Abstract
Invasive aspergillosis remains a serious complication in patients undergoing allogeneic stem cell transplantation (SCT). Since it became clear that lymphocytes provide a critical secondary defense against fungi, adoptive transfer of functionally active anti-Aspergillus T cells might be an option to restore adaptive immune effector mechanisms. Using the interferon (IFN)-γ secretion assay, we isolated human activated T cells upon stimulation with a cellular extract of Aspergillus fumigatus. Culturing this cell population for 14 days, we obtained an average of 1.1 × 107 cells from a single 100-mL blood draw in 7 of 7 healthy individuals. Within another 14 days, these cells were expanded to an average number of 2.0 × 108 T-helper 1 (TH1) cells secreting IFN-γ on stimulation with Aspergillus antigens. Testing various fungal antigen extracts, similar proportions of IFN-γ-producing CD3+/CD4+ cells were obtained upon activation with antigen extracts of A fumigatus, A flavus, A niger, and Penicillium chrysogenum, whereas no significant IFN-γ production was observed upon activation with antigen extracts of Alternaria alternata and Candida albicans. In addition, generated T cells were able to induce damage to A fumigatus hyphae, and significantly increased hyphal damage induced by human neutrophils. CD4+ T-cell-mediated alloreactivity of generated anti-Aspergillus T cells was clearly reduced compared with that of the original cell population. In conclusion, we present a simple and feasible strategy for rapid generation of a high number of functional active T cells against Aspergillus from a single blood draw. Our data suggest that functionally active T cells against Aspergillus could be a promising treatment option for patients undergoing allogeneic SCT. (Blood. 2006;107: 2562-2569)
Introduction
Invasive aspergillosis (IA) remains a major cause of morbidity and mortality in patients with hematologic malignancies, particularly in patients who have undergone allogeneic stem cell transplantation (SCT).1 Important risk factors for IA are neutropenia and defects in the phagocyte cell function.2 On the other hand, there is an increasing body of evidence that adaptive immune response also plays a critical role in the host defense against Aspergillus fumigatus, the most frequent cause of IA. For example, IA has been reported with increasing frequency in patients with advanced AIDS,3 and up to two-thirds of patients with IA diagnosed after allogeneic SCT are not neutropenic.1 In contrast to neutrophil recovery, which generally occurs within the first 2 to 3 weeks after SCT, the number of functional T cells and T-cell function increase slowly over the first few months after transplantation.4 Prolonged immune suppression due either to transplant conditioning and graft-versus-host disease (GVHD) prophylaxis or to treatment of GVHD further increase the risk of IA in SCT patients.5,6 It has recently been shown that a significant antigen-specific proliferation of interferon (IFN)-γ-producing T cells occurred in healthy individuals and in patients surviving IA,7 which confirms the crucial role of a T helper (TH) 1 reactivity in the control of infection.8-10
In patients undergoing SCT, significant progress has been made in reconstituting adaptive cell immunity with antigen-specific T cells to restore essential responses for protection against a specific pathogen. For example, an early study reported on cytomegalovirus (CMV) prophylaxis with adoptively transferred, donor-derived, CMV-specific lymphocytes in allogeneic matched sibling SCT recipients.11 No adverse events occurred, and no patient who received these cell infusions developed CMV disease. Another group treated 8 patients who lacked CMV-specific T-cell proliferation and had received unsuccessful antiviral chemotherapy for CMV infection.12 After transfer of 1 × 107 T cells/m2 , the CMV load significantly decreased in 7 evaluable patients despite cessation of antiviral medication.12 In contrast to the generation of virus-specific T cells, such as T cells specific for CMV, Epstein-Barr virus (EBV), or adenovirus,13-15 few data exist regarding functionally active anti-Aspergillus T cells.10,16-18 In addition, most of the approaches to generate antigen-specific T cells using peptide-pulsed dendritic cells (DCs)16,19 and antigen-pulsed DCs20,21 or genetically modified antigen-presenting cells (APCs)22 are labor intensive and require several weeks for the generation of a clinical product.
In this study we investigated a new, rapid-isolation strategy for functionally active anti-Aspergillus T cells. Our results suggest that high numbers of functionally active CD4+ T cells against Aspergillus can be generated from a single blood draw of randomly selected individuals within 14 days.
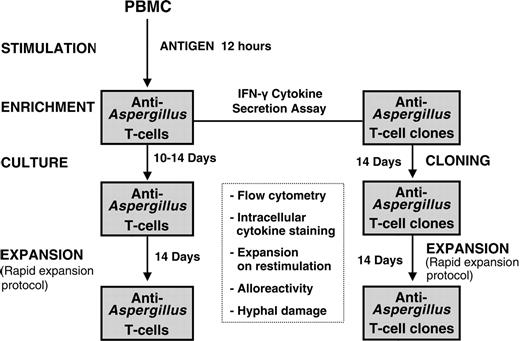
Schematic diagram for generation of functionally active anti-Aspergillus T lymphocytes. After stimulation of 1 × 108 PBMCs with the cellular extract EC SAB of A fumigatus, IFN-γ-producing cells were isolated using the IFN-γ secretion assay. Anti-Aspergillus T cells either were further expanded or were used to generate functionally active T cell clones by limiting dilution. Characterization of the functionally active T cells included flow cytometry, including intracellular, antigen-triggered cytokine staining, expansion on restimulation, hyphal damage, and assessment of alloreactivity.
