Abstract
Current monoclonal antibody therapies for multiple myeloma have had limited success, perhaps due to narrow target specificity. We have previously described the ability of polyclonal rabbit antithymocyte globulin (rATG) to induce caspase- and cathepsin-mediated apoptosis in human B and plasma cells. We now extend this observation to myeloma cells. Complement independent cell death was measured after addition of rATG (1-1000 μg/mL) to cultures of myeloma cell lines or primary CD138+ isolates from patient bone marrow aspirates. rATG induced significant levels of apoptosis in myeloma cells as assayed by caspase induction, annexin V binding, subdiploid DNA fragmentation, plasma-membrane permeability, and loss of mitochondrial-membrane potential. Addition of complement greatly augmented myeloma-cell death. Binding of rATG to individual myeloma cell-surface proteins, primarily CD38, CD52, CD126, and CD138, was demonstrated by competitive inhibition experiments with targeted monoclonal antibodies. Three pathways of cell death were identified involving caspase activation, cathepsin D, and the genistein sensitive tyrosine kinase pathway. F(ab′)2 fragments of rATG had reduced proapoptotic activity, which was restored by coincubation with Fc fragments, and anti-CD32 or anti-CD64 antibodies. We conclude that rATG is an effective agent for in vitro induction of apoptosis in multiple myeloma, and that exploratory clinical trials may be warranted.
Introduction
Developing effective cytotoxic monoclonal antibody therapies against multiple myeloma has been hampered by lack of target molecules that are unique and constitutively expressed on all myeloma cells. Therapies felt to have promise have included anti-CD20 (rituximab, or the toxin-conjugated variant),1,2 anti-CD40,3 anti-CD52 (alemtuzumab),4 anti-CD74,5 anti-CD126 (atlezumab),6,7 and anti-CD138.8 Unfortunately, these agents have often shown limited utility against myeloma.
Monoclonal antibodies may have had limited efficacy against myeloma tumor cells for at least 3 reasons. Many of the targeted surface markers, for example CD52 and CD20, are down-regulated in mature plasma cells and expressed on only a subset of myelomas.2,9 In addition, antibody depletion of cells bearing myeloma specific markers such as CD138 (syndecan-1) does not prevent reemergence of the primary clone from CD138- bone marrow precursors. Myeloma bone marrow aspirates depleted of CD138+ cells will, after several weeks, generate new CD138-expressing myeloma cells identical to those depleted.10 These putative “myeloma stem cells” express several surface markers similar to those found in post-germinal center B cells, along with molecular markers common to their more differentiated CD138+ progeny.10 Finally, some myeloma cells escape complement-dependent antibody-mediated cytotoxicity by expressing complement cascade inhibitors, such as CD59,11 or by resistance to proapoptotic signals.12,13
Polyclonal antibody preparations may have several advantages over monoclonal therapeutic agents, including the ability to target multiple surface proteins and simultaneously trigger several parallel or additive pathways for cell death. This may be a distinct advantage when attempting to eradicate myeloma cells which emerge from a common less-differentiated precursor10,14 and may be responsive to coordinate activation of several cell-death pathways.13,15
We have recently described the ability of rabbit polyclonal antithymocyte globulin (rATG) to induce apoptosis of normal naive, memory, and activated B cells, in addition to normal human plasma cells.16,17 ATG is prepared by immunizing rabbits with nonfractionated human thymocytes isolated by Ficoll density gradient centrifugation. This crude innoculum contains CD20+ B and CD138+ plasma cells,17,18 which constitute approximately 5% of thymocytes.19-21 Consistent with this finding, we have described the presence of anti-CD20-, anti-CD38-, and anti-CD138-specific activity in rATG.17 In this report, we describe the induction of apoptosis, as well as complement-mediated cell lysis, by rATG in fresh myeloma cells and myeloma cell lines, identify some of the antigens against which the activity is directed, and describe several pathways of rATG-induced cell death in myeloma cells.
Patients, materials, and methods
Human subject protection
This study was approved by the Research Subjects Review Board at the University of Rochester Medical Center. Informed consent was obtained from all participants. Research data were coded such that subjects could not be identified, directly or through linked identifiers, in compliance with the Department of Health and Human Services Regulations for the Protection of Human Subjects (45 CFR 46.101(b)4 ).
Cell lines and culture conditions
Myeloma cell lines were obtained from the American Tissue Culture Collection (ATCC; Manassas, VA) repository. Culture media contain RPMI, HEPES, l-glutamine, and different concentrations of heat-inactivated fetal bovine serum (hiFBS): MC1 media had 10% hiFBS, MC2 had 15%, and MC3 had 20%. Cell lines and cultured conditions included: MCI (RPMI-8226, ARH-77, NCI-H929), MC2 (U266), and MC3 (MC-CAR), and were obtained from ATCC. All cells were incubated in 5% CO2 at 37°C and saturated humidity. CD40L-stimulated human B cells and CpG-generated human plasma cells were prepared and cultured as previously described.17,22,23 Human bone marrow stromal cells were isolated from bone marrow aspirates of healthy volunteers and cultured as previously described.24
Sources of myeloma cells and cell lines
Primary myeloma cells were isolated from patient bone marrow aspirates. Cells were initially separated by Ficoll density gradient centrifugation and washed in phosphate-buffered saline (PBS) twice. CD138+ cells were then incubated with anti-CD138 antibodies coupled with magnetic beads and positively selected on a magnetic affinity column (Miltenyi Biotech, Auburn, CA) as previously described.17,23 The resulting cells were then washed twice in PBS and used for experiments. The percentage of myeloma infiltrate in the bone marrow was quantified by flow cytometric analysis of κ- and λ-light chain expression as previously described.17,23
Antibody reagents and flow cytometry
Flow cytometric analysis was performed with a FacsCalibur dual laser cytometer using CellQuest acquisition (both from Becton Dickinson, San Jose, CA) and Cytomation analysis software (Summit, Boulder, CO). The following antibodies were used for staining (murine monoclonal from BD Pharmingen (San Diego, CA), unless otherwise noted): fluorescein-conjugated goat F(ab′)2 (anti-heavy chain) anti-human IgG Fc (Jackson ImmunoResearch, Bar Harbor, ME), unconjugated goat F(ab′)2 anti-human heavy- and light-chain immunoglobulin G (IgG; Jackson ImmunoResearch), anti-CD3 PE (clone HIT3a), fluorescein-conjugated and unconjugated human IgG Fc fragments (Jackson ImmunoResearch), PE anti-CD19 (HIB19), PE and CyChrome anti-CD20 (2H7), FITC-anti-CD27 (M-T271), CyChrome anti-CD38 (HIT2), PE anti-CD40 (5C3), PE-anti-human leukocyte antigen (HLA)-ABC, (G46-2.6), CyChrome (TU36) and unconjugated (G46-6) anti-HLA-DR, PE anti-CD52 (YTH34.5; Serotec, Kidlingston, United Kingdom), PE anti-CD80 (L307.4), PE anti-CD95 (DX2), and PE anti-CD138 (Miltenyi Biotec). PE-, FITC-, or CyChrome-conjugated murine IgG1κ was used as isotype control. PE- and CyChrome-conjugated streptavidin (BD Pharmingen) were used as second-step reagents for biotinylated antibodies.
