) for 1 hour at 37°C and 5% CO2 before stimulating with rhMIF.
We performed adhesion assays using primary human dermal microvascular endothelial cells, HMVECs. HMVECs (1 × 104/200 μL) were plated in fibronectin (Sigma Aldrich) coated 96-well plates. The media was switched to 0.1% BSA in EBM after 2 hours. HMVECs were stimulated with rhMIF (50 nM) for 12 hours, and cell adhesion assays were performed after dye-tagging HL-60 cells with calcAM as described.
Transfection of HMVECs with small interference RNA (siRNA) directed against MIF
HMVECs (1 × 104/200 μL) were plated on fibronectin-coated 96-well plates, and the medium was switched to 0.1% BSA in EBM to minimize any effects of serum. When HMVECs were 70% confluent, they were transfected for 24 hours with siRNA directed against MIF or control-scrambled MIF siRNA (Accession no. NM_002415; Invitrogen) using TransIT-siQUEST transfection reagent from Mirus (Madison, WI) according to the manufacturer's instructions (see Table 1 for MIF siRNA sequence). After 24 hours, HMVECs were stimulated with MIF (50 nM) for 12 hours, and cell-surface ELISAs were performed as described in “Cell-surface ELISAs for adhesion molecule expression.”
Cell lysis and immunoblotting
MNs were stimulated with rhMIF for various time periods to examine the up-regulation of signaling molecules. At the end of each period, the cells were lysed with 175 μL extraction buffer containing protease inhibitors (Protease inhibitor cocktail, 1 tablet/10 mL PBS; Boehringer Mannheim, Mannheim, Germany). The protein concentration in each sample was determined with Pierce bicinchoninic acid (BCA) protein assay kits (Rockford, IL).
MNs were stimulated for 12 hours to elucidate the up-regulation of VCAM-1 and ICAM-1 by rhMIF. For inhibitor studies, MNs were preincubated 1 hour with each respective inhibitor (10 μM) and PDTC (100 μM) prior to stimulation. We used the following inhibitors: PD, an Erk1/2 inhibitor, LY, a PI3K inhibitor, PP2, a Src inhibitor, and PDTC, an NFκB inhibitor. Cell lysates in Laemmli sample buffer (15 μg total protein) were boiled for 5 minutes and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% polyacrylamide) followed by Western blot analysis as previously described.27,28 Rabbit anti–human phospho-Src,-Akt, and -NFκB were from Cell Signaling Technology (Beverly, MA). Mouse anti–human ICAM-1 and sheep anti–human VCAM-1 were from R&D Systems. Blots were scanned using an imaging densitometer (Bio-Rad) and UN-SCAN-IT 5.1 software (Silk Scientific, Orem, UT). The immunoblots were stripped and reprobed with rabbit anti–human β-actin (Sigma Aldrich) to verify equal loading.
Immunofluorescence
MNs (2.5 × 105/100 μL) were plated on sterile 8-well Labtek chambers overnight in RPMI with 5% FBS. Medium was switched to serum-free RPMI, and cells were stimulated with rhMIF for 8 hours. Cells were then washed once with PBS and fixed at –20°C in cold methanol for 15 minutes. Cells were blocked in blocking buffer having 1% BSA and 30% goat serum in PBS. Mouse anti–human VCAM-1 and ICAM-1 (1:200 in PBS) as primary antibody or control IgG were added to the blocking buffer for 2 hours at 37°C and washed with PBS 3 times. Goat anti–mouse IgG-FITC antibody (Becton Dickinson, Franklin Lakes, NJ) and goat anti–mouse IgG–R-Phycoerythrin (Jackson ImmunoResearch Laboratories, West Grove, PA) as the secondary antibodies at a dilution of 1:200 in PBS were added. The slides were kept at 37°C for 45 minutes, and nuclear staining was performed with 4′,6-diamidino-2-phenylindole-dihydrochloride (DAPI; Molecular Probes, Eugene, OR). The slides were washed with PBS 3 times and mounted with fluorescence mounting medium on slides. The slides were kept at –20°C in the dark. Immunofluorescence was detected using an Olympus BX51 Fluorescence Microscope System with DP Manager imaging software (Olympus America, Melville, NY).12,28 To define the role of signaling pathways in up-regulating VCAM-1 and ICAM-1 expression, MNs were stimulated for 20 minutes with rhMIF. Immunofluorescence was performed using phospho-Src (Cell Signaling Technology) and phospho-NFκB (Santa Cruz Biotechnology) as described.
Results
rhMIF up-regulates MN VCAM-1 and ICAM-1 expression
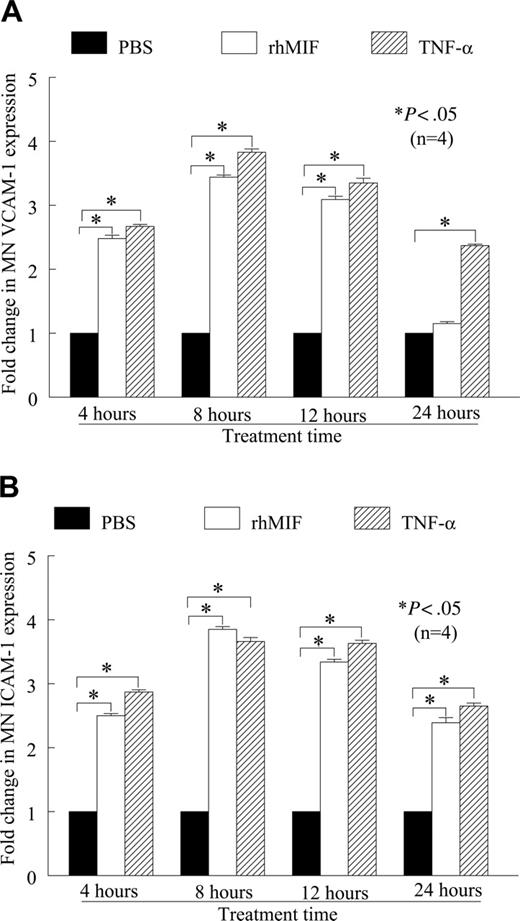
We found that rhMIF (50 nM) induced human peripheral blood MN VCAM-1 and ICAM-1 expression in a time-dependent manner, as determined by cell-surface ELISA (Figure 1A-B). Both VCAM-1 and ICAM-1 expression were significantly higher compared with nonstimulated (NS) MNs, starting from 4 hours. The maximum expression of these adhesion molecules was between 8 and 12 hours of stimulation and was comparable to the positive control, TNF-α. We found an approximate 2.5-fold increase in VCAM-1 and an approximate 3-fold increase in ICAM-1 expression compared with NS (P < .05). rhMIF-induced expression of MN VCAM-1 was reduced to basal levels at 24 hours, whereas ICAM-1 expression remained significantly higher, (P < .05), even after 24 hours.