Schematic diagram for generation of functionally active anti-Aspergillus T lymphocytes. After stimulation of 1 × 108 PBMCs with the cellular extract EC SAB of A fumigatus, IFN-γ-producing cells were isolated using the IFN-γ secretion assay. Anti-Aspergillus T cells either were further expanded or were used to generate functionally active T cell clones by limiting dilution. Characterization of the functionally active T cells included flow cytometry, including intracellular, antigen-triggered cytokine staining, expansion on restimulation, hyphal damage, and assessment of alloreactivity.
Patients, materials, and methods
Patients
After informed consent, 100 mL blood from 5 healthy volunteers was obtained for isolation and generation of anti-Aspergillus T cells. In addition, 2 buffy coats of healthy blood donors were kindly provided by the local Transfusion Medicine Department of Tübingen, Germany. The ages of the 6 men and 1 woman ranged from 24 to 50 years. None of the subjects had a history of a suspected or proven invasive fungal infection, and the volunteers did not have atopy history, in particular fungal allergies. The protocol was approved by the ethics committee of the University of Frankfurt.
Aspergillus fumigatus antigens
The water-soluble cellular extract (EC SAB) and the ethanol precipitate (PP SAB) of A fumigatus were prepared as described before.23 Instead of Sabouraud medium (2% glucose and 1% Peptone [Biokar, Beauvais, France]), Brian medium (1000 mL NH4NO3 [2.4 g], KH2PO4 [10 g], asparagine [10 g], MgSO4 7H2O [2 g], glucose [50g], H2O [1000 mL], plus 1.3 mL ZnSO4 7H2O [20 g], CuSO4 5H2O [2 g], Co(NO3)2 6H2O [1 g], H2O [1000 mL], plus 1.3 mL CaCl2 [50 g] and H2O [1000 mL]) was used for the production of the cellular extract EC BRI and the precipitate PP BRI.24 All antigens were tested endotoxin-free by the limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
T-cell proliferation assay
The proliferative response to Aspergillus antigens was assessed as follows: Peripheral blood mononuclear cells (PBMCs), obtained from 10 mL blood from healthy donors, was separated by Ficoll/Paque (BioWhittaker) density gradient centrifugation. Then, 1 × 105 cells were placed in a 96-well round-bottom plate containing 200 μL RPMI-1640 (Gibco BRL, Eggenstein, Germany) supplemented with 10% heat-inactivated human serum and different A fumigatus antigens at a concentration between 1 μg/mL and 25 μg/mL. Control stimuli included tetanus toxoid (Chiron Behring, Marburg, Germany), phytohemagglutinin (PHA) (Murex; Life Technology, Karlsruhe, Germany) and Escherichia coli-derived recombinant human (rh) interleukin (IL)-2 (Proleukin; Chiron, Ratingen, Germany) in concentrations of 20 μg/mL, 10 ng/mL, and 50 U/mL, respectively. After 4 days of stimulation, the culture was pulsed with 0.037 MBq (1 μCi) [3H]thymidine (Amersham, Braunschweig, Germany) overnight and then harvested onto glass fiber filters. The radioactivity was counted by liquid scintillation. The results are expressed as the mean value of at least triplicate cultures. A proliferation index score greater than 3 was considered to indicate a positive proliferative response.
Isolation of anti-Aspergillus T cells
PBMCs from healthy donors were isolated by Ficoll/Paque (BioWhittaker) density gradient centrifugation from an initial 100-mL heparinized blood volume (Figure 1). PBMCs were adjusted at a final concentration of 1 × 107 cells/mL in cytotoxic T lymphocyte (CTL) medium containing RPMI 1640 medium, 10% heat-inactivated human serum, 100 IU/mL penicillin G, and 100 μg/mL streptomycin (Gibco BRL). Cells were coincubated with Aspergillus antigens for 12 hours in a 37°C humidified incubator. Magnetic enrichment of cytokine-secreting cells was performed as previously described by means of the Cytokine Secretion Assay (CSA; Miltenyi Biotec, Bergisch Gladbach, Germany).25 Briefly, cells were labeled with anti-IFN-γ monoclonal antibody (Miltenyi Biotec), suspended in CTL medium at 1 × 106 cells/mL, and incubated for the IFN-γ capturing period (45 minutes). Cells were subsequently labeled with anti-IFN-γ phycoerythrin (PE), incubated for 15 minutes at 4°C, and washed with phosphate-buffered saline (PBS; BioWhittaker) containing 10% bovine serum albumin (BSA; Sigma, St Louis, MO) and 2 mM EDTA (BioWhittaker). Thereafter, cells were magnetically labeled with anti-PE microbeads (Miltenyi Biotec), incubated for 15 minutes at 4°C, and washed with PBS. Cells were resuspended in 3 mL buffer and then selected with the MIDI MACS device (Miltenyi Biotec) equipped with separation columns according to the manufacturer's instructions.
Culture of selected cells
Cell integrity was verified by visual observation using conventional microscopy. Viable cells were counted using trypan blue. The isolated cells were placed in a 24-well plate (Nunc, Wiesbaden, Germany) with 30 Gy-irradiated autologous PBMCs (2.5 × 107) in 2 mL CTL medium containing 50 IU/mL rhIL-2, and were cultured for up to 14 days at 37°C. The cultures were supplemented, aseptically, with fresh medium and 50 U/mL rhIL-2 every other day.
Rapid expansion of anti-Aspergillus T cells
In order to generate high numbers of anti-Aspergillus T cells within a short time, the cultures were further expanded as previously described with some modifications.26 In brief, 1 × 105 to 3 × 105 anti-Aspergillus T cells were placed in a 25-cm2 cell culture flask (Corning, Corning, New York) containing 25 mL CTL medium in the presence of OKT-3 (30 ng/mL; Janssen-Cilag, Neuss, Germany), 30 Gy-irradiated allogeneic PBMCs (2.5 × 107), and 60 Gy-irradiated T2 feeder cells (5 × 106). The cultures were supplemented with fresh medium only on day 4, and with 50 U/mL rhIL-2 on days 1, 4, 7, and 10.