Rabbit IgG (Sigma, St Louis, MO), ATG (rabbit) rabbit anti-human thymoglobulin (Genzyme, Cambridge, MA), rituximab (Biogen Idec, Cambridge, MA), and alemtuzumab (Berlex, Montville, NJ) were reagents used in the induction of apoptosis. rATG was generously provided by Genzyme (Cambridge, MA), or obtained independently by the investigators. Critical experiments were verified across 5 different lots of rATG (TH032-1, TH052-01, TH089-01F3, TH108-01, and TH111-01). Caspase substrates z-VAD-fmk and z-FA-fmk were obtained from Cell Technology (Minneapolis, MN). Annexin V and TOPRO-3 were purchased from BD Pharmingen (San Diego, CA) and Molecular Probes (Eugene, OR), respectively. Dexamethasone was purchased from Sigma and stock solution prepared by dissolving in DMSO. Bortezomib (Millennium Pharmaceuticals, Cambridge, MA) was purchased from the pharmacy and 10 mM stock was prepared by resuspension in 0.9% saline.
Measurement of apoptosis
Induction and measurement of apoptosis was performed as previously described. For each experiment, 105 cells/well were cultured in 48-well flat-bottom plates in their respective medium. To test their capacity for induction of apoptosis, the following agents were added to the medium: rATG (100-1000 μg/mL), rituximab (250 μg/mL), alemtuzumab (250 μg/mL), or nonimmunized rabbit IgG (100 μg/mL) as a negative control. Cells were then incubated for 1 to 18 hours at 37°C. For apoptosis experiments using bone marrow stromal cells, myeloma cells were cultured in 24-well flat-bottom plates either directly in contact with stromal cells, or in a transwell that allowed the cells to share media without direct contact.
Induction of apoptosis was assessed by 4 different methods. (1) Caspase induction was measured by adding fluorescently tagged substrates (Apo-Alert; Cell Technology) for caspase 3 (z-DEVD-fmk), caspase 8 (z-IETD-fmk), or caspase 9 (z-LEHD-fmk) to cell-culture medium at a final concentration of 1 μg/mL 1 hour prior to flow cytometry. Caspase induction was assessed by FL2 channel shift. Experiments included controls with the nonlabeled pancaspase inhibitor z-VAD-FMK (100 μg/mL) added to cell-culture medium 1 hour prior to adding rATG and incubated for 18 hours. (2) Loss of plasma-membrane polarity was assessed by flow cytometric analysis of annexin V. Cells were washed twice in PBS + bovine serum albumin (BSA) buffer and labeled with FITC-conjugated annexin V in the presence of the DNA-binding dye TOPRO-3 (1 ng/mL), and immediately analyzed by flow cytometry. (3) Loss of mitochondrial-membrane potential was measured by quantifying the fluorescence intensity of the mitochondrion-selective probe tetramethylrhodamine methyl ester (TMRM; Molecular Probes), which is taken up by depolarized mitochondria. TMRM (4 μL of a 50-μM solution) was added to the culture medium 1 hour prior to analysis after incubating the cells in rATG (250 μg/mL) for 18 hours at 37°C. (4) Subdiploid DNA content was measured by TOPRO-3 binding as previously described.17,23
Assay of complement augmentation of rATG-induced cell death
To determine whether complement would augment rATG-induced apoptosis, we cultured myeloma cell lines in the presence of varying amounts of rabbit complement. Myeloma cells were isolated by Ficoll centrifugation as previously described. Using 48-well flat-bottom plates, 105 cells/well from different myeloma lines were incubated with either rIgG (100 μg/mL), rATG (250 μg/mL) or rATG plus complement (C12CC, 16 μg/mL; Serotec) at 37°C for 1 or 18 hours prior to labeling for immediate flow cytometry.
Assay of myeloma-cell-specific antibodies in rATG
To assess if rATG contained specific antibodies directed against myeloma-cell-specific surface markers, we performed competitive binding experiments as previously described.17,23 Cultured myeloma cell lines were isolated and purified by Ficoll-Paque centrifugation and 105 cells/well were preincubated with either rabbit IgG (100 μg/mL) or rATG (250 μg/mL) on ice for 1 hour. Cells were then washed with staining buffer and incubated with 10 μL human AB (Rh-) serum for 15 minutes on ice. After washing, cells were probed with specific fluorochrome-conjugated antibodies (PE-IgG1 isotype control, PE-anti-HLA-ABC, CyChrome and unconjugated anti-HLA-DR, PE-anti-CD16, PE-anti-CD19, PE and CyChrome anti-CD20, FITC-anti-CD27, PE-anti-CD30, PE-anti-CD32, CyChrome-anti-CD38, PE-anti-CD40, PE-anti-CD52, PE-anti-CD64, PE-anti-CD80, PE-anti-CD95, PE-anti-CD126, and PE-anti-CD138) on ice for 40 minutes. Cells were then washed and resuspended in 500 μL staining buffer and analyzed by flow cytometry.
Preparation of F(ab′)2 fragments of rATG
F(ab′)2 fragments of rATG and unimmunized rabbit IgG were prepared as previously described17 by pepsin digestion using the Immunopure F(ab′)2 kit (Pierce Chemical, Rockford, IL). Lyophilized rATG with vehicle was resuspended in sterile distilled water (20 mg/mL). For F(ab′)2 fragment preparation, rATG was dialyzed against a 20-mM sodium acetate buffer (pH 4.5) and 0.5 mL was then added to an equal volume of digestion buffer (M cysteine, 20 mM sodium acetate [pH 4.5]) and a slurry of immobilized pepsin and incubated at 37°C for 5 hours. The slurry was centrifuged and the supernatant passed over a protein A column to bind undigested Ig and Fc fragments. F(ab′)2 fragments were eluted in the unbound column fraction as assessed by absorbance at 280 nm and extensively dialyzed against PBS (pH 7.0). Digestion was confirmed by polyacrylamide gel electrophoresis. The final F(ab′)2 reagent was used at concentrations equimolar to that of intact rATG.