rhMIF up-regulates MN VCAM-1 and ICAM-1 expression in a dose-dependent manner
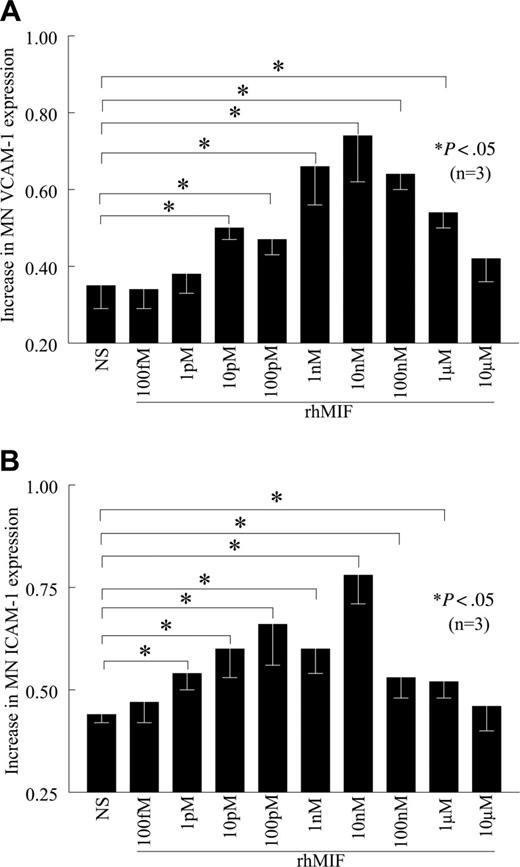
rhMIF up-regulates VCAM-1 expression starting from 10 pM, with a maximum response observed at 10 nM, which gradually declined (Figure 2A). rhMIF up-regulates MN ICAM-1 expression starting at 1 pM (Figure 2B). We did not find the up-regulation of VCAM-1 and ICAM-1 by rhMIF in femtomolar and micromolar doses, suggesting that rhMIF-induced VCAM-1 and ICAM-1 expression is a dose-dependent phenomenon.
Src, PI3K, and NFκB mediate rhMIF-induced MN VCAM-1 up-regulation
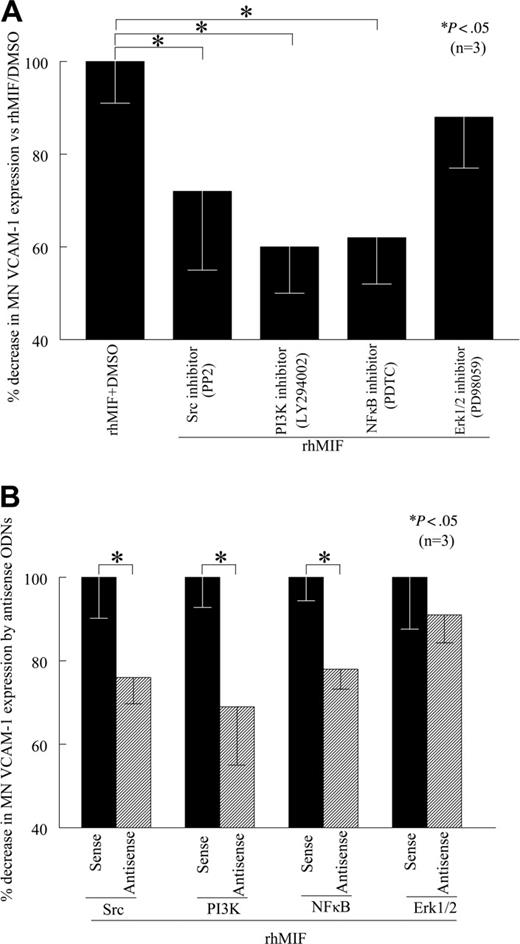
To determine the signaling events critical for rhMIF-induced VCAM-1 up-regulation, we performed cell-surface ELISAs in the presence of relatively specific kinase inhibitors. We found that PP2, LY, and PDTC, inhibitors of Src, PI3K, and NFκB pathways, respectively, significantly (P < .05) inhibited rhMIF-induced MN expression of VCAM-1, whereas PD, an Erk1/2 inhibitor, did not inhibit MN VCAM-1 expression, suggesting that Erk1/2 is not involved in VCAM-1 up-regulation by rhMIF (Figure 3A). An NFκB inhibitor (PDTC), a PI3K inhibitor (LY), and a Src inhibitor (PP2) decreased rhMIF-induced MN VCAM-1 up-regulation by 38%, 40%, and 28%, respectively. This suggests that NFκB, PI3K, and Src play a major role in rhMIF-induced expression of VCAM-1. To confirm the specificity of the signaling pathways involved in VCAM-1 up-regulation, we transfected human MNs with sense and antisense ODNs of Src, PI3K, NFκBp65, and Erk1/2 for 24 hours. We found that MNs transfected with antisense ODNs of Src, PI3K, and NFκB showed significantly less rhMIF-induced VCAM-1 expression compared with the MNs transfected with corresponding sense ODNs (Figure 3B; P < .05). In contrast, we did not find any difference in rhMIF-induced VCAM-1 expression in MNs transfected with Erk1/2 sense and antisense ODNs.