T-cell cloning of anti-Aspergillus T cells
After enrichment, anti-Aspergillus T-cell clones from randomly selected donors were produced by limiting dilution, using a protocol previously reported with some modifications.27 Briefly, 3.0 × 102 isolated cells were placed in 200 mL CTL medium containing 30 Gy-irradiated PBMCs (1.0 × 108), 60 Gy-irradiated T2 feeder cells (7.5 × 106), OKT-3 (30 ng/mL), and rhIL-2 (50 IU/mL). Then, 200 μL of this dilution were transferred to each well of a 96-well plate (Becton Dickinson, Heidelberg, Germany). Fourteen days after plating, the cells from wells that demonstrated growth based on visual inspection were analyzed by fluorescence-activated cell sorting (FACS) and expanded by the rapid expansion protocol.
Characterization of phenotype and intracellular cytokine staining
The phenotype of the isolated cells was characterized by means of flow cytometry. Antibodies against the following epitopes were used: CD3, CD4, CD8, CD14, CD19, CD28, CD38, CD45RA, CD45RO, CD56, CD69, TCRα/β, TCRγ/δ, and human leukocyte antigen (HLA)-DR. To stain the antibodies, the following fluorochromes were used: fluorescein-isothiocyanate (FITC), PE, peridinin-chlorophyll-protein complex (PerCP), or allophycocyanin. All antibodies were purchased from Becton Dickinson, except for CD4 allophycocyanin (DakoCytomation, Glostrup, Denmark). The flow cytometric analysis was performed using a 4-color cytometer (FACSCalibur, Becton Dickinson).
Intracellular cytokine staining (ICC) was performed as recently described28 with some modifications. PBMCs (1 × 106/mL) were stimulated for 6 hours with EC SAB (5 μg/mL), and generated T cells were stimulated with autologous EC SAB-loaded APCs (effector-stimulator cell ratio, 5:1 and 10:1, respectively). Both cell populations were costimulated with the monoclonal antibodies CD28 and CD49d (Becton Dickinson; 2 μg/mL each). Monocyte-derived APCs were obtained by separating adherent cells from PBMCs according to standard protocols and were stimulated with Aspergillus antigens for 12 to 14 hours. APCs with and without control antigens (CMV lysate [Biodesign, Dunn, Asbach, Germany], or tetanus toxoid) were used as negative controls. Positive controls were performed by stimulation with phorbol 12-myristate 13-acetate (PMA, 0.5 μg/mL; Sigma) and ionomycin (1 μg/mL; Sigma). For testing whether the generated T cells were specific to Aspergillus fumigatus, the cells were also stimulated with various antigen extracts of Aspergillus flavus (CBS56965), Aspergillus niger (CBS73388), Alternaria alternata (1563IP), Penicillium chrysogenum (CBS 73 388), and Candida albicans (444IP), all of which were cultivated in Sabouraud medium under conditions identical to the production of EC SAB. Antigen extracts were tested at concentrations of 1 μg/mL and 5 μg/mL protein. Brefeldin A (10 μg/mL; Sigma) was added for the last 5 hours of incubation. Samples were permeabilized and stained with fluorochrome-labeled anti-CD3, anti-CD4, anti-CD8, or anti-human IFN-γ, anti-IL-2, anti-IL-4, or anti-IL-10 antibodies (Becton Dickinson and Miltenyi Biotec) and were analyzed using FACSCalibur.
CFSE staining
Fluorescent labeling of PBMCs and anti-Aspergillus T cells was achieved as previously described with some modifications.28 Briefly, cells were washed and labeled with 0.6125 μm carboxy-fluorescein diacetate succinimidyl ester (CFDASE; Molecular Probes, Eugene, OR), unbound CF-DASE, or the deacetylated form, CFSE. Labeling was quenched with the addition of RPMI 1640 medium containing 15% human serum. Cells were washed twice and 1 × 106 cells were plated in each well of a round-bottom 24-well plate. Autologous APCs were added, either unstimulated or stimulated overnight with control antigen (tetanus toxoid) or Aspergillus-antigen EC SAB. To test alloreactivity, allogeneic APCs were added to the labeled PBMCs or to anti-Aspergillus T cells. All APCs were irradiated with 30 Gy and used at a concentration of 1 to 2 × 105 per well. After 1 day of culture, rhIL-2 (5 U/mL) was supplemented. On days 0, 4, and 7, cells were harvested, counted, and analyzed using the 4-color flow cytometer (FACSCalibur) as described under “Characterization of phenotype and intracellular cytokine staining.”
Cytokine measurement
The concentration of cytokines in the supernatant was measured using the BD Cytometric Bead Array (CBA) Multiplex Assay (Becton Dickinson). Serum levels of cytokines associated with a TH1 response, such as IL-2 and IFN-γ, or a TH2 response, such as IL-4 and IL-10, were analyzed by flow cytometry (FACSCalibur) according to the manufacturer's instructions. Supernatants with cytokine concentrations above 5000 pg/mL were not further diluted.