Immunohistochemical staining of thymic tissue
A random sample of 10 blocks was selected from 41 normal human pediatric thymuses removed from patients younger than 10 years old in 2001. New paraffin-cut sections were cut and immunoperoxidase staining was performed by previously published methods25,26 using a streptavidin-biotin detection system, horseradish peroxidase, and 7-aminoethylcarbizole (7-AEC) as the substrate. The primary antibodies were: CD3 (1:100 primary dilution; DAKO, Carpinteria, CA), CD20 (1:800 dilution, clone L26; DAKO), CD73α (1:100; DAKO), and CD138 (1:100, syndecan-1, clone B-B4; Serotec).25 Images were photographed with a Nikon Labophot microscope using a × 40 (0.75 NA) and × 100 (1.30 NA) oil objective lens (Melville, NY). Images were recorded with a Nikon Coolpix digital camera using the manufacturer's software. JPEG images were viewed with Adobe Photoshop (San Jose, CA) and contrast adjustments made.
Statistical analysis
Statistical analyses was performed using Statistica version 6 software (Statsoft, Tulsa OK) on a Windows XP operating system (Microsoft, Redmond, WA). Comparisons between groups of apoptotic conditions were performed using the Student t test (2-sided).
Results
Induction of myeloma-cell apoptosis by rATG
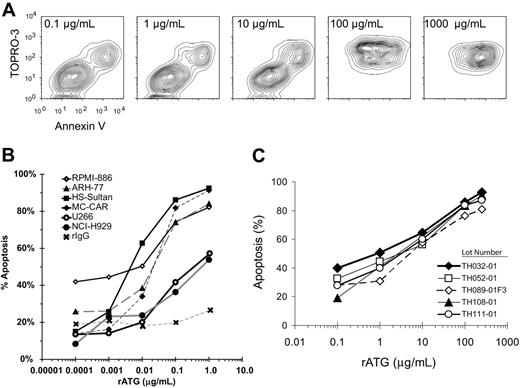
Incubation of myeloma cells at increasing concentrations of rATG demonstrated a progression from live (Annexinneg TOPROneg), to apoptotic (Annexinpos TOPROneg), and finally to late apopototic/necrotic (Annexinpos TOPROpos) phases (Figure 1A). The dose-response curve (Figure 1B) shows rATG at concentrations of greater than or equal to 250 μg/mL to be most effective at inducing apoptosis of myeloma cell lines. This compares with concentrations of rATG at 100 μg/mL for induction of apoptosis of CD40L-activated B cells.17 No lot-to-lot variation was observed when we compared the ability of 5 lots of rATG to induce apoptosis in the MC-CAR myeloma cell line (Figure 1C), as well as in CD40L-stimulated preplasmablasts (data not shown).
rATG-induced apoptosis in myeloma cell lines. (A) To determine the effective dose that renders apoptotic induction, different concentrations of rATG were added to myeloma cells. ARH-77 cells were isolated by Ficoll-Paque centrifugation, and 105 cells/well were placed in 48-well flat-bottom plates. Cells were incubated with various concentrations of rATG (0.0001-1 μg/mL) at 37°C for 18 hours and assayed with annexin/TOPRO. Incubation of myeloma cells at increasing concentrations of rATG demonstrated a progression from live (annexinneg TOPROneg) to apoptotic (annexinpos TOPROneg), and finally to late apopototic/necrotic (annexinpos TOPROpos) phases. (B) Concentration-dependent lysis of human myeloma cell lines. Multiple cell lines were incubated with rATG at varying concentrations for 18 hours in complement-free medium, and apoptosis was measured by annexin V binding. Data points are from n = 5 independent experiments. (C) Absence of lot-to-lot variation in apoptotic potential of rATG. MC-CAR myeloma cells were incubated with rATG for 18 hours; apoptosis was assessed by annexin V staining in 2 independent experiments for each data point. Five lots of rATG were tested.
rATG-induced apoptosis in myeloma cell lines. (A) To determine the effective dose that renders apoptotic induction, different concentrations of rATG were added to myeloma cells. ARH-77 cells were isolated by Ficoll-Paque centrifugation, and 105 cells/well were placed in 48-well flat-bottom plates. Cells were incubated with various concentrations of rATG (0.0001-1 μg/mL) at 37°C for 18 hours and assayed with annexin/TOPRO. Incubation of myeloma cells at increasing concentrations of rATG demonstrated a progression from live (annexinneg TOPROneg) to apoptotic (annexinpos TOPROneg), and finally to late apopototic/necrotic (annexinpos TOPROpos) phases. (B) Concentration-dependent lysis of human myeloma cell lines. Multiple cell lines were incubated with rATG at varying concentrations for 18 hours in complement-free medium, and apoptosis was measured by annexin V binding. Data points are from n = 5 independent experiments. (C) Absence of lot-to-lot variation in apoptotic potential of rATG. MC-CAR myeloma cells were incubated with rATG for 18 hours; apoptosis was assessed by annexin V staining in 2 independent experiments for each data point. Five lots of rATG were tested.
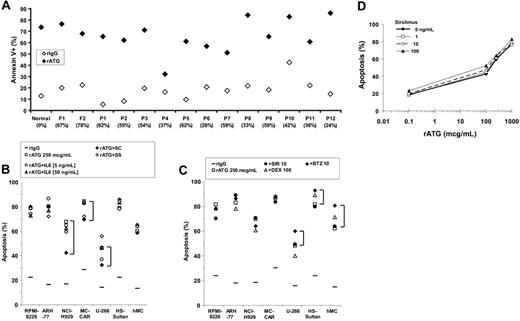
We then obtained myeloma cells from bone marrow aspirates of primary and relapsed patients and tested these for rATG-induced apoptosis. rATG at 250 μg/mL was effective at inducting apoptosis of previously frozen (F) or fresh (P) CD138+ myeloma cells obtained from patient bone marrow aspirates (Figure 2A). The degree of apoptosis induced varied between 53% and 84%, with only one sample appearing relatively resistant with 31% apoptosis.