rhMIF up-regulates VCAM-1 and ICAM-1 expression on human PB MNs by cell-surface ELISA. (A) MNs (1 × 106 cells/well) were incubated in 96-well plates in RPMI with 5% FBS for 2 hours at 37°C. Medium was switched to serum free for 6 to 8 hours to achieve quiescence. MNs were stimulated with rhMIF (50 nM), and cell-surface ELISAs were performed. rhMIF induced a time-dependent increase in MN VCAM-1 expression. rhMIF-induced VCAM-1 expression became significantly higher at 4 hours, and it decreased to the basal level after 24 hours (*P < .05). TNF-α served as a positive control. Fold change in VCAM-1 expression compared with PBS is shown. (B) rhMIF induced expression of ICAM-1 in a time-dependent manner. rhMIF increased ICAM-1 expression at 4 hours, and the maximum response was between 8 and 12 hours. ICAM-1 expression in MNs remained significantly higher even after 24 hours compared with PBS (*P < .05). Panel B shows fold increase in ICAM-1 up-regulation compared with PBS. Data represent the mean of 4 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF up-regulates VCAM-1 and ICAM-1 expression on human PB MNs by cell-surface ELISA. (A) MNs (1 × 106 cells/well) were incubated in 96-well plates in RPMI with 5% FBS for 2 hours at 37°C. Medium was switched to serum free for 6 to 8 hours to achieve quiescence. MNs were stimulated with rhMIF (50 nM), and cell-surface ELISAs were performed. rhMIF induced a time-dependent increase in MN VCAM-1 expression. rhMIF-induced VCAM-1 expression became significantly higher at 4 hours, and it decreased to the basal level after 24 hours (*P < .05). TNF-α served as a positive control. Fold change in VCAM-1 expression compared with PBS is shown. (B) rhMIF induced expression of ICAM-1 in a time-dependent manner. rhMIF increased ICAM-1 expression at 4 hours, and the maximum response was between 8 and 12 hours. ICAM-1 expression in MNs remained significantly higher even after 24 hours compared with PBS (*P < .05). Panel B shows fold increase in ICAM-1 up-regulation compared with PBS. Data represent the mean of 4 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces MN ICAM-1 expression via Src, PI3K, and NFκB
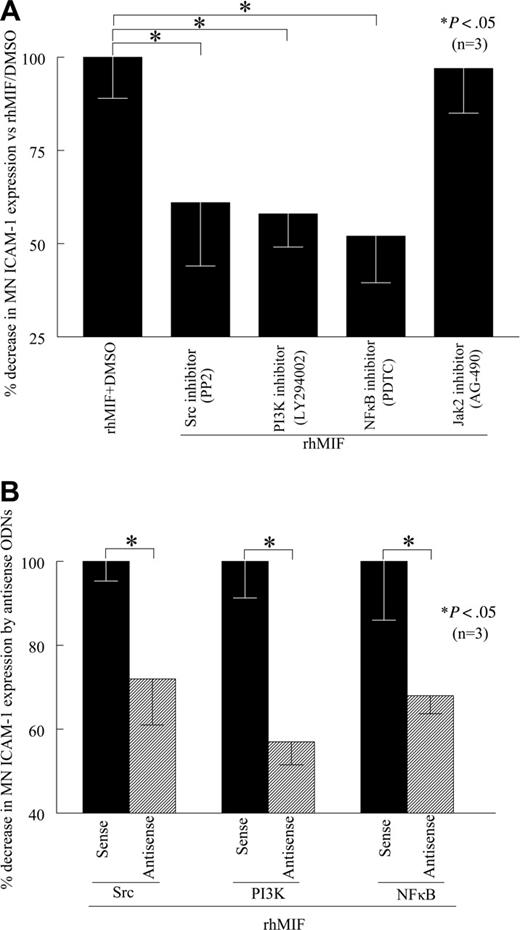
To evaluate the role of signaling cascades in rhMIF-induced MN ICAM-1 expression, we performed cell-surface ELISAs in the presence of chemical signaling inhibitors. We found that PP2, a Src inhibitor, LY, a PI3K inhibitor, PDTC, an NFκB inhibitor, significantly inhibited rhMIF-induced MN ICAM-1 expression, whereas AG-490, a Jak2 inhibitor, did not inhibit rhMIF-induced MN ICAM-1 expression, suggesting that the Jak2 pathway is not involved (Figure 4A). This decrease in MN ICAM-1 expression was 39% by a Src inhibitor (PP2), 42% by a PI3K inhibitor (LY), and 48% by an NFκB inhibitor (PDTC). The Jak2 inhibitor (AG-490) did not reduce rhMIF-induced ICAM-1 expression. To confirm our results, we transfected MNs with Src, PI3K, and NFκBp65 sense and antisense ODNs for 24 hours. Likewise, rhMIF-induced ICAM-1 expression was significantly reduced in MNs transfected with antisense ODNs compared with sense ODNs, suggesting the key role of these signaling intermediates in up-regulating rhMIF-induced MN ICAM-1 expression (Figure 4B).
rhMIF induces the adhesion of HL-60 cells to endothelial cells
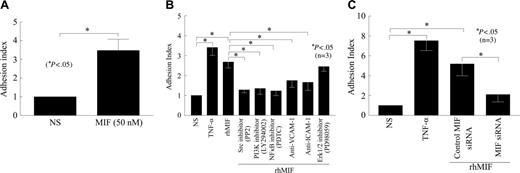
We evaluated the functional significance of the adhesion molecules induced by rhMIF with an adhesion assay using HMVECs/HMEC-1 cells and HL-60 cells. The endothelial cells were treated for 1 hour with inhibitors before they were stimulated with rhMIF or positive control, TNF-α. Following stimulation with rhMIF (50 nM) for 8 hours, the adhesion index was increased approximately 1.7-fold as compared with nonstimulated cells. The Src inhibitor (PP2), the NFκB inhibitor (PDTC), or the PI3K inhibitor (LY) significantly inhibited HL-60 cell adhesion to HMVECs/HMEC-1 cells (Figure 5A-B; P < .05). This decrease in adhesion of HL-60 cells to HMEC-1 cells was 52% by a Src inhibitor (PP2), 50% by the NFκB inhibitor (PDTC), and 54% by the PI3K inhibitor (LY). rhMIF-induced cell adhesion was decreased by 35% with anti–ICAM-1 and 42% with anti–VCAM-1. Antibody to E-selectin did not inhibit this adhesion (data not shown), suggesting that ICAM-1 and VCAM-1 account for the majority of rhMIF-induced HL-60–HMEC-1 adhesion, whereas E-selectin and Jak2 are not critical to this interaction.