Assessment of hyphal damage by the colorimetric assay XTT
Antifungal activity was assessed as hyphal damage by means of the colorimetric assay with (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]2H-tetrazolium-5-carboxyanilide) sodium salt (XTT; Sigma) plus coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone; Sigma) using A fumigatus AF 4215 (no. MYA1163; American Type Culture Collection, Manassas, VA) as described before.29,30 In order to exclude erroneous results due to allogeneic cell-cell interaction we have chosen an autologous test system. Conidia of A fumigatus (1.5 × 104 per well) were plated in a 96-well flat-bottom cell cluster (Becton Dickinson) and incubated at 37°C for 18 hours to allow germination. On the following day, various combinations of polymorphonuclear leukocytes (PMNs), APCs, and generated T-cell clones were added. PMNs were isolated by Ficoll density gradient centrifugation with subsequent hypotonic lysis of erythrocytes. Enrichment of APCs was performed as described under “Characterization of phenotype and intracellular cytokine staining.” Functionally active T cells against Aspergillus and PMNs were added at an effector-to-target (E/T) ratio of 5:1. APCs were added at a concentration range of 1.3 to 3.7 × 104 per well. For the assessment of hyphal damage, a total of 4 clones were tested, and each experiment was performed at least 3 times. In addition, each condition was tested in triplicate. After incubation at 37°C with 5% CO2 for 2 hours, the plates were washed twice with sterile water to lyse the cells. Then, 150 μL PBS containing 0.25 mg/mL XTT and 40 μg/mL coenzyme Q were added and incubated for 1 hour at 37°C with 5% CO2. Following incubation, 100 μL were transferred to a new plate and the change in color (absorbance) was assessed spectrophotometrically at 450 nm using a 690-nm reference. Antihyphal activity was calculated according to the formula:29 percent hyphal damage = (1 - X/C) × 100, where X is the absorbance of experimental wells and C is the absorbance of control wells with hyphae only.
Statistics
Data were analyzed using the software package GraphPad Prism (GraphPad Software, San Diego, CA). Comparisons were performed by 1-way analysis of variance (ANOVA) followed by Dunnett correction for multiple comparisons. A 2-sided P value of less than .05 was considered as statistically significant.
Results
Lymphoproliferative response
To determine whether different A fumigatus antigens induce specific proliferative response, PBMCs from 7 healthy individuals were stimulated with various concentrations of EC SAB, PP SAB, EC BRI, and PP BRI, and control stimuli, which included tetanus toxoid, PHA, and rhIL-2. Most individuals showed a strong proliferation to tetanus toxoid (5 of 7 donors tested; proliferation index mean ± SEM 22.1 ± 7.3), PHA (7 of 7; 54.9 ± 13.9) and rhIL-2 (6 of 7; 25.2 ± 5.6) (Figure 2). Although no significant proliferative response was evident with different concentrations of the Aspergillus antigens PP SAB, EC BRI, and PP BRI, a proliferation index greater than 3 was observed using the cellular extract EC SAB. For example, at 1 μg/mL, in 4 of 7 donors tested, proliferation index was 4.0 ± 0.8, and at 5 μg/mL, in 4 of 7 donors, it was 5.3 ± 1.7 (Figure 2). Therefore, EC SAB was used for subsequent experiments.
T-cell responses to various antigens in healthy volunteers. Anti-Aspergillus T-cell responses were assessed by a lymphoproliferation assay. Stimulation with IL-2, PHA, and tetanus toxoid was used as control. A proliferation index greater than 3 was considered to indicate a positive lymphoproliferative T-cell response. Columns represent means ± SEM.
T-cell responses to various antigens in healthy volunteers. Anti-Aspergillus T-cell responses were assessed by a lymphoproliferation assay. Stimulation with IL-2, PHA, and tetanus toxoid was used as control. A proliferation index greater than 3 was considered to indicate a positive lymphoproliferative T-cell response. Columns represent means ± SEM.
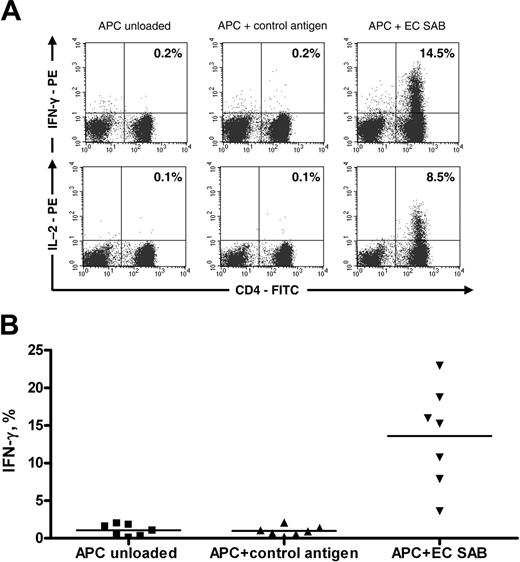
Functionally active anti-Aspergillus CD4+ T cells are enriched after selection and culture. (A) IFN-γ (top row) and IL-2 (bottom row) staining of anti-Aspergillus CD4+ lymphocytes from 1 donor. (B) Percentage of IFN-γ-positive anti-Aspergillus CD4+ lymphocytes from 7 of 7 different donors. Cells were stimulated with unloaded autologous APCs (first column) or autologous APCs prestimulated with either control antigen (tetanus toxoid; second column) or EC SAB (third column). The horizontal bars represent the mean.
Functionally active anti-Aspergillus CD4+ T cells are enriched after selection and culture. (A) IFN-γ (top row) and IL-2 (bottom row) staining of anti-Aspergillus CD4+ lymphocytes from 1 donor. (B) Percentage of IFN-γ-positive anti-Aspergillus CD4+ lymphocytes from 7 of 7 different donors. Cells were stimulated with unloaded autologous APCs (first column) or autologous APCs prestimulated with either control antigen (tetanus toxoid; second column) or EC SAB (third column). The horizontal bars represent the mean.
