Abstract
We investigated the effects of aging on the IL-7-mediated CD8+ T-cell survival pathway and of IL-7 therapy on T-cell immunity. Cells expressing IL-7 receptor (IL-7R) αhigh and αlow were identified in a CD45RA+ effector memory (EMCD45RA+, CD45RA+CCR7-) CD8+ T-cell subset. Elderly subjects (65 years and older) had an increased frequency of EMCD45RA+ IL-7Rαlow CD8+ T cells, leading to decreased STAT5 phosphorylation and survival responses to IL-7 compared with young subjects (40 years and younger). These EMCD45RA+ IL-7Rαlow cells were largely antigen experienced (CD27-CD28-), replicatively senescent (CD57+), and perforinhigh CD8+ T cells that had decreased IL-7Rα mRNA, independent of guanine and adenine binding protein α (GABPα) and growth factor independence-1 (GFI1) expression. In measuring T-cell receptor (TCR) repertoires of EMCD45RA+ CD8+ T cells, the elderly had a limited repertoire in IL-7Rαhigh and IL-7Rαlow cells, whereas the young had a diverse repertoire in IL-7Rαhigh but not in IL-7Rαlow cells. These findings suggest that aging affects IL-7Rα expression by EMCD45RA+ CD8+ T cells, leading to impaired signaling and survival responses to IL-7, and that IL-7 therapy may improve the survival of EMCD45RA+ CD8+ T cells with a diverse TCR repertoire in the young but not in the elderly.
Introduction
The proper development and maintenance of naive and memory CD8+ T cells are essential for host defense.1,2 IL-7, a member of the common cytokine-receptor γ-chain (γc) family, is critically implicated in the generation and maintenance of naive and memory CD8+ and CD4+ T cells.3-8 IL-7 is produced by multiple stromal tissues, including epithelial cells in the thymus and bone marrow.7 The IL-7R complex consists of 2 chains, the high-affinity IL-7Rα chain and γc.9 IL-7 binding to the receptor induces sequential phosphorylation of Jak1, Jak3, and STAT5, leading to the up-regulation of Bcl-2, which promotes cell survival.10,11 IL-7 regulates peripheral homeostasis of CD4+ and CD8+ T cells by promoting cell survival. When CD8+ T cells from IL-7R intact and knockout (KO) mice were adoptively transferred to wild-type mice, cells from IL-7R KO mice had decreased survival compared with those from IL-7R intact mice.3 Furthermore, in mice infected with lymphocyte choriomeningitis virus, IL-7Rαhigh CD8+ T cells had better survival and differentiation into memory cells than IL-7Rαlow CD8+ T cells.5
Alterations in T-cell immunity occur with aging. These alterations include thymic atrophy and changes in T-cell subsets and function, which likely contribute to increased risk for infection in the elderly.12,13 For example, in 1998, 82 989 deaths in persons 65 and older in the United States were attributed to influenza and pneumonia, compared with 5969 deaths in persons aged 15 to 64.14 In addition, CD4+ and CD8+ T-cell responses to the influenza vaccine were decreased in elderly subjects compared with young subjects.15,16 Although the underlying mechanism for altered T-cell immune responses with aging is largely unknown, IL-7 may have a role in developing such immune changes given that IL-7 is critically involved in the development and maintenance of T cells7,8 and that IL-7 levels in the thymus and serum are reduced with aging.15,17
A potential role for IL-7 therapy in rejuvenating T-cell immunity has been suggested.7,18,19 In fact, a recent mouse study demonstrates that IL-7 could serve as an effective vaccine adjuvant by augmenting responses to subdominant antigens and improving the survival of memory CD8+ T cells.19 However, before such therapy is initiated in an elderly patient, it is critical to investigate whether the patient has a defect in other steps involved in the IL-7-mediated CD8+ T-cell survival pathway because this defect may not be corrected by providing exogenous IL-7. We addressed this issue by measuring IL-7R expression, signaling, and cell survival in different subsets of CD8+ T cells in response to IL-7 in young and elderly subjects. In addition, the characteristics of CD8+ T-cell subsets, as defined by markers including IL-7Rα, was investigated by measuring cell-surface molecules, function, and TCR repertoire. The results of our study show that aging affects IL-7Rα expression by EMCD45RA+ CD8+ T cells, leading to impaired signaling and survival responses to IL-7, and that IL-7 therapy may selectively improve the survival of EMCD45RA+ CD8+ T cells with a diverse TCR repertoire in the young but not in the elderly.
Subjects, materials, and methods
Human subjects
Healthy elderly (65 years and older; n = 29) and young (40 years and younger; n = 27) subjects were recruited for this study (mean ± SD, 74.6 ± 4.95 and 31.7 ± 5.87 years). There was no sex difference between the 2 groups (P = .343, χ2 test). Subjects who were taking immunosuppressive drugs or who had a disease potentially affecting the immune system were excluded.15,20 Informed consent was obtained from all subjects. This work was approved by the institutional review committees of Yale University and the Veterans Administration New England Health Care System, West Haven Campus.
Flow cytometry and cell sorting
Peripheral-blood mononuclear cells (PBMCs) were stained with goat anti-human IL-7Rα and with mouse anti-human IL-15Rα, -IL-2/15Rβ, -γc, -IL-21R, or isotype antibodies (R&D Systems, Minneapolis, MN). Cells were washed and stained with donkey anti-goat IgG and donkey anti-mouse IgG antibodies (Molecular Probes, Carlsbad, CA) and with antibodies to CD8, CD45RA, and CCR7 (BD PharMingen, San Jose, CA). Some cells were additionally stained with antibodies to CD27 (e-bioscience, San Diego, CA), CD28, CD57, perforin, or isotype controls (all from BD PharMingen). In measuring intracellular STAT5 and phospho-STAT5 (P-STAT5), PBMCs were first stained with antibodies to CD8, CD45RA, and CCR7. Cells were then stimulated for 10 minutes with recombinant human IL-7 (10 pg/mL-10 ng/mL; BD PharMingen) or PBS and were stained with antibodies to STAT5 (Santa Cruz Biotechnology, Santa Cruz, CA), P-STAT5 (BD PharMingen), or isotype antibodies.