rhMIF increases VCAM-1 and ICAM-1 expression on human PB MNs in a concentration-dependent manner. (A) MNs were stimulated with different concentrations of rhMIF for 12 hours. rhMIF-induced VCAM-1 expression was significantly higher between 10 pM and 1 μM (A). We did not observe VCAM-1 expression at 10 μM. (B) Similarly, rhMIF-induced ICAM-1 expression was significantly higher compared with nonstimulated MNs between 1 pM and 1 μM. We found the maximum up-regulation of VCAM-1 and ICAM-1 by rhMIF between 1 and 100 nM (A-B). NS indicates nonstimulated. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF increases VCAM-1 and ICAM-1 expression on human PB MNs in a concentration-dependent manner. (A) MNs were stimulated with different concentrations of rhMIF for 12 hours. rhMIF-induced VCAM-1 expression was significantly higher between 10 pM and 1 μM (A). We did not observe VCAM-1 expression at 10 μM. (B) Similarly, rhMIF-induced ICAM-1 expression was significantly higher compared with nonstimulated MNs between 1 pM and 1 μM. We found the maximum up-regulation of VCAM-1 and ICAM-1 by rhMIF between 1 and 100 nM (A-B). NS indicates nonstimulated. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces MN VCAM-1 expression via Src kinase, PI3K, and NFκB. (A) To define the signaling mechanisms involved in VCAM-1 up-regulation by rhMIF, MNs were incubated in 96-well plates. Signaling inhibitors (10 μM) were added to the cells an hour before stimulating with rhMIF and remained in the medium during the experiments. MNs were stimulated with rhMIF for 8 to 12 hours in the presence and absence of different signaling inhibitors. rhMIF-induced VCAM-1 expression was significantly inhibited by an Src inhibitor, PP2, a PI3K inhibitor, LY, and a NFκB inhibitor, PDTC, (*P < .05). PD, an Erk1/2 inhibitor, did not inhibit VCAM-1 expression, suggesting that rhMIF induces VCAM-1 expression in MNs via Src, PI3K, and NFκB, whereas Erk1/2 is not involved in VCAM-1 expression. VCAM-1 up-regulation by rhMIF was more than 2-fold compared with nonstimulated MNs. (B) To confirm our results, we transfected MNs with sense and antisense ODNs of Src, PI3K, NFκB, and Erk1/2 before stimulating cells with rhMIF using lipofectAmine Plus reagent in cell-surface ELISAs. MNs (1 × 106 cells/well) were incubated in 96-well plates in RPMI with 5% FBS for 2 hours at 37°C, and medium was switched to serum free. MNs were transfected with ODNs for 24 hours before stimulation with rhMIF (50 nM). MNs were stimulated with rhMIF for 8 to 12 hours. rhMIF-induced VCAM-1 expression was significantly decreased by antisense ODNs of Src, PI3K, and NFκB compared with MNs transfected with corresponding sense ODNs (*P < .05) using an ELISA. We did not find a decrease in rhMIF-induced VCAM-1 expression by antisense ODNs of Erk1/2. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces MN VCAM-1 expression via Src kinase, PI3K, and NFκB. (A) To define the signaling mechanisms involved in VCAM-1 up-regulation by rhMIF, MNs were incubated in 96-well plates. Signaling inhibitors (10 μM) were added to the cells an hour before stimulating with rhMIF and remained in the medium during the experiments. MNs were stimulated with rhMIF for 8 to 12 hours in the presence and absence of different signaling inhibitors. rhMIF-induced VCAM-1 expression was significantly inhibited by an Src inhibitor, PP2, a PI3K inhibitor, LY, and a NFκB inhibitor, PDTC, (*P < .05). PD, an Erk1/2 inhibitor, did not inhibit VCAM-1 expression, suggesting that rhMIF induces VCAM-1 expression in MNs via Src, PI3K, and NFκB, whereas Erk1/2 is not involved in VCAM-1 expression. VCAM-1 up-regulation by rhMIF was more than 2-fold compared with nonstimulated MNs. (B) To confirm our results, we transfected MNs with sense and antisense ODNs of Src, PI3K, NFκB, and Erk1/2 before stimulating cells with rhMIF using lipofectAmine Plus reagent in cell-surface ELISAs. MNs (1 × 106 cells/well) were incubated in 96-well plates in RPMI with 5% FBS for 2 hours at 37°C, and medium was switched to serum free. MNs were transfected with ODNs for 24 hours before stimulation with rhMIF (50 nM). MNs were stimulated with rhMIF for 8 to 12 hours. rhMIF-induced VCAM-1 expression was significantly decreased by antisense ODNs of Src, PI3K, and NFκB compared with MNs transfected with corresponding sense ODNs (*P < .05) using an ELISA. We did not find a decrease in rhMIF-induced VCAM-1 expression by antisense ODNs of Erk1/2. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
siRNA directed against MIF inhibits rhMIF-induced adhesion of HL-60 cells to HMVECs
To determine the significance of MIF in adhesion assays, we transfected HMVECs with MIF siRNA for 24 hours using TransIT-siQUEST transfection reagent. HMVECs were stimulated with rhMIF for 12 hours. We found a substantial decrease in rhMIF-induced adhesion of HL-60 dye-tagged with calcAM to HMVECs transfected with siRNA directed against MIF compared with HMVECs transfected with control-scrambled MIF siRNA (P < .05). This inhibition was greater than 2-fold as determined by the adhesion index (Figure 5C).
rhMIF induces Src, PI3k, and NFκB phosphorylation in MNs
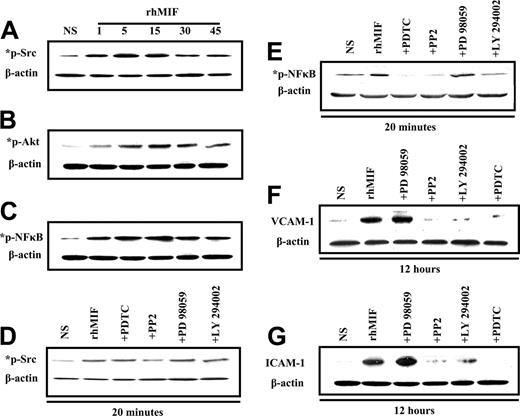
MNs were stimulated with rhMIF (50 nM). rhMIF activated phospho-Src, -PI3K, and -NFκB in MNs in a time-dependent manner, with the maximal response at 10 to 15 minutes. This activation was blocked in pretreated MNs by the inhibitors of Src, PI3K, and NFκB as shown in Figure 6A-E.
rhMIF induces MN VCAM-1 and ICAM-1 expression via Src, PI3K, and NFκB by Western blots
We found that rhMIF increased the expression of VCAM-1 and ICAM-1 in MNs in 12 hours. For inhibitor studies, cells were treated for 1 hour before stimulating with rhMIF. rhMIF induces MN expression of VCAM-1 and ICAM-1 via Src, PI3K, and NFκB, because we found a marked decrease in VCAM-1 and ICAM-1 expression in the presence of the inhibitors of Src, PI3K, and NFκB. This suggests that Src, PI3K, and NFκB play an important role in rhMIF-induced up-regulation of adhesion molecules, but Erk1/2 is not involved, because we did not observe a decrease in VCAM-1 and ICAM-1 up-regulation by an inhibitor of Erk1/2.