Anti-Aspergillus CD4+ T cells respond on stimulation with antigen extracts of A fumigatus, A flavus, A niger, and P chrysogenum, but not on stimulation with A alternata and C albicans. IFN-γ staining of anti-Aspergillus CD4+ lymphocytes from 1 donor on stimulation with antigen extracts of A fumigatus (A), A flavus (B), A niger (C), A alternata (D), P chrysogenum (E), and C albicans (F).
Anti-Aspergillus CD4+ T cells respond on stimulation with antigen extracts of A fumigatus, A flavus, A niger, and P chrysogenum, but not on stimulation with A alternata and C albicans. IFN-γ staining of anti-Aspergillus CD4+ lymphocytes from 1 donor on stimulation with antigen extracts of A fumigatus (A), A flavus (B), A niger (C), A alternata (D), P chrysogenum (E), and C albicans (F).
Generation and culture of anti-Aspergillus T cells
Anti-Aspergillus T cells were enriched from 1.0 × 108 PBMCs of healthy individuals using the IFN-γ secretion assay after stimulation with EC SAB at 5 μg/mL. In 7 of 7 donors, an average of 1.1 × 107 cells (range, 0.4 × 107-2.8 × 107 cells) was obtained after culturing for 10 to 14 days.
Phenotype and functional enumeration of functionally active anti-Aspergillus T cells
Flow cytometric analysis of anti-Aspergillus T cells that were obtained from PBMCs stimulated with EC SAB revealed a median of 91.3% CD3+/CD4+ lymphocytes (range, 30.6%-95.6%; 7 of 7 donors). Antigen-triggered production of cytokines in T cells can be used to determine frequency and functionality of antigen-specific T cells. Therefore, IFN-γ was detected by means of intracellular cytokine staining after activation with EC SAB (Figure 3A). Although we did not observe a significant proportion of IFN-γ-producing cells after enrichment (data not shown), the percentage of IFN-γ-producing CD3+/CD4+ cells after culturing for 10 to 14 days was 15.3% (median; range, 3.6%-22.9%; n = 7), which was significantly higher than the percentage of this cell population detected among negative controls stimulated with CMV lysate or tetanus toxoid (median, 0.9%; range, 0.2%-2.1%; P < .001) (Figure 3B). When ICC detecting IL-2 was used in this setting, an average of 8.5% (range, 1.8%-9.2%; 3 of 3 donors) IL-2-producing CD3+/CD4+ cells were seen (Figure 3A). Testing various fungal antigen extracts, similar proportions of IFN-γ-producing CD3+/CD4+ cells were obtained upon activation with antigen extracts of A fumigatus, A flavus, A niger, and P chrysogenum, whereas no significant IFN-γ production was observed upon activation with antigen extracts of A alternata and C albicans (n = 4) (Figure 4). In addition, IFN-γ production was detected in none of the settings in CD3+/CD8+ cells (data not shown).
Generation of functionally active T-cell clones against Aspergillus
Functionally active anti-Aspergillus T cells were cloned by limiting dilution to exclude possible contamination with small fractions of unspecific T cells, B cells, and natural killer (NK) cells. Overall, a total of 4 clones of functionally active anti-Aspergillus T cells derived from 2 healthy individuals were generated. Flow cytometry revealed a cell population of CD3+/CD4+ cells (median, 98.2%; range, 96.9%-99.3%). In addition, these cells homogenously expressed CD45RO, CD28, CD38, CD69, HLA-DR, and the T-cell receptor (TCR) α/β, indicating a memory, activated, T-helper cell population. Functional assessment by ICC revealed that an average of 8.7% of these cells (range, 6.6%-18.5%; n = 4) secreted IFN-γ on stimulation with EC SAB.
The concentration of IFN-γ in the supernatant of proliferating anti-Aspergillus T cells was highly elevated, with levels higher than 5000 pg/mL (n = 3), whereas the level of IL-4 (median, 14 pg/mL; range, 9-49 pg/mL) and IL-10 (median, 19 pg/mL; range, 11-26 pg/mL) was low. The assessment of IL-2 was not included in the analysis, since rhIL-2 was routinely added to the cultures.
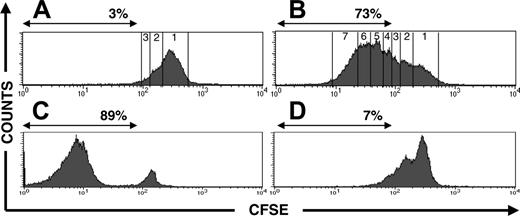
Restimulation of generated functionally active anti-Aspergillus T cells and loss of alloreactivity after enrichment and expansion of anti-Aspergillus T cells. (A-B) CFSE-labeled generated anti-Aspergillus T cells were cocultured with autologous APCs, which were either unloaded (A) or preincubated with EC SAB (B). The CSFE staining and the expansion rate are shown in 1 representative donor after 4 days. The number of divisions and the percentages of lymphocytes that underwent at least 3 cell cycles are indicated. Multiple cell divisions can be detected in anti-Aspergillus T cells after activation with EC SAB-loaded APCs, as indicated by loss of intensity in the CFSE signal. In contrast, only a marginal number of anti-Aspergillus T cells expanded in the same time period when cocultured with unloaded APCs. (C-D) CFSE-labeled unselected CD4+ T-cells (C) or anti-Aspergillus CD4+ T cells (D) were coincubated with third-party APCs to assess alloreactivity. Whereas most unselected CD4+ T cells underwent multiple cell divisions, purified anti-Aspergillus CD4+ T cells show a weak proliferative response in the same time period (D).