In determining Bcl-2 and cell survival, PBMCs were sorted into naive (CD45RA+CCR7+), central memory (CM, CD45RA-CCR7+), effector memory (EM, CD45RA-CCR7-), and EMCD45RA+ (CD45RA+CCR7-) CD8+ T cells using a FACSAria (BD Immunocytometry, San Jose, CA). Cells were incubated for 6 days in the presence of recombinant human IL-7 (100 ng/mL) or PBS. Cells were stained with antibodies to Bcl-2 (BD PharMingen) and with annexin V and 7-AAD using a commercially available kit (BD PharMingen). In measuring cell proliferation, sorted cells were labeled with carboxyfluorescein diacetate (CFSE; Molecular Probes, Eugene, OR), as described previously.21 Cells were incubated for 6 days in a 96-well tissue culture plate coated with anti-CD3 antibodies (BD PharMingen) at 10 μg/mL or with PBS in the presence of anti-CD28 antibodies (10 μg/mL; BD PharMingen). Stained cells were analyzed on a FACSCalibur or an LSRII (BD Biosciences Immunocytometry Systems). Collected flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR).
ELISA
Plasma levels of IL-15 and IL-21 were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems and Caprologics, Hardwick, MA, respectively) according to each manufacturer's instructions.
Real-time PCR
Total RNA was isolated from sorted cells and used for cDNA synthesis. Polymerase chain reaction (PCR) was performed by 1 × SYBR Green mix (Qiagen, Valencia, CA). All results were normalized to β-actin mRNA. Primers used in real-time PCR were as follows: IL-7Rα forward, 5′-TGGACGCATGTGAATTTATC-3′, and reverse, 5′-CATTCACTCCAGAAGCCTTT-3′; GABPα forward, 5′-AGCATCAGTGCAATCTGCTA-3′, and reverse, 5′-TTCCCAGGTGAGCTTCTATC-3′; GFI1 forward, 5′-TGACTTGGGGAAGGAATTTA-3′, and reverse, 5′-CCAGTGATGAGGTTTTCACA-3′.
Complementarity-determining region 3 length distribution analysis
CDR3 length distribution analysis was performed using approximately 1.5 × 105 cells from each sorted CD8+ T-cell subset, as previously described.22 Briefly, cDNA was amplified by PCR through 35 cycles (94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute) with primers specific to 24 different Vβ families and a 6-FAM-labeled BC primer.23,24 Fluorescent PCR products were analyzed using an automatic sequencer with GeneMapper software (Applied Biosystems, Foster City, CA).
Statistical analysis
Mann-Whitney U and Student 2-tailed t tests were used for analyses of differences between the young and the elderly. A correlation between the frequencies of IL-7Rαhigh cells and P-STAT5high cells was determined using Pearson correlation. All statistical analyses were performed using SPSS 12.0 (SPSS, Chicago, IL).
Results
Altered frequency of IL-7Rαhigh and IL-7Rαlow cells in EMCD45RA+ CD8+ T-cell subset in the elderly
We determined the expression of IL-7Rα and γc by CD8+ T-cell subsets using flow cytometry. In accordance with our published data,20 the frequency (mean ± SEM) of naive CD8+ T cells was decreased and the frequency of CM CD8+ T cells was similar between elderly and young subjects (naive, 5.32% ± 1.0% vs 35.4% ± 2.6%; CM, 5.31% ± 0.7% vs 5.4% ± 0.6%; P < .001 and P = .879, respectively, by Student t test). The frequencies of EM and EMCD45RA+ CD8+ T cells were higher in the elderly than in the young (EM, 52.7% ± 3.6% vs 36.1% ± 2.6%; EMCD45RA+, 36.7% ± 3.6% vs 23.1% ± 1.9%; P = .003 and P = .001, respectively, by Student t test). Young and elderly subjects had homogeneous expression of IL-7Rα by naive and CM CD8+ T-cell subsets (Figure 1A). In contrast, such receptor expression by EM and EMCD45RA+ CD8+ T-cell subsets was heterogeneous (Figure 1A). Thus, we compared the median fluorescence intensity (MDFI) of IL-7Rα expression by the 4 cell subsets between the young and the elderly. Elderly subjects had decreased IL-7Rα expression by naive and EMCD45RA+ CD8+ T-cell subsets compared with young subjects (Figure 1B; MDFI ± SEM, 215.8 ± 8.9 vs 252.6 ± 8.6 and 77.6 ± 18.6 vs 176.5 ± 28.6, respectively). However, the 2 groups had similar IL-7Rα expression by CM- and EM-cell subsets (Figure 1B). The expression of γc by the 4 CD8+ T-cell subsets was also similar between the young and the elderly (Figure 1C). We analyzed the frequency of IL-7Rαhigh and IL-7Rαlow cells in EM and EMCD45RA+ CD8+ T-cell subsets in both groups because these 2 subsets had heterogeneous expression of IL-7Rα (Figure 1A). Of interest, the frequency of IL-7Rαhigh cells was decreased and the frequency of IL-7Rαlow cells was increased in elderly subjects compared with young subjects (Figure 1D; IL-7Rαhigh cells, 37.5% ± 7.4% vs 59.1% ± 5.4%). However, in the EM CD8+ T-cell subset, the frequency of IL-7Rαhigh and IL-7Rαlow cells was not different between the 2 groups (data not shown). These findings indicate that an age-associated alteration in IL-7Rα expression occurs in naive and EMCD45RA+ CD8+ T-cell subsets and that such a change in the latter subset stems from an altered frequency of IL-7Rαhigh and IL-7Rαlow cells with aging.