rhMIF induces MN ICAM-1 up-regulation via Src kinase, PI3K, and NFκB. (A) To investigate the signaling cascades involved in ICAM-1 up-regulation by rhMIF, we performed cell-surface ELISAs using chemical signaling inhibitors. rhMIF-increased ICAM-1 expression was significantly inhibited by an Src inhibitor, PP2, a PI3K inhibitor, LY, and a NFκB inhibitor, PDTC, (*P < .05) but not by a Jak2 inhibitor, AG-490, suggesting that rhMIF induces ICAM-1 expression in MNs via Src, PI3K, and NFκB. rhMIF induced a 3-fold increase in MN ICAM-1 expression compared with NS. (B) rhMIF-induced ICAM-1 expression was significantly decreased by antisense ODNs of Src, PI3K, and NFκB compared with MNs transfected with sense ODNs of Src, PI3K, and NFκB in 8 to 12 hours (*P < .05). Panel B shows the percentage of inhibition in ICAM-1 expression by antisense ODNs of Src, PI3K, and NFκB compared with corresponding sense ODNs. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces MN ICAM-1 up-regulation via Src kinase, PI3K, and NFκB. (A) To investigate the signaling cascades involved in ICAM-1 up-regulation by rhMIF, we performed cell-surface ELISAs using chemical signaling inhibitors. rhMIF-increased ICAM-1 expression was significantly inhibited by an Src inhibitor, PP2, a PI3K inhibitor, LY, and a NFκB inhibitor, PDTC, (*P < .05) but not by a Jak2 inhibitor, AG-490, suggesting that rhMIF induces ICAM-1 expression in MNs via Src, PI3K, and NFκB. rhMIF induced a 3-fold increase in MN ICAM-1 expression compared with NS. (B) rhMIF-induced ICAM-1 expression was significantly decreased by antisense ODNs of Src, PI3K, and NFκB compared with MNs transfected with sense ODNs of Src, PI3K, and NFκB in 8 to 12 hours (*P < .05). Panel B shows the percentage of inhibition in ICAM-1 expression by antisense ODNs of Src, PI3K, and NFκB compared with corresponding sense ODNs. Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces the adhesion of HL-60 cells to HMVECs/HMEC-1 cells. (A) To evaluate the functional significance of rhMIF-induced ICAM-1 and VCAM-1 expression, we performed cell adhesion assays using a human myelomonocytic cell line, HL-60, and HMVECs. HMVECs (12.5 × 103 cells/well) were plated on fibronectin-coated 96-well plates in EBM with 10% FBS. Medium was switched to 0.1 BSA in EBM when HMVECs were 70% confluent. HMVECs were stimulated with rhMIF (50 nM) for 12 hours, and cell adhesion assays were performed. HL-60 cells (8 × 106 cells/well) labeled with calcAM were added to each well and incubated for 1 hour with HMVECs. Plates were carefully washed 4 times with PBS, and fluorescence was determined by a fluorescent plate reader set to 495 nm for excitation and 517 nm for emission. Adhesion was expressed in relative fluorescence units. We found an approximate 2.5-fold significant increase in the adhesion index in rhMIF-stimulated HMVECs compared with nonstimulated cells (A). For better comparisons of the differentially treated groups, the adhesion of HL-60 cells to nonstimulated HMEC-1 cells was chosen as a reference. The adhesion index was, therefore, defined as the ratio of adhesion of HL-60 cells to stimulated HMEC-1 cells (in relative fluorescence units) to adhesion of HL-60 cells to unstimulated HMEC-1 cells (in relative fluorescence units). (B) To elucidate the signaling mechanisms involved rhMIF-induced VCAM-1 and ICAM-1 expression in the functional assays, we performed cell adhesion assays in the presence and absence of signaling inhibitors. In this assay we used an endothelial cell line, HMEC-1. HMEC-1 cells were incubated with signaling inhibitors, anti–ICAM-1, and anti–VCAM-1 or isotype mouse-matched control (2.5 μg/mL) for 1 hour before they were stimulated with rhMIF (50 nM) or 1.15 nM TNF-α (positive control) for 8 hours at 37°C, 5% CO2. HL-60 cells (2.5 × 106 cells/mL, 100 μL) labeled with calcAM were added and incubated for 1 hour with HMEC-1 cells, and adhesion assays were performed. The Src inhibitor (PP2), the PI3K inhibitor (LY), the NFκB inhibitor, anti–ICAM-1, and anti–VCAM inhibited HL-60 cell adhesion to HMEC-1 cells (Figure 5B), but the Erk1/2 inhibitor (PD) did not inhibit rhMIF-induced adhesion. Data from 3 separate experiments are presented as the mean (n) ± SE. *P < .05 was considered significant. (C) To confirm the role of rhMIF in up-regulating these adhesion molecules, we used siRNA directed against MIF. HMVECs were plated on fibronectin-coated 96-well plates and transfected with siRNA directed against MIF or control-scrambled MIF siRNA for 24 hours using TransIT-siQUEST transfection reagent. HMVECs were stimulated with rhMIF (50 nM) for 12 hours, and cell adhesion assays were performed (C). Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
rhMIF induces the adhesion of HL-60 cells to HMVECs/HMEC-1 cells. (A) To evaluate the functional significance of rhMIF-induced ICAM-1 and VCAM-1 expression, we performed cell adhesion assays using a human myelomonocytic cell line, HL-60, and HMVECs. HMVECs (12.5 × 103 cells/well) were plated on fibronectin-coated 96-well plates in EBM with 10% FBS. Medium was switched to 0.1 BSA in EBM when HMVECs were 70% confluent. HMVECs were stimulated with rhMIF (50 nM) for 12 hours, and cell adhesion assays were performed. HL-60 cells (8 × 106 cells/well) labeled with calcAM were added to each well and incubated for 1 hour with HMVECs. Plates were carefully washed 4 times with PBS, and fluorescence was determined by a fluorescent plate reader set to 495 nm for excitation and 517 nm for emission. Adhesion was expressed in relative fluorescence units. We found an approximate 2.5-fold significant increase in the adhesion index in rhMIF-stimulated HMVECs compared with nonstimulated cells (A). For better comparisons of the differentially treated groups, the adhesion of HL-60 cells to nonstimulated HMEC-1 cells was chosen as a reference. The adhesion index was, therefore, defined as the ratio of adhesion of HL-60 cells to stimulated HMEC-1 cells (in relative fluorescence units) to adhesion of HL-60 cells to unstimulated HMEC-1 cells (in relative fluorescence units). (B) To elucidate the signaling mechanisms involved rhMIF-induced VCAM-1 and ICAM-1 expression in the functional assays, we performed cell adhesion assays in the presence and absence of signaling inhibitors. In this assay we used an endothelial cell line, HMEC-1. HMEC-1 cells were incubated with signaling inhibitors, anti–ICAM-1, and anti–VCAM-1 or isotype mouse-matched control (2.5 μg/mL) for 1 hour before they were stimulated with rhMIF (50 nM) or 1.15 nM TNF-α (positive control) for 8 hours at 37°C, 5% CO2. HL-60 cells (2.5 × 106 cells/mL, 100 μL) labeled with calcAM were added and incubated for 1 hour with HMEC-1 cells, and adhesion assays were performed. The Src inhibitor (PP2), the PI3K inhibitor (LY), the NFκB inhibitor, anti–ICAM-1, and anti–VCAM inhibited HL-60 cell adhesion to HMEC-1 cells (Figure 5B), but the Erk1/2 inhibitor (PD) did not inhibit rhMIF-induced adhesion. Data from 3 separate experiments are presented as the mean (n) ± SE. *P < .05 was considered significant. (C) To confirm the role of rhMIF in up-regulating these adhesion molecules, we used siRNA directed against MIF. HMVECs were plated on fibronectin-coated 96-well plates and transfected with siRNA directed against MIF or control-scrambled MIF siRNA for 24 hours using TransIT-siQUEST transfection reagent. HMVECs were stimulated with rhMIF (50 nM) for 12 hours, and cell adhesion assays were performed (C). Data represent the mean of 3 individual experiments (n) ± SEM. *P < .05 was considered significant.