Restimulation of generated functionally active anti-Aspergillus T cells and loss of alloreactivity after enrichment and expansion of anti-Aspergillus T cells. (A-B) CFSE-labeled generated anti-Aspergillus T cells were cocultured with autologous APCs, which were either unloaded (A) or preincubated with EC SAB (B). The CSFE staining and the expansion rate are shown in 1 representative donor after 4 days. The number of divisions and the percentages of lymphocytes that underwent at least 3 cell cycles are indicated. Multiple cell divisions can be detected in anti-Aspergillus T cells after activation with EC SAB-loaded APCs, as indicated by loss of intensity in the CFSE signal. In contrast, only a marginal number of anti-Aspergillus T cells expanded in the same time period when cocultured with unloaded APCs. (C-D) CFSE-labeled unselected CD4+ T-cells (C) or anti-Aspergillus CD4+ T cells (D) were coincubated with third-party APCs to assess alloreactivity. Whereas most unselected CD4+ T cells underwent multiple cell divisions, purified anti-Aspergillus CD4+ T cells show a weak proliferative response in the same time period (D).
Hyphal damage to A fumigatus induced by anti-Aspergillus T cells, polymorphonuclear leukocytes, and APCs, alone or in combination. The combination of PMNs, anti-Aspergillus T cells, and APCs resulted in the highest hyphal damage compared with all other settings (P < .001). Columns represent means ± SEM. AAT indicates anti-Aspergillus T cell.
Hyphal damage to A fumigatus induced by anti-Aspergillus T cells, polymorphonuclear leukocytes, and APCs, alone or in combination. The combination of PMNs, anti-Aspergillus T cells, and APCs resulted in the highest hyphal damage compared with all other settings (P < .001). Columns represent means ± SEM. AAT indicates anti-Aspergillus T cell.
Expansion of functionally active T cells against Aspergillus
Because therapeutic strategies using functionally active anti-Aspergillus T cells may require a high number of specific cells, the isolated anti-Aspergillus T cells were further expanded. In a 2-week time span, the number of anti-Aspergillus T cells was expanded up to 500-fold. Functional assessment by ICC revealed that from an original volume of 100 mL peripheral blood, an average of 2.0 × 108 IFN-γ-secreting, functionally active anti-Aspergillus T cells (range, 0.7 × 108-9.4 × 108 cells; n = 5) was obtained. Before and after expansion, anti-Aspergillus T cells cells did not significantly differ in phenotype and in the production of IFN-γ upon stimulation with EC-SAB (data not shown). In addition, cryopreservation in liquid nitrogen for up to 6 months did not affect functionally active anti-Aspergillus T cells and T-cell clones. After thawing, the ability of anti-Aspergillus T cells to expand as well as to produce IFN-γ upon stimulation was not markedly different compared to fresh anti-Aspergillus T cells (data not shown).
Generated anti-Aspergillus T cells expand after restimulation
To assess whether the generated functionally active anti-Aspergillus CD4+ T cells can appropriately divide after being stimulated with an endogenously processed antigen, T cells were labeled with CFSE and cocultured for 4 to 7 days with autologous APCs, which had been preincubated with EC SAB. The intensity of CFSE staining was controlled before stimulation of the CFSE labeled cells with Aspergillus antigen-loaded APCs (data not shown). Analysis of CFSE staining of 4 different samples on day 4 demonstrated that more than 73% of the isolated and expanded anti-Aspergillus T cells of the generated clone underwent at least 3 cell divisions (Figure 5B). In contrast, only a marginal number (up to 3%) of anti-Aspergillus T cells expanded in the same time period when cocultured with unloaded APCs (Figure 5A). Furthermore, no proliferative response was detected when CD4+ T-helper cells of the original fraction were coincubated with unloaded APCs or with APCs preincubated with EC SAB (data not shown).
Reduced alloreactivity
Adoptive transfer of donor-derived T cells into recipients of allogeneic SCT may induce GVHD. We therefore investigated whether the generated functionally active T cells have reduced alloreactivity compared with the original cell fraction. In all 3 clones tested, up to 90% of unspecific and unselected CD4+ T cells elicited a strong proliferation response against third-party APCs, as measured by loss of intensity in the CFSE signal (Figure 5C). In contrast, in all 3 experiments, purified functionally active anti-Aspergillus CD4+ T cells coincubated with allogeneic APCs revealed only a marginal expansion in the same time period (Figure 5D), which was comparable with the expansion observed when purified anti-Aspergillus CD4+ T cells were cocultured with unloaded autologous APCs (n = 3; data not shown).
The reduced alloreactivity of the generated functionally active anti-Aspergillus T cells compared with the CD4+ T cells of the original fraction is also supported by analysis of the cytokine concentrations in the supernatant. While low levels of IFN-γ were detected in the supernatant of anti-Aspergillus T cells coincubated with unloaded APCs (median, 14 pg/mL; range, 4-19 pg/mL; n = 3) or with allogeneic APCs (median, 16 pg/mL; range, 6-18 pg/mL; n = 3), significantly higher concentrations of IFN-γ were measured when PBMCs were cocultured with unloaded third-party APCs (median, 905 pg/mL; range, 246-4746 pg/mL; n = 3).
Antifungal activity of purified and expanded T cells
To address the question whether expanded T cells have the capacity to damage hyphae of A fumigatus and to what extent they increase the antifungal activity of PMNs, the colorimetric XTT assay was performed with 4 different T-cell clones. In this setting, PMNs showed a similar hyphal damage when tested alone (mean ± SEM, 14.2% ± 2.1%), in combination with APCs (15.1% ± 1.4%), or in combination with the expanded T cells (15.0% ± 2.0%) (Figure 6). A comparable hyphal damage was seen when the expanded T cells were coincubated with APCs (14.2% ± 1.7%). In contrast, the combination of APCs and expanded T cells with PMNs resulted in a significantly higher hyphal damage compared with all other settings (23.3% ± 2.8%; P < .001). APCs alone or expanded T cells alone showed the weakest capacity to induce hyphal damage (7.4% ± 1.1% and 11.3% ± 1.8%, respectively).