IL-7Rα and γc expression by CD8+ T-cell subsets in elderly and young subjects. IL-7Rα and γc expression was measured on naive (CD45RA+CCR7+), central memory (CM, CD45RA+CCR7+), effector memory (EM, CD45RA-CCR7-), and EMCD45RA+ (CD45RA+-CCR7-) CD8+ T-cell subsets in healthy elderly and young subjects using flow cytometry. (A) Representative histograms of IL-7Rα (shaded) and isotype control (open) staining. MDFI of IL-7Rα (B) and γc (C) staining was compared between the elderly (○, n = 10) and the young (•, n = 10). (D) The frequency of IL-7Rαhigh and IL-7Rαlow cells in the EMCD45RA+ CD8+ T-cell subset was compared between elderly (○, n = 10) and young (•, n = 10) subjects. P values were obtained with the Mann-Whitney U test.
IL-7Rα and γc expression by CD8+ T-cell subsets in elderly and young subjects. IL-7Rα and γc expression was measured on naive (CD45RA+CCR7+), central memory (CM, CD45RA+CCR7+), effector memory (EM, CD45RA-CCR7-), and EMCD45RA+ (CD45RA+-CCR7-) CD8+ T-cell subsets in healthy elderly and young subjects using flow cytometry. (A) Representative histograms of IL-7Rα (shaded) and isotype control (open) staining. MDFI of IL-7Rα (B) and γc (C) staining was compared between the elderly (○, n = 10) and the young (•, n = 10). (D) The frequency of IL-7Rαhigh and IL-7Rαlow cells in the EMCD45RA+ CD8+ T-cell subset was compared between elderly (○, n = 10) and young (•, n = 10) subjects. P values were obtained with the Mann-Whitney U test.
Age-associated decrease in IL-7Rα expression by EMCD45RA+ CD8+ T cells leads to impaired STAT5 signaling, Bcl-2 up-regulation, and cell survival in response to IL-7
We next addressed whether altered IL-7Rα expression by naive and EMCD45RA+ CD8+ T-cell subsets in the elderly had a physiologic consequence in cell signaling and survival. We stimulated PBMCs with IL-7 and measured P-STAT5 in the CD8+ T-cell subsets using flow cytometry. In naive and CM subsets, the phosphorylation of STAT5 was homogeneous (Figure 2A). In contrast, EM and EMCD45RA+ subsets with IL-7Rαhigh and IL-7Rαlow cells had 2 different T-cell populations with P-STAT5high and P-STAT5low, reflecting their IL-7Rα expression (Figure 2A). In the latter 2 subsets, a strong correlation between the frequencies of IL-7Rαhigh cells and P-STAT5high cells was observed in young and elderly subjects (Figure 2B). A similar correlation was observed between the frequencies of IL-7Rαlow cells and P-STAT5low cells in the same subsets (data not shown).
Measuring P-STAT-5, Bcl-2, and cell survival in CD8+ T-cell subsets in response to IL-7 in elderly and young subjects. PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CD45RA, and CCR7. (A-C) Cells were incubated for 10 minutes with recombinant human IL-7 (10 pg/mL∼10 ng/mL) or PBS. P-STAT5 in naive, CM, EM, and EMCD45RA+ CD8+ T-cell subsets was measured using flow cytometry. (A) Representative histograms of P-STAT5 (shaded) and isotype control (open) staining. (B) Correlation between the frequencies of IL-7Rαhigh cells and P-STAT5high cells. (C) MDFI of P-STAT5 staining is compared between elderly (○, n = 10) and young (•, n = 10) subjects. (D-E) Cells stained with antibodies to CD8, CD45RA, and CCR7 were sorted into naive, CM, EM, and EMCD45RA+ CD8+ T cells using a FACSAria. Cells were incubated for 6 days in the presence of recombinant human IL-7 (100 ng/mL) or PBS. (D) Cells were permeabilized and stained with antibodies to Bcl-2 (representative data from 4 separate subjects in each group). (E) Cells were stained with annexin V and 7-AAD to identify live cells (annexin V- and 7-AAD-). The difference in the frequency of live cells between samples treated with IL-7 and PBS was calculated from each sample (○ indicates elderly; •, young). P values were obtained with the Pearson correlation (B) or the Mann-Whitney U test (C,E).
Measuring P-STAT-5, Bcl-2, and cell survival in CD8+ T-cell subsets in response to IL-7 in elderly and young subjects. PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CD45RA, and CCR7. (A-C) Cells were incubated for 10 minutes with recombinant human IL-7 (10 pg/mL∼10 ng/mL) or PBS. P-STAT5 in naive, CM, EM, and EMCD45RA+ CD8+ T-cell subsets was measured using flow cytometry. (A) Representative histograms of P-STAT5 (shaded) and isotype control (open) staining. (B) Correlation between the frequencies of IL-7Rαhigh cells and P-STAT5high cells. (C) MDFI of P-STAT5 staining is compared between elderly (○, n = 10) and young (•, n = 10) subjects. (D-E) Cells stained with antibodies to CD8, CD45RA, and CCR7 were sorted into naive, CM, EM, and EMCD45RA+ CD8+ T cells using a FACSAria. Cells were incubated for 6 days in the presence of recombinant human IL-7 (100 ng/mL) or PBS. (D) Cells were permeabilized and stained with antibodies to Bcl-2 (representative data from 4 separate subjects in each group). (E) Cells were stained with annexin V and 7-AAD to identify live cells (annexin V- and 7-AAD-). The difference in the frequency of live cells between samples treated with IL-7 and PBS was calculated from each sample (○ indicates elderly; •, young). P values were obtained with the Pearson correlation (B) or the Mann-Whitney U test (C,E).