Multiple mechanisms of rATG-induced apoptosis
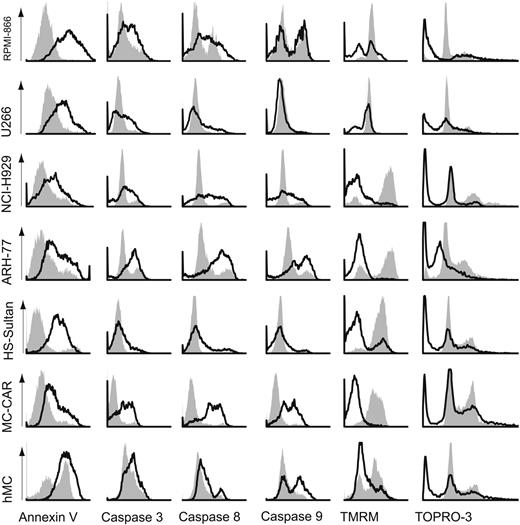
Because phosphatidyl serine externalization in B cells can under some conditions reflect activation as well as apoptosis, we measured rATG-induced apoptosis by several assays: loss of plasma-membrane polarization by annexin V binding to the outer leaflet, caspases 3, 8, and 9 induction, loss of mitochondrial-membrane potential measured by uptake of the TMRM dye, and subdiploid DNA content. Using these assays, we compared rATG with unimmunized rabbit IgG at 250 μg/mL on 5 myeloma cell lines. rATG induced high levels of apoptosis in U266 and RPMI-8226 (Figure 3), but apparently by different mechanisms. The cell lines RPMI-8226, NCI-H929, ARH-77, HS-Sultan, and MC/CAR all show a significant loss of mitochondrial-membrane potential. Similar results were found in primary myeloma cells. All cell lines and the primary myeloma cells showed induction of caspases 8 and 9, with the exception of the U266 and HS-Sultan lines. Interestingly, caspase 3 induction was only modest in HS-Sultan and primary myeloma cells. All cells showed subdiploid DNA fragmentation.
Does the bone marrow stroma protect myeloma cell lines against rATG-induced apoptosis?
The bone marrow microenvironment may protect myeloma cells from proapoptotic signals, either via secreted factors such as interleukin-6 (IL-6), or by direct cell contact.27 Thus, we next tested whether the presence of bone marrow stroma provided any protection against rATG-induced apoptosis. First, myeloma cell lines were incubated with 250 μg/mL rATG in the presence or absence of varying concentrations of IL-6 (Figure 2B). No difference in the efficacy of rATG-induced apoptosis was observed. Next, myeloma cell lines were cultured in transwell plates so that they were exposed to stromal-cell-secreted factors (SS), or in direct contact with primary bone marrow stromal cultures (SC; Figure 2B). We found several patterns of stromal-cell effect on apoptosis. NCI-H929 cells had reduced apoptosis in rATG when cultured in contact (SC) with stroma, but this effect was lost when cultured in transwell plates. In contrast, MC-CAR and U266 cells had modest reductions in apoptotic sensitivity with either contact or noncontact coculture.
rATG-induced apoptosis of primary myeloma-cell isolates from bone marrow aspirates. (A) CD138+-expressing cells were selected by immunomagnetic bead affinity columns from fresh normal (N), fresh patient with myeloma (P), or previously frozen (F) Ficoll density gradient-prepared bone marrow aspirates, with each data point composed of 2 replicates. Cells were incubated with 100 μg/mL rATG or unimmunized rabbit IgG serum in complement-inactivated free medium for 18 hours, and apoptosis measured by annexin V+TOPRO-3+ staining. (B) Effect of IL-6, bone marrow stromal secreted factors (SS), or contact (SC) on rATG-induced apoptosis. Myeloma cell lines or human primary myeloma cells (hMc) were incubated with rATG alone (□) or in combination with 5 ng/mL IL-6 (○) and 50 ng/mL IL-6 (•), or cultured in direct contact (SC; ♦)or in a transwell culture system sharing media but no cell-cell contact (SS; ⋄) with human primary bone marrow stromal cells. While IL-6 and soluble stromal-cell factors had no effect on rATG-induced apoptosis, stromal-cell contact modestly reduced rATG-induced apoptosis for NCI-H292, MC-CAR, and the U266 myeloma cell lines (brackets). (C) Incubation of myeloma cell lines or primary myeloma cells from bone marrow aspirates with rATG (250 μg/mL; □) and dexamethasone (100 μg/mL;▵), sirolimus (10 ng/mL; •), or bortezomib (10 nM; ♦) did not significantly augment rATG-induced apoptosis. (D) Sirolimus does not augment rATG-induced apoptosis of the myeloma cell line RPMI-8226. RPMI-8226 cells were incubated with clinically relevant concentrations of sirolimus and increasing concentrations of rATG. Data points represent 2 independent experiments.
rATG-induced apoptosis of primary myeloma-cell isolates from bone marrow aspirates. (A) CD138+-expressing cells were selected by immunomagnetic bead affinity columns from fresh normal (N), fresh patient with myeloma (P), or previously frozen (F) Ficoll density gradient-prepared bone marrow aspirates, with each data point composed of 2 replicates. Cells were incubated with 100 μg/mL rATG or unimmunized rabbit IgG serum in complement-inactivated free medium for 18 hours, and apoptosis measured by annexin V+TOPRO-3+ staining. (B) Effect of IL-6, bone marrow stromal secreted factors (SS), or contact (SC) on rATG-induced apoptosis. Myeloma cell lines or human primary myeloma cells (hMc) were incubated with rATG alone (□) or in combination with 5 ng/mL IL-6 (○) and 50 ng/mL IL-6 (•), or cultured in direct contact (SC; ♦)or in a transwell culture system sharing media but no cell-cell contact (SS; ⋄) with human primary bone marrow stromal cells. While IL-6 and soluble stromal-cell factors had no effect on rATG-induced apoptosis, stromal-cell contact modestly reduced rATG-induced apoptosis for NCI-H292, MC-CAR, and the U266 myeloma cell lines (brackets). (C) Incubation of myeloma cell lines or primary myeloma cells from bone marrow aspirates with rATG (250 μg/mL; □) and dexamethasone (100 μg/mL;▵), sirolimus (10 ng/mL; •), or bortezomib (10 nM; ♦) did not significantly augment rATG-induced apoptosis. (D) Sirolimus does not augment rATG-induced apoptosis of the myeloma cell line RPMI-8226. RPMI-8226 cells were incubated with clinically relevant concentrations of sirolimus and increasing concentrations of rATG. Data points represent 2 independent experiments.