Discussion
Invasive aspergillosis remains a serious complication in patients undergoing allogeneic SCT, showing an increasing incidence over the last decade.1,31 Despite improvement of prophylactic strategies and development of new antifungal drugs, IA is still associated with high morbidity and unacceptable mortality. Not long ago, the general belief was that neutrophil-mediated immunity was the single most important defense against filamentous fungi, such as A fumigatus, but it became clear that T lymphocytes provide a critical secondary defense against these organisms. Animal models suggest that the reconstitution of Aspergillus-specific immune responses after allogeneic SCT is protective against the development of IA.9,10,32 Therefore, Aspergillus-specific T-cell immunity, transferred through the infusion of ex vivo-generated, donor-derived, anti-Aspergillus T cells might be beneficial for recipients of allogeneic SCT. The approach of adoptively transferring antigen-specific T cells after allogeneic SCT is well established for patients suffering from CMV disease or EBV-associated posttransplantation lymphoproliferative disease,12,33 but there are few data regarding patients with invasive fungal infection. This might be due, at least in part, to the antigenic properties of A fumigatus, which is rather complex and less well characterized compared with viruses such as CMV. Only a few of the hundreds of (glycol)proteins of A fumigatus reported in the literature have been characterized at a molecular and biochemical level.34 We therefore initially screened blood samples from healthy individuals for their ability to proliferate in response to various cellular extracts of A fumigatus. Although there may be a high variability in cellular extracts of A fumigatus, it has been attributed to experimental conditions used, such as the composition of the culture medium or to what extent the antigenic components are purified and concentrated, and it does not appear to be strain specific.34 Of the antigens tested, we chose the cellular extract EC SAB as stimulus for our novel approach to generate functionally active anti-Aspergillus T cells. This antigen had the highest proliferation index and had been shown promise in inducing TH1 responses.7 Interestingly, the addition of proteinase completely suppresses the stimulating effect of EC SAB (data not shown), indicating that 1 or more proteins are the antigenic component of this preparation.
It is important to note that the T cells we have generated with a cellular extract derived from A fumigatus responded also upon stimulation with A flavus, A niger, and P chrysogenum, but not upon stimulation with A alternata and C albicans. This cross-reactivity is also observed in the clinical setting, since the widely used galactomannan enzyme-linked immuosorbent assay (ELISA), which uses the monoclonal antibody EB-A2, reacts with antigens of both Aspergillus and Penicillium species.35 In a recent study, 35 haploidentical hematopoietic transplant recipients with evidence of invasive aspergillosis, indicated by the presence of pulmonary infiltrates and positive galactomannan results, but not by the isolation of Aspergillus species, received Aspergillus-specific T-cell therapy.18 The authors suggested that the pathogen-specific T cells, generated with extracts of A fumigatus, controlled antigenemia and infectious mortality, but unfortunately, no data were presented showing whether these T cells were Aspergillus-specific indeed. Therefore, one cannot exclude that the generated cells were functionally active against other filamentous fungi than A fumigatus. This possibility is supported by our in vitro data and has to be addressed in future clinical studies. To this end, however, the administration of T-cell clones that protect against an array of pathogens but do not cause graft-versus-host disease would be the ultimate goal in the supportive care of transplant recipients. For further insight into the pathogen-host interaction, the characterization of Aspergillus antigen(s), which elicit anti-Aspergillus human T-cell responses and may be produced as recombinant proteins, is the current focus of our research. Despite the fact that unselected lymphocytes proliferated upon the stimulation with EC SAB, we and others36 were not able to detect cytokine production at the single-cell level by flow cytometry. It is conceivable that the latter approach is less sensitive, since we could culture an average of 2.0 × 108 IFN-γ-secreting functionally active anti-Aspergillus T cells from a single 100-mL blood draw. To date, most reports on the generation of anti-Aspergillus T cells have used dendritic cells (DCs) pulsed with live fungi or fungal extract.10,17 One group recently generated DCs from healthy donors within 48 hours that were capable of inducing T-cell proliferation upon stimulation with Aspergillus.17 Unfortunately, the authors do not report the volume of peripheral blood used or the number of functionally active T cells obtained after 28 days of culture, including 3 restimulations. Although the number of functionally active anti-Aspergillus T cells required for prevention or treatment of IA after allogeneic SCT is not yet defined, studies of adoptive immunotherapy for posttransplantation viral infections suggest that an adequate number of generated T cells is crucial.37 It is important to note that in the present study the generated functionally active anti-Aspergillus T cells do not represent terminally differentiated CD4+ T cells; restimulation experiments led to several cell divisions, as demonstrated by the dilution of CFSE dye and a corresponding cell expansion. Thus, adoptive transfer of the generated T cells into SCT recipients may allow further expansion if the T cells are stimulated by Aspergillus antigen-presenting cells in vivo.
In agreement with the findings of a recent study, functional assessment of CD3+/CD8+ lymphocytes did not reveal intracellular production of IFN-γ.16 Other studies assessing the phenotype of the cells reported that the number of CD3+/CD8+ cells significantly increased in mice receiving conidia-pulsed DCs, and that the Aspergillus effectors generated in vitro by stimulation with autologous DCs were of mixed CD3+/CD4+or CD3+/CD8+ phenotype, depending on the stimulus used.10,17 Additional studies need to be performed in order to elucidate the exact role of CD3+/CD8+ cells in the antifungal host response to A fumigatus and the optimal CD4+/CD8+ ratio for the transfer of adaptive immunity in patients after allogeneic SCT.