Elderly subjects had significantly fewer P-STAT5 in the EMCD45RA+ subsets than did young subjects (Figure 2C). This difference was greater when higher doses of IL-7 were used for cell stimulation. A similar trend was observed in the naive subset but did not reach statistical significance (Figure 2C). In contrast, for CM and EM subsets, both groups had comparable levels of P-STAT5. There was no difference in total STAT5 among the 4 subsets in young and elderly subjects as measured by flow cytometry (data not shown). These findings demonstrate that the magnitude of IL-7Rα expression by CD8+ T cells dictates the extent of subsequent cell signaling and that an age-associated alteration in IL-7Rα expression by the EMCD45RA+ CD8+ T-cell subset impairs IL-7 signaling.
Cellular characteristics of EM and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. (A-C) PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CCR7, CD45RA, IL-7Rα, CD27, CD28, CD57, perforin, or isotype antibodies. Expression of CD27, CD28, CD57 (shaded histograms, B), perforin (shaded histograms, C) and isotype (open histograms, B-C) antibodies by naive, CM, EM IL-7Rαhigh, EM IL-7Rαlow, EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T-cell subsets was determined by gating on each cell subset. Numbers in dot plots (A) indicate the percentage of positive cells in each quadrant. Results are representative data from 5 separate subjects in each group. (D-E) PBMCs from a healthy elderly subjects were stained with antibodies to CD8, CD45RA, and CCR7 and were sorted into naive, EM, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow. (D) Cells were stained with CFSE and stimulated for 6 days with antibodies to CD3 and CD28. Stimulated cells were stained with antibodies to CD57. Numbers in histograms indicate the percentages of proliferating cells. Results are representative data from 3 young and 2 elderly subjects. (E) Sorted cells were incubated for 6 days in the presence of IL-7 (100 ng/mL) and were stained with annexin V and 7-AAD. Numbers in dot plots indicate the percentage of cells in each quadrant. Results are representative data from 2 young and 2 elderly subjects.
Cellular characteristics of EM and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. (A-C) PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CCR7, CD45RA, IL-7Rα, CD27, CD28, CD57, perforin, or isotype antibodies. Expression of CD27, CD28, CD57 (shaded histograms, B), perforin (shaded histograms, C) and isotype (open histograms, B-C) antibodies by naive, CM, EM IL-7Rαhigh, EM IL-7Rαlow, EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T-cell subsets was determined by gating on each cell subset. Numbers in dot plots (A) indicate the percentage of positive cells in each quadrant. Results are representative data from 5 separate subjects in each group. (D-E) PBMCs from a healthy elderly subjects were stained with antibodies to CD8, CD45RA, and CCR7 and were sorted into naive, EM, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow. (D) Cells were stained with CFSE and stimulated for 6 days with antibodies to CD3 and CD28. Stimulated cells were stained with antibodies to CD57. Numbers in histograms indicate the percentages of proliferating cells. Results are representative data from 3 young and 2 elderly subjects. (E) Sorted cells were incubated for 6 days in the presence of IL-7 (100 ng/mL) and were stained with annexin V and 7-AAD. Numbers in dot plots indicate the percentage of cells in each quadrant. Results are representative data from 2 young and 2 elderly subjects.
To investigate whether an alteration in IL-7Rα expression and STAT5 phosphorylation in CD8+ T-cell subsets could affect Bcl-2 regulation and cell survival in elderly subjects, Bcl-2 up-regulation and cell survival were also determined in sorted CD8+ T-cell subsets in response to IL-7. As with IL-7Rα expression and STAT5 phosphorylation, Bcl-2 was up-regulated homogeneously in naive and CM subsets in response to IL-7 (Figure 2D). However, in EM and EMCD45RA+ CD8+ T-cell subsets, Bcl-2 up-regulation was heterogeneous, reflecting IL-7Rα expression by these cell subsets. The up-regulation of Bcl-2 in EMCD45RA+ CD8+ T-cell subset was lower in elderly subjects (Figure 2D). We next measured cell survival in young and elderly subjects from sorted CD8+ T-cell subsets incubated for 6 days with IL-7 (100 ng/mL) or PBS. Cells in the 4 subsets incubated with IL-7 had higher numbers of live cells than cells incubated with PBS. In the EMCD45RA+ CD8+ T-cell subset, the increase in the frequency of live cells was lower in elderly than in young subjects (Figure 2E; 19.9% ± 2.4% vs 26.8% ± 1.01%), whereas the frequency of such cells in the other 3 subsets increased similarly in both groups. These findings indicated that elderly subjects had decreased cell survival responses to IL-7 in the EMCD45RA+ CD8+ T-cell subset that stemmed from decreased IL-7Rα expression, STAT5 phosphorylation, and Bcl-2 up-regulation.