Immunoblotting of MNs stimulated with rhMIF for various time points. (A) MNs were stimulated with rhMIF (50 nM) for time periods of 1 minute to 45 minutes. rhMIF induced a marked increase in Src, Akt, and NFκB phosphorylation in a time-dependent manner compared with nonstimulated cells. This phosphorylation was inhibited by relatively specific chemical inhibitors (A-E). (B) Western blotting was performed with MNs stimulated with rhMIF (50 nM) for 12 hours to examine the up-regulation of VCAM-1 and ICAM-1. For inhibitor studies, MNs were pretreated with PP2, LY, PD, and PDTC for 1 hour prior to stimulating with rhMIF. All inhibitors were used at 10 μM concentration except PDTC (100 μM). rhMIF induced a marked increase in VCAM-1 and ICAM-1 in MNs at 12 hours. rhMIF-induced MN VCAM-1 and ICAM-1 up-regulations were abrogated by the inhibitors of Src, PI3K, and NFκB, but an inhibitor of Erk1/2 did not affect the expression of the adhesion molecules (F-G). Each blot represents 1 of 3 experiments.
Immunoblotting of MNs stimulated with rhMIF for various time points. (A) MNs were stimulated with rhMIF (50 nM) for time periods of 1 minute to 45 minutes. rhMIF induced a marked increase in Src, Akt, and NFκB phosphorylation in a time-dependent manner compared with nonstimulated cells. This phosphorylation was inhibited by relatively specific chemical inhibitors (A-E). (B) Western blotting was performed with MNs stimulated with rhMIF (50 nM) for 12 hours to examine the up-regulation of VCAM-1 and ICAM-1. For inhibitor studies, MNs were pretreated with PP2, LY, PD, and PDTC for 1 hour prior to stimulating with rhMIF. All inhibitors were used at 10 μM concentration except PDTC (100 μM). rhMIF induced a marked increase in VCAM-1 and ICAM-1 in MNs at 12 hours. rhMIF-induced MN VCAM-1 and ICAM-1 up-regulations were abrogated by the inhibitors of Src, PI3K, and NFκB, but an inhibitor of Erk1/2 did not affect the expression of the adhesion molecules (F-G). Each blot represents 1 of 3 experiments.
rhMIF induces the expression of VCAM-1 and ICAM-1 in MNs via Src and NFκBp65 by immunofluorescence
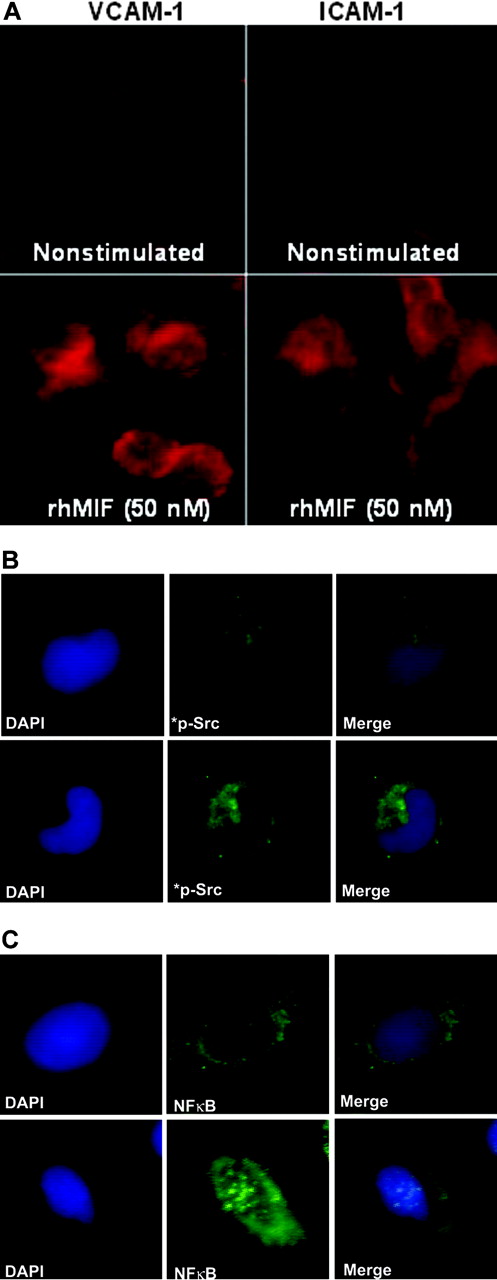
We performed immunofluorescence after stimulating MNs with rhMIF for 8 to 12 hours. rhMIF induced VCAM-1 and ICAM-1 expression on MNs compared with nonstimulated MNs. VCAM-1 and ICAM-1 expression were clearly visible on MNs at the cell surface (Figure 7A).
To evaluate the role of signaling cascades, we performed immunofluorescence with MNs stimulated with rhMIF for 20 minutes using phosphospecific rabbit polyclonal anti–human Src and anti–human NFκB. rhMIF activates phospho-Src and phospho-NFκBp65, as detected by immunofluorescence, compared with PBS, further confirming the hypothesis that rhMIF has a direct effect on MNs and its effect is mediated through Src and NFκB (Figure 7B-C).
Discussion
MIF is a proinflammatory cytokine which plays a critical role in the initiation and progression of chronic inflammatory and immune-mediated diseases such as RA and atherosclerosis. Leukocyte recruitment and angiogenesis are key factors implicated in the perpetuation and proliferation of these diseases. We and others have shown that MIF is a very potent angiogenic factor as well as playing a role in autoimmune diseases.10-14
This study elucidates rhMIF-induced up-regulation of VCAM-1 and ICAM-1 in MNs as well as the signaling cascades involved. We found that rhMIF increases the expression of adhesion molecules in a time- and concentration-dependent manner, starting from 4 hours with maximum response between 8 and 12 hours. VCAM-1 expression was reduced to basal levels in 24 hours, whereas ICAM-1 levels remained significantly higher compared with nonstimulated MNs even after 24 hours (Figure 1A-B). rhMIF increased VCAM-1 expression at 10 pM and ICAM-1 expression at 1 pM, suggesting that different times and doses of rhMIF are required for up-regulation of these adhesion molecules. Matsui et al10 have shown that anti-MIF antibody ameliorates autoimmune myocarditis via decreasing the expression of VCAM-1 on endothelial cells and via decreasing the inflammatory cell infiltration into the heart. That study provides indirect evidence of VCAM-1–MIF interaction. In contrast, we demonstrate a direct role of rhMIF in increasing VCAM-1 and ICAM-1 expression on MNs. Denkinger et al11 have demonstrated that anti-MIF antibody treatment prevents the up-regulation of VCAM-1 on endothelial cells in the central nervous system and ameliorates experimental autoimmune encephalomyelitis. In contrast to Matsui et al10 and our report, this group did not find any difference in the expression of ICAM-1 in anti-MIF treated or control groups. Another study determined that anti-MIF treatment prevented the up-regulation of VCAM-1 and ICAM-1 in the rat kidney and blocked the development of autoimmune glomerulonephritis.19 Our findings provide direct evidence for the role of MIF in up-regulating MN VCAM-1 and ICAM-1 expression.