Delineation of apoptotic pathways triggered by rATG. Myeloma cell lines and primary myeloma cells were incubated with 250 μg/mL unimmunized rabbit IgG (filled areas) or rATG (open areas) and assayed at 18 hours. Representative histograms are shown (from n = 5 experiments/cell type). Loss of mitochondrial-membrane potential was a prominent feature of all cell lines with the exception of U266, which exhibited primarily caspase-3 activation. Caspase-3 activation was seen to varying degrees in all cell lines and primary cells. All myeloma cell lines and primary cells exhibited subdiploid DNA fragmentation.
Delineation of apoptotic pathways triggered by rATG. Myeloma cell lines and primary myeloma cells were incubated with 250 μg/mL unimmunized rabbit IgG (filled areas) or rATG (open areas) and assayed at 18 hours. Representative histograms are shown (from n = 5 experiments/cell type). Loss of mitochondrial-membrane potential was a prominent feature of all cell lines with the exception of U266, which exhibited primarily caspase-3 activation. Caspase-3 activation was seen to varying degrees in all cell lines and primary cells. All myeloma cell lines and primary cells exhibited subdiploid DNA fragmentation.
Does coincubation with sirolimus, dexamethasone, or bortezomib increase rATG-induced apoptosis?
Recent work has indicated that the mTOR inhibitor sirolimus (rapamycin),28-30 dexamethasone,31,32 and proteasome inhibitor bortezomib33 sensitize myeloma cells to apoptosis. We were therefore interested to see if there was any synergy between rATG and these agents in induction of apoptosis (Figure 2C). No increase in rATG-induced apoptosis was seen with either sirolimus (10 ng/mL), bortezomib (10 nM), or dexamethasone (100 μM). Myeloma cell lines were incubated with clinically relevant concentrations of sirolimus and increasing concentrations of rATG (Figure 2C). No increase in rATG-induced apoptosis was seen over a sirolimus concentration range of 0.1 to 100 ng/mL, and similar results were obtained with dexamethasone and bortezomib (data not shown).
Complement and FcR ligation augment rATG-induced myeloma-cell death
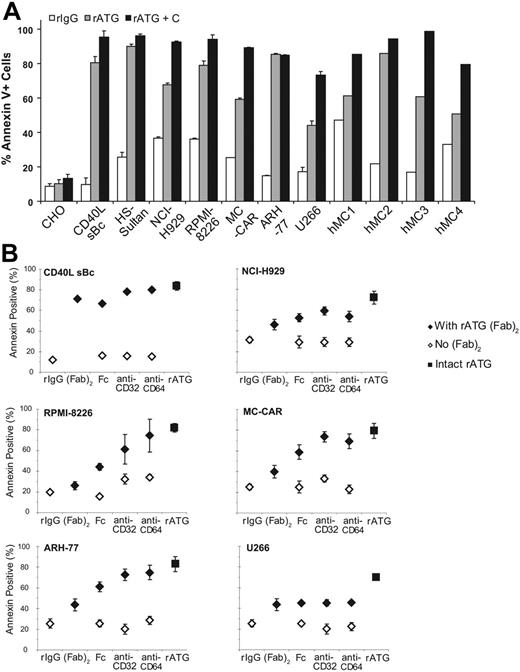
When given in vivo, rATG is a potent inducer of complement-mediated cell death. To assess the effect of complement, myeloma cells were incubated with rATG or unimmunized rabbit IgG with and without the addition of complement (Figure 4A). After 1 hour, addition of complement significantly augmented rATG-induced cell death of the RPMI-8226, NCI-H929, MC-CAR, and U266 cell lines. Small but statistically significant changes in cell death were seen with CD40L-stimulated B cells and the B-cell chronic lymphocytic leukemia (B-CLL) line H-Sultan, while no additional cell death was seen in the ARH-77 cell line compared with rATG alone. Similar increases in cell lysis in CD138+ primary myeloma cells from bone marrow aspirates were observed when complement was added to rATG. In some cases (hMC3, hMC4) the presence of complement increased cell death by as much as 45% over rATG alone.
Effects of complement upon rATG-induced cell death. (A) Addition of complement augments the ability of rATG to kill CD138+ myeloma cells. Once isolated by Ficoll centrifugation and washed, 105 cells/well were incubated with rIgG control, rATG alone, or rATG plus rabbit complement. Compared with rATG alone, rATG with added complement significantly increased death of CD40L-stimulated B cells, H-Sultan, NCI-H929, RPMI-8226, MC-CAR, and U266 cell lines. However, it had no effect on CHO or ARH-77 cell lines. Error bars indicate the mean ± standard deviation. (B) rATG F(ab′)2 fragment activity was assessed by reconstituting with anti-FcR-specific monoclonal antibodies. CD40L-stimulated human B-cell blasts or myeloma cell lines (105 cells/well) were incubated with 250 μg/mL rATG F(ab′)2 fragments alone or coincubated with either human Fc fragments, anti-CD32, or anti-CD64 (♦). The response is measured against the intact rATG molecule (▪). Controls without rATG F(ab′)2 but with unimmunized rIgG, Fc fragments, anti-CD32, or anti-CD64 are also shown (⋄). rATG F(ab′)2 fragment with or without human Fc fragment showed a weaker apoptotic induction potential than intact rATG (P < .05, all samples). For CD40L sBc, RPMI-8226, and NCI-H929 cells, rATG F(ab′)2 fragments coincubated with anti-CD32 or anti-CD64 increased apoptosis of myeloma cells, although still somewhat less than intact rATG. No effect was observed on U266 cells.
Effects of complement upon rATG-induced cell death. (A) Addition of complement augments the ability of rATG to kill CD138+ myeloma cells. Once isolated by Ficoll centrifugation and washed, 105 cells/well were incubated with rIgG control, rATG alone, or rATG plus rabbit complement. Compared with rATG alone, rATG with added complement significantly increased death of CD40L-stimulated B cells, H-Sultan, NCI-H929, RPMI-8226, MC-CAR, and U266 cell lines. However, it had no effect on CHO or ARH-77 cell lines. Error bars indicate the mean ± standard deviation. (B) rATG F(ab′)2 fragment activity was assessed by reconstituting with anti-FcR-specific monoclonal antibodies. CD40L-stimulated human B-cell blasts or myeloma cell lines (105 cells/well) were incubated with 250 μg/mL rATG F(ab′)2 fragments alone or coincubated with either human Fc fragments, anti-CD32, or anti-CD64 (♦). The response is measured against the intact rATG molecule (▪). Controls without rATG F(ab′)2 but with unimmunized rIgG, Fc fragments, anti-CD32, or anti-CD64 are also shown (⋄). rATG F(ab′)2 fragment with or without human Fc fragment showed a weaker apoptotic induction potential than intact rATG (P < .05, all samples). For CD40L sBc, RPMI-8226, and NCI-H929 cells, rATG F(ab′)2 fragments coincubated with anti-CD32 or anti-CD64 increased apoptosis of myeloma cells, although still somewhat less than intact rATG. No effect was observed on U266 cells.