Age-related increased frequency of EMCD45RA+ IL-7Rαlow cells likely stems from accumulated antigen-experienced CD8+ T cells with replicative senescence
To investigate the mechanisms for increased frequency of EMCD45RA+ IL-7Rαlow CD8+ T cells with aging, we determined the cellular characteristics of the CD8+ T-cell subsets. In young and elderly subjects, EM and EMCD45RA+ IL-7Rαlow CD8+ T cells were largely antigen experienced and perforinhigh (CD27-CD28- perforinhigh) with replicative senescence (CD57+)25,26 (Figure 3A-C). Such molecular changes were more notable in elderly subjects (Figure 3A-C). In measuring the proliferation of EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in response to anti-CD3 and anti-CD28 antibodies, IL-7Rαlow cells proliferated minimally whereas IL-7Rαhigh cells proliferated to the same extent as naive CD8+ T cells in elderly (Figure 3D) and young (data not shown) subjects. These findings suggest that the increased frequency of EMCD45RA+ IL-7Rαlow CD8+ T cells likely stems from the age-related accumulation of antigen-experienced and replicatively senescent CD8+ T cells. This notion is further supported by studies showing the down-regulation of IL-7Rα by TCR triggering in T cells,27 which we also observed (Figure 4A-B). Of interest, CD57+ cells in the EMCD45RA+ IL-7Rαhigh CD8+ T-cell subset proliferated significantly in response to anti-CD3 and anti-CD28 antibodies, whereas cells expressing the same molecule in the EMCD45RA+ IL-7Rαlow CD8+ T-cell subset proliferated poorly (Figure 3D). This finding suggests that some CD57+ CD8+ T cells are functional in terms of cell proliferation and that IL-7Rα can be a marker for identifying such cells. We also measured phosphorylated STAT5 signaling and survival rates of IL-7Rαhigh and IL-7Rαlow cells in the EMCD45RA+ CD8+ T-cell subset in young and elderly subjects after stimulating cells with IL-7. As expected, IL-7Rαhigh cells had better survival rates (Figure 3E) and phosphorylation signaling (data not shown) in response to IL-7 than did IL-7Rαlow cells, which further supports the role of IL-7Rα in promoting CD8+ T-cell survival in response to IL-7.
Alterations in the expression of IL-7Rα, GABPα, and GFI1 mRNA in naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells in response to cell stimulation. PBMCs from healthy young subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and were sorted into naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells. The expression of IL-7Rα, GABPα, and GFI1 mRNA in freshly isolated (0 hour), 24-hour PBS- and PHA-incubated naive (A) and EMCD45RA+ IL-7Rαhigh (B) cells was measured using real-time PCR. Graphs show the mean expression (n = 2) and standard deviation (SD) of the indicated genes. Data were normalized to β-actin expression in individual samples.
Alterations in the expression of IL-7Rα, GABPα, and GFI1 mRNA in naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells in response to cell stimulation. PBMCs from healthy young subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and were sorted into naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells. The expression of IL-7Rα, GABPα, and GFI1 mRNA in freshly isolated (0 hour), 24-hour PBS- and PHA-incubated naive (A) and EMCD45RA+ IL-7Rαhigh (B) cells was measured using real-time PCR. Graphs show the mean expression (n = 2) and standard deviation (SD) of the indicated genes. Data were normalized to β-actin expression in individual samples.
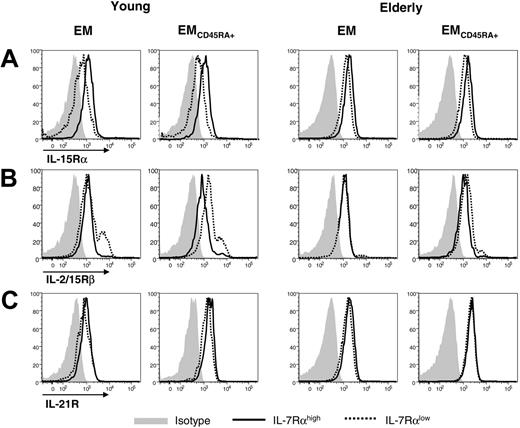
Expression of IL-15Rα, IL-2/15Rβ, and IL-21R by EM and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CCR7, CD45RA, IL-7Rα, IL-15Rα, IL-2/15Rβ, IL-21R, or isotype antibodies. The expression of IL-15Rα (A), IL-2/15Rβ (B), and IL-21R (C) was determined by gating on IL-7Rαhigh and IL-7Rαlow cells in EM and EMCD45RA+ CD8+ T-cell subsets. Results are representative data from 4 separate subjects in each group.
Expression of IL-15Rα, IL-2/15Rβ, and IL-21R by EM and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. PBMCs from healthy elderly and young subjects were stained with antibodies to CD8, CCR7, CD45RA, IL-7Rα, IL-15Rα, IL-2/15Rβ, IL-21R, or isotype antibodies. The expression of IL-15Rα (A), IL-2/15Rβ (B), and IL-21R (C) was determined by gating on IL-7Rαhigh and IL-7Rαlow cells in EM and EMCD45RA+ CD8+ T-cell subsets. Results are representative data from 4 separate subjects in each group.
EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells make similar use of IL-15 and IL-21 in the elderly
IL-7Rαlow cells in the EM and EMCD45RA+ CD8+ T-cell subsets were maintained despite these cells having impaired proliferation to TCR stimulation (Figure 3D) and decreased IL-7Rα expression, critical for cell survival. IL-15 and IL-21 are also members of the γc cytokine family and potently promote the proliferation of CD8+ T cells.28-30 Thus, we investigated whether IL-7Rαlow cells in the EM and EMCD45RA+ CD8+ T-cell subsets had altered expression of IL-15Rα, IL-2/15Rβ, and IL-21R compared with IL-7Rαhigh cells. In young subjects, the expression of IL-15Rα by IL-7Rαhigh cells in the EM and EMCD45RA+ CD8+ T-cell subsets was higher than by IL-7Rαlow cells, but the difference was smaller in elderly subjects (Figure 5A). This suggests that the use of IL-15 by IL-7αhigh and IL-7αlow cells through IL-15Rα is similar in the elderly, though such cytokine use by IL-7Rαhigh cells can be higher than by IL-7Rαlow cells in the young. The expression of IL-2/15Rβ by EMCD45RA+ IL-7Rαlow CD8+ T cells was higher (young subjects) and similar (elderly subjects) compared with IL-7Rαhigh cells (Figure 5B), suggesting that the use of IL-15 by EMCD45RA+ IL-7Rαlow cells through IL-2/IL-15Rβ is at least comparable to that of IL-7Rαhigh cells. The expression of IL-21R by IL-7Rαhigh and IL-7αlow cells in EM and EMCD45RA+ CD8+ T-cell subsets was similar in the 2 groups (Figure 5C). Because any alteration in IL-15 and IL-21 levels can affect CD8+ T-cell homeostasis, we also measured plasma levels of these cytokines in both groups of subjects. They had comparable plasma levels (IL-15, 1.75 pg/mL ± 0.15 vs 1.49 pg/mL ± 0.20; IL-21, 225 ng/mL ± 77 vs 272 ng/mL ± 73; P = .322 and P = .676, respectively), indicating the unaltered age-related availability of such cytokines in blood. These findings suggest that IL-7Rαlow CD8+ T cells in the elderly have unaltered IL-15- and IL-21-mediated CD8+ T-cell homeostasis than do IL-7Rαhigh cells, which may account for the maintenance of IL-7Rαlow CD8+ T cells in vivo.