rhMIF induces VCAM-1 and ICAM-1 on MNs via Src and NFκB as determined by immunofluorescence. MNs were plated in 8-well chamber slides overnight. MNs were stimulated with rhMIF (50 nM) for 10 hours and studied for VCAM-1 and ICAM-1 expression using FITC-conjugated and PE-conjugated secondary antibodies. VCAM-1 and ICAM-1 expression was visible on the cell surface (A). To investigate Src activation by rhMIF, MNs were stimulated for 20 minutes. Panels B and C show immunopositivity for phospho-Src and phospho-NFκBp65 compared with NS cells. DAPI was added to stain nuclei. We have merged the DAPI and phosphorylation images in panels B and C to show the nuclear and cytoplasmic localization of phospho-NFκBp65 and phospho-Src. NS indicates nonstimulated. Images were captured using an Olympus BX fluorescence microscope with attached Olympus camera (Olympus, Melville, NY) and a 100 ×/1.3 numeric aperture objective, with oil as an imaging medium. Images were assembled using Adobe Photoshop software, version 7.01 (Adobe Systems, San Jose, CA).
rhMIF induces VCAM-1 and ICAM-1 on MNs via Src and NFκB as determined by immunofluorescence. MNs were plated in 8-well chamber slides overnight. MNs were stimulated with rhMIF (50 nM) for 10 hours and studied for VCAM-1 and ICAM-1 expression using FITC-conjugated and PE-conjugated secondary antibodies. VCAM-1 and ICAM-1 expression was visible on the cell surface (A). To investigate Src activation by rhMIF, MNs were stimulated for 20 minutes. Panels B and C show immunopositivity for phospho-Src and phospho-NFκBp65 compared with NS cells. DAPI was added to stain nuclei. We have merged the DAPI and phosphorylation images in panels B and C to show the nuclear and cytoplasmic localization of phospho-NFκBp65 and phospho-Src. NS indicates nonstimulated. Images were captured using an Olympus BX fluorescence microscope with attached Olympus camera (Olympus, Melville, NY) and a 100 ×/1.3 numeric aperture objective, with oil as an imaging medium. Images were assembled using Adobe Photoshop software, version 7.01 (Adobe Systems, San Jose, CA).
We found that rhMIF affects MNs by up-regulating VCAM-1 and ICAM-1 in a time-dependent manner. This increase was about 2-fold for VCAM-1 and 3-fold for ICAM-1 compared with control groups (Figures 1, 2). Lin et al18 found that MIF up-regulates ICAM-1 on endothelial cells. We found that rhMIF up-regulates both VCAM-1 and ICAM-1 in endothelial cells and RA synovial tissue fibroblasts (data not shown). Similarly, Gregory et al42 have described an in vivo model in which they found that LPS-induced leukocyte-endothelial cell interactions and recruitment in the inflamed microcirculation in the cremaster muscle are reduced in MIF-null mice compared with wild-type mice. That study demonstrated that anti–P-selectin attenuated leukocyte-endothelial cell interactions and rolling, suggesting that MIF mediates leukocyte emigration and rolling via P-selectin. However, no differences in LPS-induced P-selectin expression in MIF-null and wild-type mice were found. This group did not examine VCAM-1 expression and its role in leukocyte rolling and emigration in wild-type mice treated with LPS or TNF-α in the same model. We have demonstrated MNs VCAM-1 and ICAM-1 expression by rhMIF using cell-surface ELISAs (Figure 1A-B). We performed Western blots with MNs stimulated with rhMIF for 12 hours and found a marked increase in VCAM-1 and ICAM-1 expression by rhMIF compared with nonstimulated MNs (Figure 6 F-G). We confirmed our results by immunofluorescence (Figure 7A).
To evaluate the functional significance of rhMIF-induced expression of adhesion molecules, we performed cell adhesion assays using HMVEC/HMEC-1 and HL-60 cells. The endothelial cells were stimulated with rhMIF (50 nM) or positive control, TNF-α, for 8 to 12 hours. We found that the adhesion index of rhMIF-stimulated cells was significantly higher compared with nonstimulated cells (Figure 5). Anti–ICAM-1 and anti–VCAM-1 inhibited rhMIF-induced HL-60 cell adhesion to HMEC-1 cells virtually completely, whereas anti–E-selectin did not inhibit this adhesion, implying that rhMIF-induced adhesion of HL-60 cells to HMEC-1 cells is mediated by ICAM-1 and VCAM-1, whereas E-selectin is not critical (data not shown). We confirmed our results by performing cell adhesion assays with HMVECs transfected with siRNAdirected against MIF. We found more than a 2-fold decrease in rhMIF-induced adhesion index in HMVECs transfected with MIF siRNA compared with HMVECs transfected with control siRNA. Huang et al43 have reported that MIF up-regulation in acute gastric ulcers correlated with marked macrophage and neutrophil accumulation and that anti-MIF attenuated acute gastric ulcers by decreasing TNF-α, ICAM-1, and nitric oxide synthase mRNA, and protein expression. Our results suggest that MIF may participate in leukocyte adhesion which may then contribute to leukocyte recruitment.
MIF stimulates release of proinflammatory cytokines by macrophages such as TNF-α, IL-1β, IL-6, and IL-8 and up-regulates MMP-1, MMP-3, MMP-9, and MMP-13 in RA ST fibroblasts.5,26,27,44,45 MIF ablation ameliorated rodent models of arthritis, encephalomyelitis, and autoimmune myocarditis.7-11 Leukocyte migration in the cremaster muscle or the synovial microcirculation is decreased in MIF gene–deficient mice compared with wild-type mice, following exposure to LPS and carrageenan, respectively.42 The basic mechanism common to each of these models is leukocyte adhesion and recruitment mediated by cell adhesion molecules.