We next examined whether FcR binding augmented the degree of ATG-induced apoptosis. Both plasma and myeloma cells express CD32 and thus binding of the Fc portion of rATG might be expected to aid in crosslinking of proapoptotic surface molecules and augment apoptotic signaling of rATG. We therefore examined the contribution of the Fc portion of rATG to apoptosis induction by comparing the apoptotic efficacy of intact rATG and F(ab′)2 rATG. Incubation of RPMI-8226, U266, and NCI-H929 cells with rATG F(ab′)2 fragments resulted in lower levels of apoptosis compared with the intact molecule (Figure 4B). Coincubation of CD40L sBc, RPMI-8226, and NCI-H929 cells with rATG F(ab′)2 and anti-CD32 or anti-CD64, but not human Fc fragments, restored the apoptotic activity of the F(ab′)2 fragments to a similar or higher magnitude than the intact rATG. The U266 myeloma cell line appeared to be much more resistant to rATG F(ab′)2 fragment-induced apoptosis.
What are the targets of rATG on myeloma cells?
The observation that rATG can induce myeloma-cell death by complement-independent and -dependent mechanisms suggests that rATG has activity against specific surface markers on myeloma cells. To determine if rATG contained antibodies directed at cell-surface proteins linked to proapoptotic pathways or growth factor-receptor pathways (eg, IL-6R), we performed competitive binding studies using myeloma cell lines. Given previous data describing the emergence of a putative myeloma stem cell capable of regenerating CD138+ myeloma clones,10,14 we also tested for rATG activity against a variety of preplasma cell-surface markers expressed on naive and CD40L-activated B cells. Cells and cell lines used in these experiments were selected for expression of the target molecule of interest. Cells were incubated in either rATG or unimmunized rabbit Ig for 1 hour at 37°C, followed by labeling with monoclonal antibodies specific for known proapoptotic B-cell and myeloma cell-surface proteins, as well as several B-cell and myeloma cell-specific markers (Figure 5). While a negative result in such assays does not exclude the possibility that rATG contains antibodies directed at an alternate epitope, a positive result strongly suggests the presence of antibodies directed against epitopes recognized by both the tagged monoclonal and unlabeled rATG polyclonal antibody preparations. We observed inhibition of indicator antibody binding for HLA-ABC, HLA-DR, CD32 (FcRγ2), CD19, CD20, CD30, CD38, and CD95. Significantly, rATG inhibited binding of antibodies directed against the plasma cell-surface proteins CD126 (IL-6R) and CD138 (syndecan-1).
Which of the shared specificities of rATG are the likely targets for rATG activity?
We next phenotyped the 6 cell lines and a cross-section of 5 myeloma-cell aspirates to determine the frequency of target antigen expression (Figure 5). HLA-ABC, CD30, CD32, CD38, CD95, CD126, and CD138 were all expressed in a significant percentage of myeloma cells and cell lines, demonstrating that most myeloma cells displayed target antigens for rATG binding. Of interest, U266, which was less sensitive to rATG-induced apoptosis and complement-mediated lysis, had low expression of CD32 (FcγIIR), CD30, and CD40 compared with other cell lines and primary myeloma cells. Similarly, The NCI-H929 cell line had lower expressions of CD30, CD40, and CD95 along with a lower sensitivity to rATG-induced apoptosis.
Why does rATG contain antibodies against CD138+ myeloma and plasma cells?
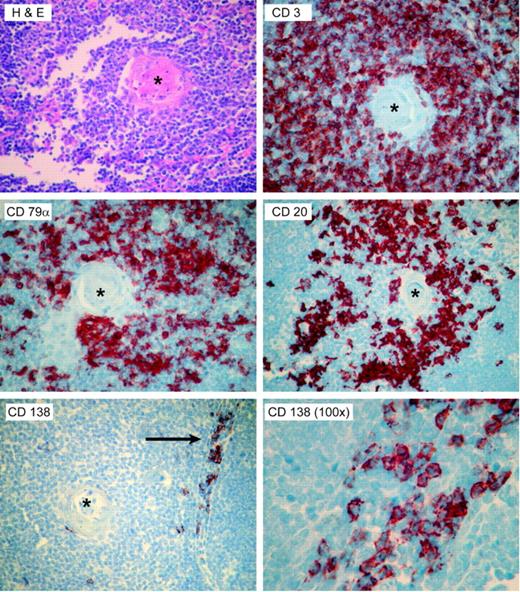
ATG is made by immunizing rabbits against unfractionated, Ficoll-isolated preparations of normal human pediatric thymocytes. The finding of B-cell- and plasma cell-specific antibodies, such as those directed against CD20, CD79α, and CD138, suggests that these preparations contain B cells. To investigate this hypothesis, we stained 10 normal pediatric thymii, excised during pediatric thoracotomy for cardiac surgery, for CD138 as well as CD20. A representative sample is shown in Figure 6. Cells staining positive for both markers were present in thymic tissues, with CD20+ B cells present around the Hassel corpuscles, and CD138+ cells near the medullary cord areas.
Which cell-death pathways are activated by rATG in CD138+ myeloma cells?
In previous work with CD40L-activated human B cells, we found that rATG triggered caspase-dependent and cathepsin D-dependent cell-death pathways. Myeloma cells are known to vary in their sensitivity to various proapoptotic stimuli and pathways, and we therefore asked which of several apoptosis pathway inhibitors could prevent rATG-induced myeloma-cell death (Table 1). Significant reductions in rATG-triggered apoptosis were observed with the cathepsin D inhibitor pepstatin A in NCI-H929 and ARH-77 lines, and with tyrosine kinase inhibitor genistein in NCI-H929 and U266 myeloma cell lines. Modest reductions in apoptosis were seen in the RPMI-8226 and U266 cell lines with the pancaspase inhibitor z-VAD-fmk. In contrast to our findings in CD40L-stimulated human B cells (sBc's), the inhibitor of lysosomal cysteine proteases (E64d) cathepsins B and D had no effect on rATG-induced apoptosis. Inhibitors of mitogen-activated protein (MAP) kinases (SB202474, SB203561) had no significant reduction in rATG-induced cell death in any of the cell lines.