EMCD45RA+ IL-7Rαlow CD8+ T cells in homeostasis have suppressed IL-7Rα mRNA expression irrespective of GABPα and GFI expression
We investigated how EMCD45RA+ IL-7Rαlow CD8+ T cells maintain low levels of IL-7Rα expression in homeostasis by measuring the gene expression of IL-7Rα and 2 transcriptional factors, guanine and adenine binding protein α (GABPα) and growth factor independence-1 (GFI1), in sorted CD8+ T-cell subsets. GABPα is required for the expression of IL-7Rα in T cells,31 whereas GFI1 is involved in suppressing IL-7Rα expression in mouse lymph node CD8+ T cells in response to IL-7.32 In our study, IL-7 withdrawal by the maintenance of cells for 24 hours in tissue culture medium—a known condition up-regulating the expression of IL-7Rα—increased IL-7Rα and GABPα mRNA expression by naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells (Figure 4A-B). In contrast, TCR stimulation for 24 hours with PHA suppressed IL-7Rα and GABPα mRNA expression by the same cell subsets compared with samples stimulated for 24 hours with PBS (Figure 4A-B). These findings indicate that GABPα is likely involved in the regulation of IL-7Rα expression in human CD8+ T cells in response to stimulations altering IL-7Rα expression. Surprisingly, IL-7 withdrawal increased the expression of GFI1, a suppressor of IL-7Rα expression in mice, by naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells. However, TCR stimulation did not change or down-regulated the expression of GFI1 by the same cell subsets compared with samples treated for 24 hours with PBS in tissue culture media (Figure 4A-B). These findings suggest that the role of GFI1 in regulating IL-7Rα expression in human CD8+ T cells may not be the same as in mouse lymph node T cells.32
We next determined mRNA expression of IL-7Rα, GABPα, and GFI1 in freshly sorted naive EMCD45RA+ IL-7Rαhigh and IL-7Rαlow cells in elderly subjects. The expression of IL-7Rα mRNA was lower in EMCD45RA+ IL-7Rαlow CD8+ T cells than in other subsets (Figure 6). However, GABPα mRNA expression was not statistically different among naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow cells, suggesting that this transcriptional factor was not accountable for the suppressed IL-7Rα mRNA expression in EMCD45RA+ IL-7Rαlow cells in homeostasis. Of interest, GFI1 mRNA expression was higher in EMCD45RA+ IL-7Rαhigh cells than in naive and EMCD45RA+ IL-7Rαlow cells, which contradicted the previous study reporting the suppressor role of GFI1 in regulating IL-7Rα expression in mouse lymph node T cells.32 These observations suggest that the mechanism involved in maintaining the low levels of IL-7Rα mRNA expression in EMCD45RA+ IL-7Rαlow CD8+ T cells in homeostasis are different from the known IL-7Rα regulatory mechanisms.
Expression of IL-7Rα, GABPα, and GFI1 mRNA in naive and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in the elderly. PBMCs from healthy elderly subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and were sorted into naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells. Expression of IL-7Rα, GABPα, and GFI1 mRNA was measured in freshly isolated naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells using real-time PCR. Graphs show the mean ± SD expression (n = 5) of the indicated genes. Data were normalized to β-actin expression in individual samples. P values were obtained with the Mann-Whitney U test.
Expression of IL-7Rα, GABPα, and GFI1 mRNA in naive and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in the elderly. PBMCs from healthy elderly subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and were sorted into naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells. Expression of IL-7Rα, GABPα, and GFI1 mRNA was measured in freshly isolated naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells using real-time PCR. Graphs show the mean ± SD expression (n = 5) of the indicated genes. Data were normalized to β-actin expression in individual samples. P values were obtained with the Mann-Whitney U test.
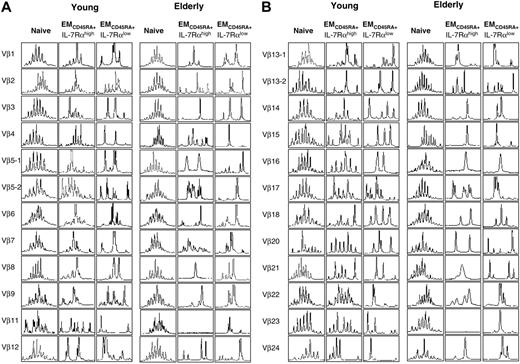
TCR repertoires of EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells are limited in the elderly
Data, including ours, indicate that survival of the CD8+ T-cell subsets can be promoted by IL-7.7 Therefore, IL-7 therapy can be considered to improve the development and survival of naive and memory CD8+ T cells. However, before attempting such therapy, it is important to determine the diversity of the TCR repertoire in IL-7Rαhigh and IL-7Rαlow CD8+ T cells because a restriction in the TCR repertoire with oligoclonal CD8+ T-cell expansion occurs with aging13 and such changes can impair the development of CD8+ T-cell immune responses to new antigens.33 In our study, young and elderly subjects had diverse TCR repertoires in naive CD8+ T cells but limited TCR repertoires in EMCD45RA+ IL-7Rαlow CD8+ T cells (Figure 6A-B). However, elderly subjects had a more limited TCR repertoire in EMCD45RA+ IL-7Rαhigh CD8+ T cells than did young subjects (Figure 7A-B). These findings suggest that IL-7 therapy may enhance the survival of EMCD45RA+ IL-7Rαhigh CD8+ T cells with a diverse TCR repertoire in the young but not in the elderly, though such therapy may increase the survival of naive CD8+ T cells with a diverse TCR repertoire in both groups.