We found that rhMIF-induced MN VCAM-1 expression was inhibited by signaling inhibitors of Src, PI3K, and NFκB but not Erk1/2. This suggests that Erk1/2 is not involved in VCAM-1 up-regulation by rhMIF. To confirm our results, we used sense and antisense ODNs to block the genes of specific interest such as Src, PI3K, and NFκB. We found that VCAM-1 up-regulation by rhMIF was decreased by Src, PI3K, and NFκB antisese ODNs, whereas Erk1/2 antisense as well as sense ODNs of Src, PI3K, and NFκB did not affect its expression. Similarly, we found that Src, PI3K, and NFκB are involved in rhMIF-induced ICAM-1 expression on MNs using chemical inhibitors as well as sense and antisense ODNs. Jak2 was not involved in rhMIF-induced MN ICAM-1 expression. To demonstrate the functional significance of this adhesion, we performed cell adhesion assays in vitro using HL-60 and HMEC-1 cells. We found that rhMIF-induced HL-60 cell adhesion to HMEC-1 cells was significantly decreased by inhibitors of Src, PI3K, NFκB, anti–VCAM-1, and anti–ICAM-1. Erk1/2 did not inhibit the adhesion of HL-60 cells to HMEC-1 cells. We examined the mechanism of MIF-induced up-regulation of adhesion molecules by performing Western blots. rhMIF up-regulated VCAM-1 and ICAM-1 in MNs in 12 hours via Src, PI3K, and NFκB, but Erk1/2 was not important. Our data define the mechanism by which MIF induces MN-endothelial adhesion.
Src kinases are activated during the interactions of MNs with endothelial cells, in endothelial cell differentiation, and in cell-matrix adhesion and cell migration.26,40,46-48 Src kinases are activated by a variety of growth factors. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor stimulate Src activation in avian endothelial cells, and sE-selectin and VEGF mediate angiogenesis via Src family tyrosine kinases.30,31 In this study, we found that rhMIF up-regulates VCAM-1 and ICAM-1 on MNs by activating Src. rhMIF also activates Src up-regulation in a time-dependent manner as determined by Western blots (Figure 6). rhMIF up-regulates phospho-Src starting at 5 minutes with a maximum response between 15 and 30 minutes. We have confirmed our data by using sense and antisense ODNs of Src in cell-surface ELISAs, cell adhesion assays, and by immunofluorescence. MIF increases MMP-13 and MMP-1 mRNA levels in rat osteoblasts and human dermal fibroblasts, respectively, via the Src kinase pathways, but rhMIF-induced Src phosphorylation was not correlated with cell adhesion molecule expression.6,49 Targeting Src may provide us a potential tool for treating diseases characterized by MIF-induced cell adhesion molecule up-regulation.
We next investigated the role of PI3K in rhMIF-induced expression of adhesion molecules. We found that rhMIF induced VCAM-1 and ICAM-1 expression via PI3K.26-28 PI3K is implicated in cell adhesion, cell survival, and angiogenesis.29,50-53 We have previously shown that rhMIF and sE-selectin mediate angiogenesis via PI3K.12,31 We have shown that IL-18 up-regulates VCAM-1 via PI3K.27 This report is in agreement with the finding that PI3K is important in cell adhesion molecule expression. We found that PI3K mediates rhMIF-induced expression of VCAM-1 and ICAM-1 on MNs by cell-surface ELISAs and cell adhesion assays. We confirmed our results using PI3K sense and antisense ODNs. rhMIF activates phospho-Akt, a downstream target of PI3K, in a time-dependent manner as determined by Western blotting. We showed maximum Akt phosphorylation induced by rhMIF at 15 minutes (Figure 6).
NFκB is a ubiquitous transcriptional factor and promotes the transcription of 150 genes.54,55 Many of theses genes are proinflammatory, including cytokines, adhesion molecules, nitric oxide synthase, and cyclooxygenase. It is activated by cytokines such as IL-1β, TNF-α, and IL-18.26,55,56 IL-1β, TNF-α, and IL-18 increase the expression of VCAM-1 and ICAM-1 via activating NFκB. These reports are consistent with our study, because we have shown that rhMIF mediates VCAM-1 and ICAM-1 up-regulation via NFκB. There are conflicting reports about the up-regulation of NFκB by MIF. Lacey et al57 have reported that MIF regulates RA ST fibroblast proliferation via Erk1/2 but not via NFκB. Similarly, Kleemann et al58 have reported that TNF-α–induced NFκB activation in 293T cells is not reduced by MIF, and MIF activates the transcriptional proteinAP-1 to up-regulate several proinflammatory genes. In contrast to those 2 reports, Daun and Cannon59 have reported that MIF antagonizes the anti-inflammatory effects of hydrocortisone in mononuclear cells by increasing NFκB activation. Our results support those of Daun and Cannon,59 because we have found rhMIF activates NFκBp65 phosphorylation in MNs in a time-dependent manner by Western blotting and immunofluorescence (Figures 6, 7). We have also found that rhMIF increases the expression of VCAM-1 and ICAM-1 in MNs via NFκB. We used chemical signaling inhibitors and sense and antisense ODNs of NFκB. This NFκB activation by MIF may be cell specific, because we and Daun and Cannon59 have found NFκB activation in MNs, whereas Lacey et al57 and Kleeman et al58 have performed experiments using RA fibroblasts and a kidney tumor cell line. There are a number of reports showing that up-regulation of VCAM-1 and ICAM-1 is highly dependent on NFκB activation and there is no role of MAPKs in the up-regulation of cell adhesion molecules.55,60,61
The significance of up-regulation of adhesion molecules on mononuclear cells is that they are shed and are angiogenic in soluble form.15,16 They promote ingress of mononuclear cells into inflammatory sites.20-22 The up-regulation of soluble adhesion molecules correlates with the severity of RA and atherosclerosis.20-22,62-64
In conclusion, MIF up-regulates VCAM-1 and ICAM-1 in human MNs via Src, PI3K, and NFκB activation. MIF or its signaling intermediates may prove to be potential therapeutic targets for the treatment of diseases characterized by leukocyte recruitment via cell adhesion molecules such as RA and atherosclerosis.
Prepublished online as Blood First Edition Paper,November 29, 2005; DOI 10.1182/blood-2005-05-2011.
Supported by funds from the Veterans Administration Research Services (A.E.K.) and the William D. Robinson and Frederick Huetwell Endowment (A.E.K.) for Arthritis Research and the Gallagher Professorship (A.E.K.) for Arthritis Research. Additional support included funds from the National Institute of Health (grants AI40987, HL58695, and AR48267) (A.E.K.), and a postdoctoral fellowship grant from the American Heart Association (AHA).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Edwin Ades of the Centers for Disease Control and Thomas Lawley of Emory University for providing the HMEC-1 cells.