Identification of rATG-binding targets by competitive inhibition of binding. NCI-H929 myeloma cells were incubated with control rabbit IgG (□) or rATG (▪) for 1 hour at 37°C followed by incubation on ice with the monoclonal antibody noted to assess for blockade of rATG binding. Myeloma cell lines and naive and CD40L-activated B cells were used as targets based on expression of the antigen of interest: RPMI-866 (IgG1, CD38), NCI-H929 (HLA-ABC, CD16, CD32, CD64, CD126 [IL-6R]), naive human B cells (CD52), CD40L-activated B cells (HLA-DR, CD19, CD20, CD27, CD30, CD40, CD80, CD95), and human myeloma cells (CD38, CD138). Significant inhibition of rATG binding to numerous B-cell-specific surface proteins from all stages of B-cell development was observed. Error bars denote 1 SD. The table shows the average fraction of myeloma cell lines and cells from 3 patients expressing the noted surface marker. Relative level of common expression of each marker among all cell types is summarized at the bottom of the table.
Identification of rATG-binding targets by competitive inhibition of binding. NCI-H929 myeloma cells were incubated with control rabbit IgG (□) or rATG (▪) for 1 hour at 37°C followed by incubation on ice with the monoclonal antibody noted to assess for blockade of rATG binding. Myeloma cell lines and naive and CD40L-activated B cells were used as targets based on expression of the antigen of interest: RPMI-866 (IgG1, CD38), NCI-H929 (HLA-ABC, CD16, CD32, CD64, CD126 [IL-6R]), naive human B cells (CD52), CD40L-activated B cells (HLA-DR, CD19, CD20, CD27, CD30, CD40, CD80, CD95), and human myeloma cells (CD38, CD138). Significant inhibition of rATG binding to numerous B-cell-specific surface proteins from all stages of B-cell development was observed. Error bars denote 1 SD. The table shows the average fraction of myeloma cell lines and cells from 3 patients expressing the noted surface marker. Relative level of common expression of each marker among all cell types is summarized at the bottom of the table.
The presence of B cells and plasma cells in the normal human thymii. Ten pediatric thymii were stained by hematoxylin and eosin, and by immunohistochemistry for the T-cell-specific marker CD3, the pan-B-cell markers CD79α and CD20, and the plasma cell-specific marker CD138. Representative photomicrographs from a single thymus are shown. The CD20+ cells appeared to cluster around the Hassel corpuscles (asterisk), while the CD138-staining plasma cells were seen in the medullary areas (arrow). Micrographs are all × 40, except as noted.
The presence of B cells and plasma cells in the normal human thymii. Ten pediatric thymii were stained by hematoxylin and eosin, and by immunohistochemistry for the T-cell-specific marker CD3, the pan-B-cell markers CD79α and CD20, and the plasma cell-specific marker CD138. Representative photomicrographs from a single thymus are shown. The CD20+ cells appeared to cluster around the Hassel corpuscles (asterisk), while the CD138-staining plasma cells were seen in the medullary areas (arrow). Micrographs are all × 40, except as noted.
Discussion
Variations in surface marker expression among patients with hematologic malignancies, including multiple myeloma, are a serious barrier to developing targeted monoclonal antibody therapies. Not only is there variation among patient populations with respect to the degree of surface marker expression, but cell-surface phenotypes may vary in different treatment phases as the malignant clone becomes more or less differentiated. In myeloma, the recognition of the “myeloma stem cell” phenomenon, where CD138 myeloma cells can emerge from CD138-, phenotypically “normal” B-cell precursors, raises doubts as to whether a single monoclonal antibody will be effective for tumor ablation. This theoretical issue, coupled with our observation that polyclonal rATG has proapoptotic activity against human plasma cells, suggested to us that rATG might be a candidate agent for treatment of myeloma.
Early laboratory investigations indicated that rATG had proapoptotic activity against B cells and human B-cell lines. Bonnefoy-Berard and colleagues showed that rATG had binding activity against CD11a, CD18, CD45, CD3, CD5, HLA-DR, CD2, CD4, CD8, and CD25, some of which were expressed by B lymphocytes.34 We have recently demonstrated that rATG induced high levels of apoptosis in human naive, memory, and activated B cells, as well as plasma cells, and identified many B-cell-specific surface markers that are targets for rATG.17 We now extend this observation to myeloma cell lines and primary cultures. In this report, we characterize in detail the activity of rATG against human myeloma cell lines and CD138+ myeloma cells from primary bone marrow aspirates. As measured by multiple assays, rATG induced complement-independent apoptosis in virtually all myeloma cell lines and primary isolates tested. This complement-independent apoptosis was partially blocked by inhibitors of cathepsin B and the pancaspase inhibitor z-VAD-fmk. The presence of complement, as one would expect in vivo, significantly increased myeloma-cell death, even for those cell lines and primary myeloma-cell isolates that did not undergo substantial apoptosis.
Polyclonal ATGs have been used as immunosuppressive agents in solid organ and bone marrow transplantation and in the treatment of graft-versus-host disease. Clinical preparations of ATG have been made by immunizing horses or rabbits with human thymocytes or activated T- or B-cell blasts. Several studies recognized that most ATG preparations contain antibodies reactive against both T and B cells.34-37 While the use of “thymocytes” as the immunogen has led most clinicians to think of ATGs as selective anti-T-cell agents, this assumption is incorrect. We have shown that rATG prepared by immunization with pediatric human thymocytes has specific activity against CD19, CD20, CD27, CD38, CD126, and CD138, surface proteins expressed by naive and activated B cells, normal plasma cells, and myeloma cells. These antibodies are a direct result of the presence of CD20+ B cells and CD138+ plasma cells in the thymocyte innocula.