TCR repertoire of naive and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. PBMCs from healthy young and elderly subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and sorted into naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells. Total RNA was isolated from sorted cells and used for measuring the TCR repertoire using spectratyping, as described in “Experimental procedures.” Results are representative data from 2 young and 2 elderly subjects.
TCR repertoire of naive and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells in elderly and young subjects. PBMCs from healthy young and elderly subjects were stained with antibodies to CD8, CD45RA, CCR7, and IL-7Rα and sorted into naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow CD8+ T cells. Total RNA was isolated from sorted cells and used for measuring the TCR repertoire using spectratyping, as described in “Experimental procedures.” Results are representative data from 2 young and 2 elderly subjects.
Discussion
This is the first study demonstrating an age-associated alteration in the frequency of EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells, leading to decreased cell signaling and survival responses to IL-7. In measuring IL-7Rα expression, naive and CM CD8+ T-cell subsets had homogeneous expression of IL-7Rα (Figure 1A), whereas 2 different cell populations, IL-7Rαhigh and IL-7Rαlow, were identified in EM and EMCD45RA+ CD8+ T-cell subsets. Of interest, elderly subjects had decreased expression of IL-7Rα by naive and EMCD45RA+ CD8+ T cells. Such a change in the latter subset was secondary to an increased frequency of IL-7Rαlow cells (Figure 1B, D), which were largely late differentiated (CD27-CD28-) and replicatively senescent cells (CD57+) (Figure 3A-C).25,26 Consistent with the expression of CD27 and CD28, costimulatory molecules for T-cell proliferation,25 the proliferative response was markedly reduced in EMCD45RA+ IL-7Rαlow cells compared with EMCD45RA+ IL-7Rαhigh cells (Figure 3D). These findings indicate that IL-7Rαlow cells are generated as a result of antigenic stimulation because TCR stimulation suppresses CD27 and CD28 expression.25 This notion is further supported by the results of studies, including ours, showing the down-regulation of IL-7Rα by TCR stimulation (Figure 4A-B)27,34 and the expansion of IL-7Rαlow CD8+ T cells in persons infected with HIV who have chronic immune activation.35 Overall, the results of our study demonstrate that the increased frequency of EMCD45RA+ IL-7Rαlow CD8+ T cells in the elderly is likely related to an accumulation of antigen-experienced and replicatively senescent CD8+ T cells with aging.
An intriguing question is how IL-7Rαlow cells in EM and EMCD45RA+ CD8+ T-cell subsets can be maintained in vivo despite low levels of IL-7Rα expression that is critical for cell survival. We addressed this question by measuring the expression of IL-15Rα, IL-2/15Rβ, and IL-21R by IL-7Rαhigh and IL-7Rαlow cells. IL-15 and IL-21 potently induce the proliferation of memory CD8+ T cells,30,36-39 whereas IL-7 predominantly promotes the survival of these cells.3-5,7,8 In our study, IL-7Rαlow cells in the EMCD45RA+ CD8+ T-cell subsets had increased (young) or similar (elderly) expression of IL-2/15Rβ compared with IL-7Rαhigh cells (Figure 5B). This finding suggests that IL-7Rαlow cells can be maintained in vivo through the use of IL-15 despite the decreased IL-7Rα expression on these cells. In fact, the crucial role of IL-2/15Rβ in IL-15-mediated cell signaling and in maintaining memory CD8+ T cells has been reported.40,41 This notion is further supported by the finding that IL-7Rαlow CD8+ T cells survive better in IL-15 intact mice than in IL-15 KO mice (S. Kaech and Nikhil Joshi, Yale University, New Haven, CT; unpublished observation, June 2005).
To measure the physiologic significance of IL-7Rα expression changes with aging, we investigated CD8+ T-cell signaling and survival in response to IL-7. First, we measured the phosphorylation of STAT5 in CD8+ T-cell subsets in response to IL-7 (Figure 2A). The effect of IL-7 on STAT5 phosphorylation in CD8+ T cells appears to be dependent on 2 factors, the quantity of IL-7Rα expressed on cells and the strength of IL-7 stimulation. The importance of the former factor is demonstrated by the correlation between the frequencies of IL-7Rαhigh cells and P-STAT5high cells in EM and EMCD45RA+ CD8+ T-cell subsets (Figure 2A-B). The quantitative role of IL-7Rα expression in determining the extent of STAT5 phosphorylation is further supported by the results of our experiment showing reduced P-STAT5 in IL-7-stimulated EMCD45RA+ CD8+ T cells in elderly subjects that contained a higher number of IL-7Rαlow cells than in young subjects. The phosphorylation of STAT5 in the CD8+ T-cell subsets also correlated with the strength of IL-7 stimulation in the young. Although a similar correlation between the 2 parameters in naive, CM, and EM CD8+ T-cell subsets was observed in the elderly, the phosphorylation of STAT5 in the EMCD45RA+ CD8+ T-cell subset reached a plateau at 1 ng/mL IL-7 (Figure 2C). This suggests that even high-dose IL-7 cannot overcome the physiologic effect of the decreased IL-7Rα expression by EMCD45RA+ IL-7Rαlow CD8+ T cells in the elderly.