Recent evidence that TH1/TH2 dysregulation and a switch to TH2 immune response contribute to the development of IA confirms the crucial role of TH1 reactivity in the control of Aspergillus infection.20,38 In addition, an increased ratio of IFN-γ/IL-10 in response to Aspergillus antigens by PBMCs from SCT patients was associated with a favorable response to antifungal therapy, suggesting that a TH1 response is beneficial.7 However, the factors that determine the differential activation of TH1orTH2 cells are not fully understood. Both the type of antigen and the manner in which these antigens are presented by the subtype of DCs (DC1 vs DC2) may result in different T-cell responses.10,39 While 1 group reported a high-level production of IL-4 associated with a TH2 response on the recombinant allergen Asp f2,39 others observed the generation of protective TH1 response on various extracts of A fumigatus or on various synthesized pentadecapeptides spanning the coding region of Asp f 16.16,17,39 In the present study, functionally active anti-Aspergillus T cells produced IFN-γ and IL-2 upon stimulation with EC SAB, as revealed by ICC and assessment of cytokine levels in the supernatant. In contrast, no significant production of IL-4 or IL-10 was observed, indicating that a TH1 response was induced. Notably, EC SAB stimulated PBMCs selected with the IL-4 or the IL-10 cytokine secretion assay and cultured in the presence of IL-4 or IL-7, interleukins involved in the differentiation and proliferation of TH2 cells,40 did not lead to a TH2 response (data not shown).
Infusion of donor-derived PBMCs, even in small numbers, may also transfer alloreactive T cells in numbers sufficient to cause severe or even lethal GVHD. On the other hand, extensive culturing of T cells or the generation of T-cell clones may eradicate alloreactive T cells and, thus, may prevent GVHD. In the present study, the majority of unspecific and not selected CD4+ T cells elicited a strong proliferation response against allogeneic APCs, whereas purified functionally active anti-Aspergillus T cells only marginally expanded when coincubated with third-party APCs. Others have reported similar findings in virus-specific T cells either by assessing the stimulation of CD4+ cells from original and expanded cell fractions,41 with intracellular cytokine staining,42 or by using third-party PHA blasts as targets in a chromium release assay.12 In addition, we detected a significant higher level of IFN-γ in the supernatant of the proliferating unspecific cells compared with functionally active anti-Aspergillus T cells. This also indicates a marked reduction in alloreactivity of functionally active anti-Aspergillus T cells, since IFN-γ is one of the hallmarks of acute GVHD.43
While the damage of Aspergillus hyphae by PMNs has been the focus of extensive research,44,45 the effector mechanisms through which T cells participate in the control of fungal infection are not fully understood. Phagocytes exhibit intrinsic antifungal activity through a combination of oxidative and complementary nonoxidative mechanisms, the latter consisting of degranulation and intracellular or extracellular release of effector molecules, defensins, and neutrophil cationic peptides.38 The degree of hyphal damage highly depends on the effector-target ratio, which was rather low in our study.45 Stringent statistical analysis including a correction for multiple comparisons showed that the combination of PMNs, T cells, and APCs exhibited a significant higher hyphal damage than each cell population alone or than the combinations of PMNs and T cells, of PMNs and APCs, and of T cells and APCs, respectively (P < .001). This might be explained by the fact that the antifungal activity of the phagocytes was increased by cytokines that were produced by functionally active anti-Aspergillus T cells upon activation of APCs. In particular, IFN-γ, secreted by activated T cells, is known to stimulate phagocytosis and activate the intracellular antifungal killing mechanisms of neutrophils and macro-phages.38,44 Still, the relatively low hyphal damage observed in vitro might significantly further be increased in vivo, in particular since functionally active anti-Aspergillus T cells have the ability to expand on restimulation. In corroborating results reported recently,16 we observed some degree of hyphal damage by T cells alone, which, however, is less powerful compared with the hyphal damage seen by neutrophils. Both CD4+ and CD8+ T cells have been shown to possess direct antifungal activity in vitro.46 For example, cytotoxic CD4+ T cells are able to carry out perforin-mediated cytotoxicity by lytic granules containing preformed perforin and granzymes.47 Clearly, the direct and indirect mechanisms of hyphal damage by functionally active anti-Aspergillus T cells warrants characterization in more detail, but our results suggest that transferring functionally active anti-Aspergillus T cells are beneficial for nonneutropenic patients who still suffer from impaired cellular immunity after allogeneic SCT.
In conclusion, we present a simple and feasible strategy for the rapid generation of a high number of functionally active human T cells against Aspergillus from a single blood draw. Before considering a clinical application, however, further studies need to focus on defining the optimal antigen(s) which reproducibly induce a TH1 response and elicit high antifungal activity, as well as to characterize the subpopulation of patients undergoing allogeneic SCT who ultimately benefit from either a prophylactic or a therapeutic adoptive transfer of functionally active anti-Aspergillus T cells.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-04-1660.
Supported by the Deutsche Leukämie-Forschungshilfe and the Allostem project EU PP6 program (“The Development of Immunotherapeutic Strategies to Treat Haematological and Neoplastic Diseases on the Basis of Optimised Allogeneic Stem Cell Transplantation”) (M.S.T., H.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Thomai Konstantinou for her technical assistance and Ralf Schubert, Christoph Koenigs, Georg Rauser, and Holger Hebart for their helpful advice in preparing this manuscript.