Of particular relevance for treatment of multiple myeloma is the presence of antibodies directed against the IL-6R and syndecan-1 (CD138). IL-6 is a potent growth factor for plasma cells and myeloma cells, supporting extramedullary growth of primary myeloma cells.38 Blockade of IL-6 signaling by antagonistic monoclonal antibodies induces apoptosis in many primary myeloma cells.39,40 Interestingly, some myeloma cell lines require blockade of heparin-binding epidermal growth factor, for which CD138 is a coreceptor. Syndecans act as coreceptors for heparin-binding growth factors (HBGFs) and may increase local concentration of these ligands, allowing enhanced receptor activation even at low ligand concentrations.41 In addition, syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma.42,43 Heparin-binding epidermal growth factor-like growth factor (HB-EGF) receptor genes ErbB1 and ErbB4 are expressed by many myeloma cell lines, and augment the ability of low levels of IL-6 to support myeloma-cell growth. Blockade of EGF signaling sensitizes these myeloma cells to anti-IL-6-or dexamethasone-induced apoptosis.44 Interestingly, EGF receptors are also present on immature thymocytes.45 In light of this and the work reported here, further investigation to determine if anti-EGF receptor antibodies are also present in rATG seems warranted.
It is interesting to note that binding of rATG to the FcR appears to increase the efficacy of rATG-induced apoptosis for many myeloma cell lines. This appears to be a consequence of FcR cross-linking and FcR ligation, as this activity can be restored when F(ab′)2 fragments of rATG are combined with either divalent antibodies that cross-link, or monovalent Fc fragments which bind to the FcγII receptor. Ligation of the FcR in B cells under certain conditions has been reported to induce apoptosis,46 and FcR heterogeneity may also explain the differential sensitivity of some patients with lupus to treatment with anti-CD20.47 Thus, particularly for myeloma cells which express the FcγII, it seems advantageous to have antibodies with a functional Fc region for both complement binding and for apoptosis induction.
Many of the specificities of rATG relevant to B cells have known roles in apoptosis signaling. Thus, a polyclonal approach may be advantageous when targeting myeloma cells, which may be heterologous in their susceptibility to any single monoclonal antibody. We observed this phenomenon in this study, with differential sensitivity among myeloma cell lines to the different modes of cell death induced by rATG (caspase- or cathepsin-mediated apoptosis, complement-mediated lysis). Another potential approach to this problem would be to create “poly-monoclonal” reagents: defined mixtures of monoclonal antibodies that target multiple surface proteins expressed at different stages of lymphocyte development.
One potential issue with this strategy is that we did not determine the relative concentrations of B-versus T-cell-directed antibodies in the rATG preparations. It is therefore possible that clinical anti-B-cell activity of rATG may be inadequate to “debulk” the pre-plasma-cell compartment of patients with a high myeloma-cell burden. In such cases, we speculate that it may be advantageous to use combination therapy with rATG and rituximab to further boost the anti-B-cell effect.
A related issue is the risk of infection with rATG therapy in myeloma given its ability to deplete the T-cell compartment. Infections related to T-cell depletion tend to be those of viral origin, including cytomegalovirus, Epstein-Barr virus, parvovirus, adenovirus, and herpesviruses. However, the experience in solid organ transplantation has shown that the T-cell compartment recovers within 1 to 2 months, making this a manageable issue. In addition, one might consider a clinical treatment strategy that involved leukopheresis and isolation of CD3+ T cells, which can then be reinfused when rATG levels fall. Finally, rATG appears to have only modest and temporary effects against normal hematopoietic cells. The extensive experience with rATG in treatment of aplastic anemia has shown that the hematologic response to ATG therapy is associated with increased numbers of hematopoietic progenitors after treatment.48 Supporting this observation is the finding that CD34+ hematopoietic stems cells from healthy individuals are Fas negative and insensitive to rATG-induced apoptosis.49 Clinical recovery of hematopoietic progenitor compartment may be also improved with administration of granulocyte colony-stimulating factor.50
The results reported here lead us to hypothesize that rATG may have utility as a component of a clinical regimen for myeloma- and plasma-cell malignancies. Further careful clinical study will be needed to examine this possibility, as positive in vitro results may not be predictive of in vivo outcomes. Response to rATG therapy may vary among patients as clonal sensitivity to the multiple apoptosis pathways varies. In addition, the marrow microenvironment may convey additional protection from rATG-induced apoptosis or complement-mediated lysis. We would suggest that any trial design include a component of in vitro screening before rATG administration.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2005-06-2269.
Supported in part by research funding from Genzyme, Cambridge, MA (M.Z.), National Institutes of Health grant no. RO1 AI049660-01A1 and U19 AI56390 (I.S.), and training grants from the Department of Surgery, Division of Solid Organ Transplantation (T.V.) and the Allergy, Immunology and Rheumatology Unit (J.H.), University of Rochester Medical Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Karen Rosell of the University of Rochester Medical Center Bone Marrow Transplant Unit for her assistance with the bone marrow aspirate samples, and all of the myeloma patients and healthy volunteers who gave cells for this work.

![Figure 5. Identification of rATG-binding targets by competitive inhibition of binding. NCI-H929 myeloma cells were incubated with control rabbit IgG (□) or rATG (▪) for 1 hour at 37°C followed by incubation on ice with the monoclonal antibody noted to assess for blockade of rATG binding. Myeloma cell lines and naive and CD40L-activated B cells were used as targets based on expression of the antigen of interest: RPMI-866 (IgG1, CD38), NCI-H929 (HLA-ABC, CD16, CD32, CD64, CD126 [IL-6R]), naive human B cells (CD52), CD40L-activated B cells (HLA-DR, CD19, CD20, CD27, CD30, CD40, CD80, CD95), and human myeloma cells (CD38, CD138). Significant inhibition of rATG binding to numerous B-cell-specific surface proteins from all stages of B-cell development was observed. Error bars denote 1 SD. The table shows the average fraction of myeloma cell lines and cells from 3 patients expressing the noted surface marker. Relative level of common expression of each marker among all cell types is summarized at the bottom of the table.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-06-2269/5/m_zh80070693620005.jpeg?Expires=1765913421&Signature=Go6hUXXZPPlAgi7pzo86xzIQd0pGDRJVnvYgtHeQQzsw8T4EMomPCjE08J~0EUtRH~W3XpN-tVHh8phEnskwI64mNqHDOFmNdGo2qYPNwf1rbk2QRsJVzA19S0sUfAUTmidmuIxvqNlZsoNSvQJiaLcffBRe3n~vwA5NlFiDFlTBf8Aoz9f7E9JIfRf1zMh4qmQwiuqLRDPfgxeRvYDtWD8NOf6so75o2Tb9h6egL52mYDI6aDrgsrsmxG4Uigtw2HLS92~XryRAGnfRCaiCBEeUVSnyXW-W95d2V8kLioJ62wbvJfVd~luKD~nAuDSj8K8n8MYI-Jpk0w~L8frXLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)