Bcl-2 is an antiapoptotic molecule that is critically involved in the IL-7-mediated cell-survival pathway.7 The results of our study demonstrate that this correlation of IL-7Rα and bcl-2 up-regulation also occurs in human peripheral CD8+ T cells. Similar to STAT5 phosphorylation, Bcl-2 up-regulation in EMCD45RA+ CD8+ T cells was lower in elderly subjects than in young subjects (Figure 2D). Furthermore, the survival response of EMCD45RA+ CD8+ T cells on IL-7 stimulation was lower in elderly than in young subjects (Figure 2E). These findings indicate the physiologic impact of the decreased IL-7Rα expression and STAT5 phosphorylation by this cell subset with aging on subsequent Bcl-2 up-regulation and cell survival.
The regulatory mechanisms for IL-7Rα expression in T cells are largely unknown. Recent studies reported a role of 2 transcriptional factors, GABPα and GFI1, in regulating IL-7Rα expression in human and mouse T cells.31,32 GABPα regulated IL-7Rα expression in T cells by binding to the GGAA motif in the IL-7R promoter in mouse splenic T cells.31 In addition, IL-7Rα expression in human and mouse T cells was suppressed by siRNA targeting of GABPα. In contrast to GABPα, GFI1 suppressed the expression of IL-7Rα in mouse lymph node T cells stimulated with prosurvival cytokines including IL-7.32 Based on these observations, we asked how IL-7Rα expression was kept low in EMCD45RA+ IL-7Rαlow cells in vivo. First, we confirmed the correlation between IL-7Rα mRNA and protein expressions in naive and EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T-cell subsets, indicating that decreased IL-7Rα protein expression by IL-7Rαlow cells was directly related to the suppression of gene expression (Figure 6). Of interest, GABPα expression was not different among the 3 CD8+ T-cell subsets despite the difference in IL-7Rα mRNA expression (Figure 6). This suggests the limited role of GABPα in regulating IL-7Rα mRNA expression in primary human CD8+ T cells in homeostasis. The result of measuring GFI1 in sorted naive, EMCD45RA+ IL-7Rαhigh, and IL-7Rαlow cells was surprising. GFI mRNA expression was higher in EMCD45RA+ IL-7Rαhigh cells than in IL-7Rαlow cells (Figure 6), which contradicted the previously reported suppressor role of this transcriptional factor in regulating IL-7Rα expression in mouse lymph node T cells.32 In fact, in our study, the expression of GFI1 was also unaltered or suppressed in naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells after 24-hour TCR stimulation, a known condition that down-regulates IL-7Rα expression,27 compared with cells stimulated for 24 hours with PBS (Figure 4A-B). This finding further questions the suppressor role of GFI1 in human peripheral CD8+ T cells. There are several possible explanations for such a difference between our findings and the results of the previous studies on GABPα and GFI1. This difference could be secondary to using T cells from different species or activation status. More likely, in homeostasis, the low levels of IL-7Rα mRNA expression in EMCD45RA+ IL-7Rαlow CD8+ T cells are maintained by an unknown factor(s) involved in the IL-7Rα regulatory mechanism.
The reduced TCR repertoire of CD8+ T cells with oligoclonal expansions has been observed with aging.13 In our study, EMCD45RA+ IL-7Rαlow CD8+ T cells had reduced TCR diversity compared with naive cells in young and elderly subjects. However, the TCR diversity of EMCD45RA+ IL-7Rαhigh CD8+ T cells was different between the young and the elderly groups. The diversity of such cells in elderly subjects was limited, suggesting oligoclonal expansions. This finding prompts caution in attempting IL-7 therapy in the elderly because such therapy may increase the survival of EMCD45RA+ IL-7Rαhigh CD8+ T cells with a limited TCR repertoire and possible oligoclonal expansions that may not be beneficial to the immune system,33 although it can still increase the survival of naive CD8+ T cells with a diverse TCR repertoire. Indeed, a recent study33 demonstrated the impaired development of CD8+ T-cell immune responses to herpes simplex virus-1 (HSV-1) infection in aged mice with oligoclonal CD8+ T-cell expansions. However, in the young, IL-7 therapy can be useful in promoting the survival of naive and EMCD45RA+ IL-7Rαhigh CD8+ T cells with a diverse TCR repertoire, leading to improved immune responses to new antigens. In fact, this notion is supported by a recent mouse study19 reporting an adjuvant role of IL-7 in augmenting CD8+ T-cell immune responses to subdominant antigens and improving the survival of memory CD8+ T cells.
In summary, our study demonstrates an age-associated alteration in the frequency of EMCD45RA+ IL-7Rαhigh and IL-7Rαlow CD8+ T cells that impairs cell signaling and survival responses to IL-7. Such an alteration stems likely from age-long antigenic CD8+ T-cell stimulation, leading to the down-regulation of IL-7Rα mRNA synthesis and maintained by an unaltered use of IL-15 through IL-15Rα and IL-2/15Rβ expression. Our findings also suggest that IL-7 therapy in the elderly may not be as effective and beneficial as in the young because a large proportion of CD8+ T cells in the former group has decreased IL-7Rα expression and limited TCR repertoire compared with the latter group. Overall, the results of our study are unique in that they provide new insight into aging and IL-7-mediated CD8+ T-cell homeostasis and into the potential implications of IL-7 therapy in the elderly.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-09-3560.
Supported in part by grants from the National Institutes of Health (K08 AR49444-02), the Hartford Foundation, the American College of Rheumatology, the Arthritis Foundation, the American Foundation for Aging Research, the Yale Pepper Center, and the Lupus Foundation of America (all for I.K.).
H.K. and I.K. designed and performed the research, analyzed the data, and wrote the paper. M.S.H. and J.M.D. performed the research and analyzed the data.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Susan Kaech and Alexia Belperron for critical reviews of this manuscript and Lynne Iannone, Christine Bailey, and Barbara Foster for assistance in the recruitment of human subjects.